Abstract
Objectives
Using individual patient-level data from the phase 3 VIALE-A trial, this study assessed the cost-effectiveness of venetoclax in combination with azacitidine compared with azacitidine monotherapy for patients newly diagnosed with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy, from a United States (US) third-party payer perspective.
Methods
A partitioned survival model with a 28-day cycle and three health states (event-free survival (EFS), progressive/relapsed disease, and death) was developed to estimate costs and effectiveness of venetoclax + azacitidine versus azacitidine over a lifetime (25-year) horizon. Efficacy inputs (overall survival (OS), EFS, and complete remission (CR)/CR with incomplete marrow recovery (CRi) rate) were estimated using VIALE-A data. Best-fit parametric models per Akaike Information Criterion were used to extrapolate OS until reaching EFS and extrapolate EFS until Year 5. Within EFS, the time spent in CR/CRi was estimated by applying the CR/CRi rate to the EFS curve. Past Year 5, patients still in EFS were considered cured and to have the same mortality as the US general population. Mean time on treatment (ToT) for both regimens was based on the time observed in VIALE-A. Costs of drug acquisition, drug administration (initial and subsequent treatments), subsequent stem cell transplant procedures, adverse events (AEs), and healthcare resource utilization (HRU) associated with health states were obtained from the literature/public data and inflated to 2021 US dollars. Health state utilities were estimated using EuroQol-5 dimension-5 level data from VIALE-A; AE disutilities were obtained from the literature. Incremental cost-effectiveness ratios (ICERs) per life-year (LY) and quality-adjusted life-year (QALY) gained were estimated. Deterministic sensitivity analyses (DSA), scenario analyses, and probabilistic sensitivity analyses (PSA) were also performed.
Results
Over a lifetime horizon, venetoclax + azacitidine versus azacitidine led to gains of 1.89 LYs (2.99 vs. 1.10, respectively) and 1.45 QALYs (2.30 vs. 0.84, respectively). Patients receiving venetoclax + azacitidine incurred higher total lifetime costs ($250,486 vs. $110,034 (azacitidine)). The ICERs for venetoclax + azacitidine versus azacitidine were estimated at $74,141 per LY and $96,579 per QALY gained. Results from the DSA and scenario analyses supported the base-case findings, with ICERs ranging from $60,718 to $138,554 per QALY gained. The results were most sensitive to varying the parameters for the venetoclax + azacitidine base-case EFS parametric function (Gompertz), followed by alternative approaches for ToT estimation, treatment costs of venetoclax + azacitidine, standard mortality rate value and ToT estimation, alternative sources to inform HRU, different cure modeling assumptions, and the parameters for the venetoclax + azacitidine base-case OS parametric function (log-normal). Results from the PSA showed that, compared with azacitidine, venetoclax + azacitidine was cost-effective in 99.9% of cases at a willingness-to-pay threshold of $150,000 per QALY.
Conclusions
This analysis suggests that venetoclax + azacitidine offers a cost-effective strategy in the treatment of patients with newly diagnosed AML who are ineligible for intensive chemotherapy from a US third-party payer perspective.
Trial Registration
ClinicalTrials.gov, NCT02993523. Date of registration: 15 December 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In a cost-effectiveness analysis of venetoclax + azacitidine versus azacitidine alone for patients newly diagnosed with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy, venetoclax + azacitidine was associated with gains of 1.89 life-years (LYs) and 1.45 quality-adjusted life-years (QALYs) over azacitidine. |
The incremental cost-effectiveness ratios for venetoclax + azacitidine versus azacitidine were $74,141 per LY and $96,579 per QALY gained, lower than the common willingness-to-pay threshold of $150,000/QALY in the USA. |
This analysis suggests that venetoclax + azacitidine is a cost-effective treatment for patients with newly diagnosed AML who are ineligible for intensive chemotherapy from a US third-party payer perspective. |
1 Introduction
Acute myeloid leukemia (AML) is a blood cancer characterized by the proliferation of immature cells in the bone marrow and blood and, subsequently, the impairment of normal blood cell production [1]. An estimated 20,240 new cases of AML and 11,400 AML-related deaths occurred in the United States of America (USA) in 2021 [2]. The overall incidence of AML, estimated at 4.3 per 100,000 persons per year in the USA, increases with age, with a median age at diagnosis of 68 years [2].
The initial treatment for newly diagnosed AML (ND-AML) consists primarily of high-intensity induction chemotherapy, followed by hematopoietic stem cell transplantation (HSCT) for those eligible [3, 4]. However, over half of patients are clinically unfit for this approach, particularly elderly patients with comorbidities, poorer health status, and other adverse prognostic risk factors [5,6,7]. Alternative treatment options are limited to less intensive regimens such as hypomethylating agents (HMAs; e.g., azacitidine) [4], although HMAs have not demonstrated high response rates (remission rates: ~ 20–30%) or significant improvement in survival compared with best supportive care or low-dose cytarabine [7,8,9,10]. As a result, patients with AML ineligible for intensive chemotherapy experience poorer clinical outcomes, with a median survival of less than 1 year and an estimated 5-year survival rate below 10% [10,11,12]. Thus, there exists a substantial unmet need for innovative treatments for this patient population.
Venetoclax is a selective small-molecule B-cell lymphoma 2 inhibitor that causes apoptosis in leukemic cells and slows disease progression [13]. In October 2020, the US Food and Drug Administration (FDA) gave full approval of venetoclax in combination with azacitidine for adults with ND-AML aged ≥ 75 years or who have comorbidities that preclude the use of intensive induction chemotherapy [14], based on the results of the phase 3, placebo-controlled, international VIALE-A trial (ClinicalTrials.gov identifier: NCT02993523) [15]. In VIALE-A, previously untreated patients with AML ineligible for induction therapy were randomized to venetoclax + azacitidine (n = 286) or azacitidine + placebo (n = 145); both groups had a mean age of 75 years and 60% of patients were male. At a median follow-up of 20.5 months, venetoclax + azacitidine demonstrated significant clinical benefits over azacitidine + placebo in terms of overall survival (OS; median: 14.7 vs. 9.6 months, respectively), complete remission (CR; 36.7 vs. 17.9%, respectively), and composite CR (CR or CR with incomplete marrow recovery (CRi); 66.4 vs. 28.3%, respectively) (all p < 0.001) [15].
While the efficacy of venetoclax + azacitidine has been demonstrated in VIALE-A, to date, only one study has examined its cost-effectiveness over a lifetime horizon in the USA. Using aggregated published data from VIALE-A [15], that study predicted an incremental quality-adjusted life-year (QALY) of 0.61 for venetoclax + azacitidine versus azacitidine, and an incremental cost-effectiveness ratio (ICER) of $260,343 per QALY gained [16]. At a willingness-to-pay (WTP) threshold of $150,000 per QALY, the study concluded that venetoclax + azacitidine was not cost-effective for patients with ND-AML unfit for intensive chemotherapy in the USA. However, the analysis was limited by the use of published aggregate data from VIALE-A, whereas individual patient-level data (IPD) would best inform the appropriate modeling approaches and assumptions on long-term efficacy. In addition, the cure assumption, commonly used in prior AML economic evaluations to inform the long-term data extrapolation [17,18,19], was not adopted. Furthermore, these results were based on reconstructed survival data from the literature, which may be less accurate than using patient-level data.
To address these limitations, the current study used IPD from VIALE-A to assess the cost-effectiveness of venetoclax + azacitidine versus azacitidine for patients with ND-AML who are ineligible for intensive chemotherapy, from a US third-party payer perspective.
2 Methods
2.1 Model Overview
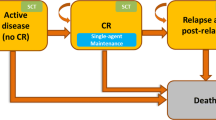
A three-state partitioned survival model (PSM) was constructed in Microsoft Excel to assess the cost-effectiveness of venetoclax + azacitidine versus azacitidine among patients with ND-AML ineligible for intensive chemotherapy. The model was conducted from the US third-party payer’s perspective and only direct costs were included. The base-case analysis considered a lifetime horizon (25 years) with costs and effectiveness discounted 3.0% annually. A 28-day cycle length was used.
In the PSM, patients transit between three mutually exclusive health states: (1) event-free survival (EFS), (2) progressive disease/relapse (PD/RL), and (3) death (Fig. 1). EFS was defined as a state without any of the following: disease progression, relapse from CR/CRi, treatment failure, or death from any cause. The proportion of patients in the EFS health state was defined by the EFS curve of each treatment. Within EFS, a proportion of time was assumed to be spent in CR/CRi, estimated by applying the CR/CRi rate to the EFS curve. PD/RL was the state after disease progression, relapse from CR/CRi, or treatment failure. The proportion of patients in the PD/RL health state was set to be equal to the difference between the proportion of living patients (based on the OS curve) and the EFS curve. Death was the absorbing state.
2.2 Model Assumptions
In the base case, the model assumed for both treatments that patients who remained in EFS at Year 5 became long-term survivors of AML. After Year 5, these patients were assumed to follow the natural mortality rate of the US general population and to incur the health state costs and utility inputs of long-term survivors (assumed equal to the inputs for patients in EFS with CR/CRi state). Year 5 was selected to be a conservative assumption cure point in the base case. The EFS curve of venetoclax + azacitidine after 2 years from VIALE-A plateaus, and all patients who remained in EFS beyond 2 years were in CR/CRi [Online Supplemental Material (OSM) Fig. 1]. In addition, a cure point between 2 and 5 years had been cited as the most clinically plausible scenario by clinical experts in existing technology assessments on cost-effectiveness in AML [17,18,19]. Furthermore, within EFS until Year 5, the model assumed a constant proportion of patients achieving CR/CRi. Given that all patients on venetoclax + azactidine who remained in EFS beyond 2 years were in CR/CRi, this assumption is considered conservative.
Costs of grade 3/4 adverse events (AEs) that affected ≥ 5% patients in any treatment arm were considered in the model. The costs of subsequent pharmacological treatments were considered for patients in the PD/RL state who received subsequent treatments in each arm. Costs of subsequent HSCT were applied to the proportion of patients who received subsequent HSCT. The effectiveness of subsequent HSCT and subsequent pharmacological treatments on OS were assumed to be reflected in the trial results because these treatments were allowed in VIALE-A after discontinuing initial treatment. Patients were assumed to incur costs related to routine monitoring, regular check-ups, and other medical resource use (hospitalizations, blood transfusions), independent of treatments received and specific to each health state. All patients incurred terminal-care costs before death.
Health state utility values were assumed to be dependent on health states and independent of treatment arm. Grade 3/4 AEs and subsequent HSCT were assumed to reduce utility among affected patients.
2.3 Model Inputs
The key model inputs are presented in Table 1.
2.3.1 Efficacy Inputs
Efficacy inputs for venetoclax + azacitidine and azacitidine, including OS, EFS, and CR/CRi rates, were based on IPD from VIALE-A (data cut-off: 4 January 2020; data on file, Genentech, Inc.). The observed OS and EFS data for each treatment were fit to six parametric functions: exponential, Weibull, Gompertz, log-normal, log-logistic, and generalized gamma.
In the base-case analysis, parametric function was selected to predict OS and EFS until Year 5 for both treatments because it has the lowest Akaike Information Criterion (AIC) value and demonstrates a good fit with the observed curves based on visual inspection (OSM Figs. 2–5). Specifically, log-normal (venetoclax + azacitidine OS), exponential (azacitidine OS), Gompertz (venetoclax + azacitidine EFS), and exponential (azacitidine EFS) distributions were used. At the end of Year 5, those who remained in EFS were assumed to be long-term AML survivors (functionally cured), with survival estimated based on the 2018 US life table [20]. The same mortality risk was applied to azacitidine to be conservative. The use of long-term AML survivors’ mortality rate reduced the uncertainty associated with the long-term extrapolation of clinical trial data. A similar approach has been employed in other cost-effectiveness studies in AML [17,18,19], with a cure time point between 2 and 5 years considered the most clinically plausible scenario. Year 5 was selected as the conservative cure assumption based on clinical inputs and literature [21,22,23] and the Kaplan–Meier curve of venetoclax + azacitidine observed from VIALE-A [15]. After Year 5, the best-fit parametric function was still used to estimate OS for both arms, bounded by the natural mortality based on the 2018 US life table [20], until OS curves reached EFS curves.
2.3.2 Utility Inputs
In VIALE-A, EuroQol Group-5 Dimension-5 Level Instrument (EQ-5D-5L) data were collected. The utility for each health state was calculated using pooled EQ-5D-5L data from both arms in VIALE-A based on the US crosswalk preference-weights from van Hout et al. [24]. A linear mixed-effects model was used to account for correlation within patients' repeated assessments.
The disutility inputs associated with grade 3/4 AEs were obtained from Wehler et al. [25], which summarized the AE disutility values from AML literature (OSM Table 1). The disutility associated with HSCT was 0.3 based on Guadagnolo et al. [26].
2.3.3 Cost Inputs
The cost inputs were from US public databases or the best data available in the literature. They were reported in or inflated to 2021 US dollars based on the Personal Consumption Expenditure Price Index for healthcare from the US Bureau of Economic Analysis [27].
Unit drug costs were obtained from Red Book® [28] and unit administration costs were obtained from the 2021 Centers for Medicare and Medicaid Services (CMS) Physician Fee Schedule [29]. The dosing schedule, dose intensity, administration route, administration frequencies, and treatment duration for venetoclax + azacitidine and azacitidine were obtained from VIALE-A. Dose intensity was applied to the drug acquisition costs and was calculated as the ratio of actual dose (obtained from trial observations that reflected any dose reduction, interruption, or hold) and full expected dose (based on the VIALE-A dosing schedule) for each treatment. The average treatment duration was considered for both treatments to capture the duration of treatment use accordant with both the clinical trial observation and the efficacy data. In VIALE-A, patients may not have discontinued treatments at the end of follow-up. However, the phase 1b study of venetoclax + azacitidine in the same population had a longer follow-up than VIALE-A (29 vs. 20.5 months, respectively), but patients had a similar mean treatment duration to that of VIALE-A (10.4 vs. 9.9 months, respectively) [30]. Such an approach has been used and accepted in prior National Institute for Health and Care Excellence (NICE) AML submissions [17, 19], thus was considered as the base case. Vial sharing was not considered in the study.
The unit AE costs were obtained from the 2016 Healthcare Cost and Utilization Project (OSM Table 1) [31]. AE rates for venetoclax + azacitidine and azacitidine were obtained from VIALE-A. Unit drug and administration costs for the subsequent pharmacological treatments were obtained from Red Book® [28] and the 2021 CMS Physician Fee Schedule, respectively [29]. The dosing schedules and treatment durations were obtained from the literature [10, 32, 33].
The proportions of patients who received HSCT in the two arms were obtained from VIALE-A. The costs of HSCT (procedure and 1-year follow-up costs for patients with AML aged ≥ 65 years) were obtained from the literature [34].
Other medical costs for each health state included hospitalization, red blood cell and platelet transfusion, and other monitoring costs. No mandatory hospitalization for venetoclax + azacitidine during the treatment initiation period was assumed based on the venetoclax prescribing information [35]. Healthcare resource utilization (HRU) related to hospitalization during subsequent cycles and to transfusions for each health state was derived from the literature [36, 37]. Daily hospitalization costs were obtained from the literature based on patients with hematologic malignancies [38]. Transfusion unit costs were obtained from the 2021 CMS Hospital Outpatient Prospective Payment Schedule [39]. The specific monitoring tests were based on the National Comprehensive Cancer Network guideline for AML, including complete blood count, chemistry panel, and bone marrow aspirate and biopsy [6]. The frequencies of each test were derived from the NICE TA399 of azacitidine during remission, stable disease, and PD states [40]. The cost of each test was obtained from the 2021 CMS Clinical Laboratory Fee Schedule [41]. Terminal care costs were derived from the literature [27, 42].
2.4 Model Outputs
2.4.1 Base-Case Analysis
Total costs were calculated separately for patients receiving venetoclax + azacitidine or azacitidine as the sum of medical costs and the costs of initial treatment, AEs associated with initial treatment, subsequent treatment, and subsequent HSCT. Total life-years (LYs) and QALYs were aggregated for each treatment arm across the modeled time horizon. QALYs were estimated as the time spent in each state weighted by the utility of each state. ICERs were calculated as the total incremental costs per LY and QALY gained.
2.4.2 Deterministic Sensitivity Analyses (DSA) and Scenario Analyses
To assess the robustness of the model results, DSAs were conducted by varying one model input or assumption at a time while holding other assumptions or parameters constant (Table 2 describes the models and key inputs). In the sensitivity analyses, CR/CRi rates, parameters for EFS and OS parametric functions for each arm, health state utilities, and patient characteristics were varied by the 95% confidence interval (CI), while drug and administration costs, AE costs and disutilities, and medical costs associated with health states were varied by ± 25% from the base-case inputs.
In scenario analyses, scenarios included alternative parametric functions for EFS and OS, approaches for time-on-treatment (ToT) estimation, sources for medical costs, hospitalization and dose-reduction assumptions for venetoclax, discount rates and time horizons, and modeling assumptions for long-term extrapolation.
2.4.3 Probabilistic Sensitivity Analysis
Probabilistic sensitivity analyses (PSA) were conducted to examine the probability of venetoclax + azacitidine being cost-effective versus azacitidine when considering different willingness-to-pay (WTP) thresholds. A Monte Carlo simulation with 1000 iterations was conducted. In each iteration, key efficacy, utility, patient characteristic, and cost inputs were randomly drawn from the specified distribution and varied simultaneously to inform the possible range of the inputs (OSM Table 2). Correspondingly, QALYs, costs, and ICERs were calculated in each iteration.
3 Results
3.1 Base Case
Over a lifetime horizon (25 years), a patient with AML receiving venetoclax + azacitidine was expected to incur total costs of $250,486, compared with $110,034 if receiving azacitidine (Table 3). For venetoclax + azacitidine, 48% of the total costs ($119,555) were initial treatment costs and 41% ($103,512) were medical costs; the remaining 11% were attributed to AEs ($25,116), subsequent HSCT ($1753), and subsequent treatment ($550). For azacitidine, 28% of the total costs ($30,654) were initial treatment costs and 52% ($56,977) were medical costs; the remaining 20% were attributed to AEs ($19,089), subsequent HSCT ($1729), and subsequent treatment ($1585).
In terms of effectiveness, venetoclax + azacitidine was associated with 2.99 LYs and 2.30 QALYs, while azacitidine was associated with 1.10 LYs and 0.84 QALYs. Treatment with venetoclax + azacitidine was associated with gains of 1.89 LYs and 1.45 QALYs over azacitidine at an additional lifetime cost of $140,452, corresponding to $74,141 and $96,579 in incremental costs per LY and QALY gained, respectively.
3.2 DSA and Scenario Analysis
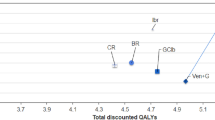
In the DSA, the model’s results remained robust. Across all scenarios evaluated, the ICER for venetoclax + azacitidine versus azacitidine ranged from $60,718 to $138,554 per QALY gained. The top 30 drivers in the DSA are summarized in Fig. 2. The model was most sensitive to varying the parameters for the venetoclax + azacitidine EFS parametric function (Gompertz), followed by using ToT IPD with best-fit parametric function, varying the treatment costs of venetoclax + azacitidine, using alternative standard mortality rate (SMR) values and exponential distribution to extrapolate ToT, an alternative HRU source based on the azacitidine submission to NICE, different long-term extrapolation assumptions applied to patients alive after Years 3 and 5, and varying the parameters for the venetoclax + azacitidine OS parametric function (log-normal). The majority of scenarios had a minimal effect on the ICER and none exceeded an ICER of $150,000 per QALY.
Deterministic sensitivity analysis (DSA) and scenario analysis sensitivity results: Top 30 drivers of incremental cost per quality-adjusted life-year (QALY) gained for venetoclax + azacitidine versus azacitidine. Costs listed in 2021 US dollars. AE adverse event, AIC Akaike Information Criterion, CI confidence interval, CR complete remission, CRi complete remission with incomplete marrow recovery, EFS event-free survival, HR hazard ratio, HRU healthcare resource utilization, NICE National Institute for Health and Care Excellence, OS overall survival, PD/RL progressive disease/relapse, SMR standard mortality rate, ToT time on treatment, US United States
3.3 Probabilistic Sensitivity Analysis
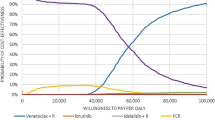
Across 1000 simulations, the estimated probability of venetoclax + azacitidine being cost-effective compared with azacitidine was 99.9% based on a WTP threshold of $150,000 per QALY (Fig. 3).
Probabilistic sensitivity analysis (PSA) results: Incremental cost-effectiveness plane (a) and cost-effectiveness acceptance curve (b) for venetoclax + azacitidine versus azacitidine. The red line indicates the willingness-to-pay threshold of $150,000 per QALY gained. ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
4 Discussion
The introduction of venetoclax + azacitidine addresses a critical unmet treatment need for patients with ND-AML who are ineligible for intensive chemotherapy, a population that has historically experienced poor outcomes with conventional low-intensity therapies. In this study, over a lifetime horizon, venetoclax + azacitidine was associated with incremental gains of 1.89 LYs and 1.45 QALYs versus azacitidine. Considering both the costs and clinical outcomes, venetoclax + azacitidine was associated with incremental costs of $74,141 per LY and $96,579 per QALY gained versus azacitidine. Although there is no consensus on the WTP threshold in the USA, the most recent value assessment framework by the Institute for Clinical and Economic Review suggested $150,000 per QALY as a value-based price benchmark [43], which has been widely used in US-based studies assessing the value and cost-effectiveness of AML therapies [44,45,46,47,48,49]. Considering the WTP threshold of $150,000 per QALY, venetoclax + azacitidine is a cost-effective treatment for patients with ND-AML ineligible for intensive chemotherapy in the USA. The results of extensive sensitivity analyses supported the base-case findings, as none of the incremental costs per QALY gained across all DSAs and scenario analyses exceeded $150,000 per QALY. Furthermore, the PSA results showed that the estimated probability of venetoclax + azacitidine being cost-effective versus azacitidine was 99.9% based on a WTP threshold of $150,000 per QALY.
This study has several distinct features that permitted robust estimates to assist healthcare payers in their coverage or reimbursement decision-making. First, this study benefited from the use of IPD for venetoclax + azacitidine and azacitidine from VIALE-A. Data from VIALE-A were directly incorporated into the model where possible. This contributed the most accurate information when estimating the costs, safety, and effectiveness of venetoclax + azacitidine and azacitidine, as well as a valid data source for utility inputs.
Second, although VIALE-A had a limited follow-up time (maximum 31 months), to reduce the uncertainty associated with long-term extrapolation of the data, an EFS cure assumption was considered. To be conservative, this cure assumption was also considered for azacitidine. The EFS cure approach is supported by the plateau of EFS curve of venetoclax + azacitidine after 2 years from VIALE-A (OSM Fig. 1), where all patients who remained in EFS beyond 2 years were in CR/CRi. Additionally, based on clinical inputs and literature [21,22,23, 50], there is a limited risk of relapse or death among patients with AML after 5 years of remission (1–3% of patients relapse).
Third, the robustness of the base-case results and the uncertainty associated with key model inputs and efficacy assumptions were examined using scenario analyses. These included the use of alternative costs scenarios, different parametric functions for OS/EFS estimation, varying time horizons and discount rates, and alternative cure assumptions. The ICER results in these analyses ranged from $60,718 to $138,554 per QALY, all below $150,000 per QALY gained. The scenario with the highest ICER used ToT IPD from VIALE-A and a best-fit parametric function (i.e., log-normal) to extrapolate the treatment duration. The ICER increased by 43.5% to $138,554 per QALY gained but remained below the WTP threshold of $150,000 per QALY. For venetoclax + azacitidine, 16 and 9% of patients were predicted to remain on treatment by the end of Years 3 and 5, respectively, which may be overestimates for this elderly population in clinical practice. Another scenario was to consider 100% hospitalization during the venetoclax ramp-up period because some patients could receive venetoclax + azacitidine in the inpatient setting in real-world practice. The ICER only increased by 5% to $101,422 per QALY gained. Finally, a cure time point from 2 to 5 years was considered clinically plausible, and a less conservative cure assumption starting from Year 3 decreased the ICER to $85,368.
To date, only one other study has assessed the cost-effectiveness of venetoclax + azacitidine in the USA among the same population [16]. That study used published aggregate VIALE-A data [15] and a Weibull function to predict the long-term efficacy outcomes for venetoclax + azacitidine. It assumed a constant relationship (i.e., a hazard ratio (HR) approach) between azacitidine and venetoclax + azacitidine to estimate OS and EFS for azacitidine. The resultant incremental QALY of 0.61 for venetoclax + azacitidine versus azacitidine equated to an ICER of $260,343 per QALY gained, above a WTP threshold of $150,000 per QALY [16]. In comparison, the present study directly used IPD from VIALE-A, which enabled a more accurate estimate of the efficacy of both treatments. The constant HR approach between venetoclax + azacitidine and azacitidine employed by the prior study was not consistent with trial observations. Specifically, by overlaying the OS and EFS curves of the two treatments, the difference between the two arms gradually increased, indicating that the treatment effect of venetoclax + azacitidine versus azacitidine was not constant over time. Thus, the HR approach may overestimate the efficacy of azacitidine. The current study fitted separate parametric models to each treatment to better capture their efficacy. Additionally, the previous study relied on Weibull distributions to project long-term OS and EFS of venetoclax + azacitidine, which is not the best-fit model if using IPD and underestimates the efficacy of venetoclax + azacitidine. More importantly, VIALE-A had limited follow-up time, and extrapolation using trial data over the entire time horizon could lead to large uncertainty and limit the ability to detect the long-term clinical benefits of venetoclax + azacitidine. Conversely, the current study used trial data for the first 5 years and SMR-adjusted natural mortality afterwards, assuming no additional clinical benefit for venetoclax + azacitidine versus azacitidine after Year 5 to reduce uncertainty. This approach has been well accepted in prior health technology assessments in AML [17, 42, 51]. The cure assumption and choice of the cure time point was supported by VIALE-A data, clinical experts, and previous literature [21,22,23].
The results of this CEA should be considered in light of several limitations. First, despite the best efforts to select the most accurate model inputs based on the trial data, some inputs, including disutility and HRU after progression, were sourced from publications other than VIALE-A. This approach may have introduced heterogeneity as there were inherent differences between patient populations across studies. Second, VIALE-A had a limited follow-up. To mitigate uncertainties from long-term extrapolation of data, the model assumed all patients who remained in EFS from Year 5 onward would have a mortality risk of the US general population and incur the medical costs and utility equal to the inputs for patients in EFS with CR/CRi state. Long-term survival was estimated using an SMR of 1 approach, considering this elderly patient population (i.e., patients aged ~ 80 years at Year 5). As a result, the choice of cure time point and SMR may introduce uncertainty to the model as the real-world AML population may become long-term survivors at varying time points and SMR. To address this uncertainty, extensive sensitivity analyses were performed, which confirmed the robustness of the results. Lastly, the model only included azacitidine as the comparator. Future studies versus other treatments, such as low-dose cytarabine, are warranted.
5 Conclusions
The results of this CEA suggest that venetoclax + azacitidine was cost-effective compared with azacitidine monotherapy, with an ICER of $96,579 over a lifetime horizon. Based on a WTP threshold of $150,000 per QALY, the estimated probability of venetoclax + azacitidine being cost-effective versus azacitidine was 99.9% in the USA. The cost-effectiveness of venetoclax + azacitidine was robust in all examined sensitivity analyses. Thus, in addition to the significant clinical benefit of venetoclax + azacitidine observed in VIALE-A, payers and other healthcare decision-makers may consider its cost-effectiveness compared with azacitidine monotherapy, when making reimbursement determinations for patients with ND-AML who are ineligible for intensive chemotherapy.
References
Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606.
Surveillance Epidemiology and End Results (SEER) Program. Cancer stat facts: leukemia—acute myeloid leukemia (AML). 2021 [cited 2021 August 13]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html.
Yu J, Jiang PYZ, Sun H, Zhang X, Jiang Z, Li Y, et al. Advances in targeted therapy for acute myeloid leukemia. Biomark Res. 2020;8(1):17.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Acute Myeloid Leukemia V.2.2021. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed August 14, 2021. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528–49.
Sadashiv SK, Hilton C, Khan C, Rossetti JM, Benjamin HL, Fazal S, et al. Efficacy and tolerability of treatment with azacitidine for 5 days in elderly patients with acute myeloid leukemia. Cancer Med. 2014;3(6):1570–8.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–9.
Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53(1):110–7.
Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61.
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–7.
Griffiths EA, Carraway HE, Chandhok NS, Prebet T. Advances in non-intensive chemotherapy treatment options for adults diagnosed with acute myeloid leukemia. Leuk Res. 2020;91:106339.
Zeidan AM, Wang R, Wang X, Shallis RM, Podoltsev NA, Bewersdorf JP, et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: a large population-based study in the United States. Blood Adv. 2020;4(10):2192–201.
Roberts AW. Therapeutic development and current uses of BCL-2 inhibition. Hematology. 2020;2020(1):1–9.
United States Food and Drug Administration. FDA grants regular approval to venetoclax in combination for untreated acute myeloid leukemia. 2020 [cited 2021 August 14]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-venetoclax-combination-untreated-acute-myeloid-leukemia.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29.
Patel KK, Zeidan AM, Shallis RM, Prebet T, Podoltsev N, Huntington SF. Cost-effectiveness of azacitidine and venetoclax in unfit patients with previously untreated acute myeloid leukemia. Blood Adv. 2021;5(4):994–1002.
National Institute for Health and Care Excellence (NICE). Gemtuzumab ozogamicin for untreated acute myeloid leukaemia [ID982]: Committee papers. 2018 [cited 2021 September 20]. Available from: https://www.nice.org.uk/guidance/ta545/evidence/appraisal-consultation-committee-papers-pdf-6596279678.
National Institute for Health and Care Excellence (NICE). Midostaurin for untreated acute myeloid leukaemia [TA523]. 2017 [cited 2021 September 20]. Available from: https://www.nice.org.uk/guidance/ta523/documents/committee-papers-2.
National Institute for Health and Care Excellence (NICE). Gilteritinib for treating relapsed or refractory acute myeloid leukaemia [TA642]. 2019 [cited 2021 September 20]. Available from: https://www.nice.org.uk/guidance/ta642/documents/committee-papers.
Arias E, Bastian B, Xu J, Tejada-Vera B. US state life tables, 2018. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2021;70(1):1–18.
Verma D, Kantarjian H, Faderl S, O’Brien S, Pierce S, Vu K, et al. Late relapses in acute myeloid leukemia: analysis of characteristics and outcome. Leuk Lymphoma. 2010;51(5):778–82.
Patel A, Agha M, Raptis A, Hou J-Z, Farah R, Redner RL, et al. Outcomes of patients with acute myeloid leukemia who relapse after 5 years of complete remission. Oncol Res. 2021;28(7):811–4.
Medeiros BC, Minden MD, Schuh AC, Schimmer AD, Yee K, Lipton JH, et al. Characteristics and outcomes of acute myelogenous leukemia patients with very late relapse (>5 years). Leuk Lymphoma. 2007;48(1):65–71.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
Wehler E, Storm M, Kowal S, Campbell C, Boscoe A. A health state utility model estimating the impact of ivosidenib on quality of life in patients with relapsed/refractory acute myeloid leukemia. Presented at the 23rd Congress of the European Hematology Association meeting during June 14–17, 2018 in Stockholm, Sweden. 2018. Available from: https://investor.agios.com/static-files/25de7161-9a10-4d8c-8a03-8bd5e7d0a5d3. Accessed 3 May 2022.
Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24(25):4116–22.
United States Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Personal Consumption Expenditure (PCE) Price Index, health care. 2021 [cited 2021 May 1]. Available from: https://apps.bea.gov/iTable/iTable.cfm?reqid=19&step=2#reqid=19&step=2&isuri=1&1921=survey.
IBM Micromedex. RED BOOK Online. 2021 [cited 2021 April 27]. Available from: https://www.micromedexsolutions.com.
Centers for Medicare and Medicaid Services. Medicare Physician Fee Schedule. 2020 [cited 2021 April 29]. Available from: https://www.cms.gov/medicare/physician-fee-schedule/search.
Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH, Pullarkat V, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208–17.
Healthcare Cost and Utilization Project. 2016 Hospital inpatient national statistics. 2018 [cited 2020 June 20]. Available from: https://hcupnet.ahrq.gov/#setup.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
Stahl M, DeVeaux M, Montesinos P, Itzykson R, Ritchie EK, Sekeres MA, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923–32.
Mau L-W, Meyer C, Burns LJ, Saber W, Steinert P, Vanness DJ, et al. Reimbursement, utilization, and 1-year survival post-allogeneic transplantation for Medicare beneficiaries with acute myeloid leukemia. JNCI Cancer Spectr. 2019;3(4):pkz048.
United States Food and Drug Administration. Highlights of prescribing information: VENCLEXTA (venetoclax). 2020 [cited 2021 April 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208573s023lbl.pdf.
Bell JA, Galaznik A, Farrelly E, Blazer M, Murty S, Ogbonnaya A, et al. Economic burden of elderly patients with acute myeloid leukemia treated in routine clinical care in the United States. Leuk Res. 2018;71:27–33.
Hagiwara M, Sharma A, Chung KC, Delea TE. Healthcare resource utilization and costs in patients with newly diagnosed acute myeloid leukemia. J Med Econ. 2018;21(11):1119–30.
Saito AM, Cutler C, Zahrieh D, Soiffer RJ, Ho VT, Alyea EP, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14(2):197–207.
American Association of Blood Banks (AABB). Centers for Medicare and Medicaid Services proposes Medicare hospital outpatient payment rates and policies for CY 2021. 2020 [cited 2021 April 29]. Available from: https://www.aabb.org/docs/default-source/default-document-library/positions/opps-2021-final-rule-summary.pdf?sfvrsn=fcc7f4c2_4.
National Institute for Health and Care Excellence (NICE). Azacitidine for treating acute myeloid leukaemia with more than 30% bone marrow blasts: Committee papers. 2016 [cited 2021 April 29]. Available from: https://www.nice.org.uk/guidance/ta399/documents/committee-papers.
Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule Files. 2020 [cited 2021 May 14]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files. Accessed 3 May 2022.
Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ, Teitelbaum AH. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8(6):75s–80s.
Institute for Clinical and Economic Review (ICER). 2020–2023 Value Assessment Framework. 2020 [cited 2021 September 20]. Available from: https://icer.org/wp-content/uploads/2021/03/ICER_2020_2023_VAF_013120-4-2.pdf. Accessed 3 May 2022.
Qi CZ, Bollu V, Yang H, Dalal A, Zhang S, Zhang J. Cost-effectiveness analysis of tisagenlecleucel for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma in the United States. Clin Ther. 2021;43(8):1300–19.
Salcedo J, Bulovic J, Young CM. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. Sci Rep. 2021;11(1):10838.
Sharma D, Xing S, Hung Y-T, Caskey RN, Dowell ML, Touchette DR. Cost-effectiveness analysis of lumacaftor and ivacaftor combination for the treatment of patients with cystic fibrosis in the United States. Orphanet J Rare Dis. 2018;13(1):172.
Le QA. Patient-level modeling approach using discrete-event simulation: a cost-effectiveness study of current treatment guidelines for women with postmenopausal osteoporosis. J Manag Care Spec Pharm. 2019;25(10):1089–95.
Lin E, Chertow GM, Yan B, Malcolm E, Goldhaber-Fiebert JD. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: a modeling study. PLOS Med. 2018;15(3): e1002532.
Chaudhary MA, Lubinga SJ, Smare C, Hertel N, Penrod JR. Cost-effectiveness of nivolumab in patients with NSCLC in the United States. Am J Manag Care. 2021;27(8):e254–60.
Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–24.
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–71.
National Institute for Health and Care Excellence (NICE). Azacitidine for treating acute myeloid leukaemia with more than 30% bone marrow blasts. Technology appraisal guidance [TA399]. 2016 [cited 2021 September 20]. Available from: https://www.nice.org.uk/guidance/ta399.
Acknowledgements
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc. Funding for this assistance was provided by Genentech, Inc. Editorial assistance was provided by Lynda McEvoy, PhD, Ashfield MedComms, an Ashfield Health company, funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Support for this study was provided by Genentech, Inc.
Conflict of interest
Keith W. Pratz is an associate professor of medicine at the Hospital of the University of Pennsylvania. Keith W. Pratz has received honoraria or consultancy fees from AbbVie, Astellas, Boston BioMedical, Jazz Pharmaceuticals, STI Pharmaceuticals, and Celgene; and institutional research funding from AbbVie, Astellas, Agios, and Millennium. Xinglei Chai, Jipan Xie, Lei Yin, and Xiaoyu Nie are employees of Analysis Group, Inc., which has received consulting fees from Genentech, Inc. Melissa Montez, Erica Iantuono, Lisa Downs, and Esprit Ma are employees of Genentech, Inc. and hold stock/options.
Compliance with ethical standards
This study is a post hoc analysis of previously collected data. No institutional board review was required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The research data are confidential and will not be shared.
Code availability
Code associated with this research will not be shared.
Author contributions
Conceptualization: all authors; methodology: all authors; formal analysis and investigation: XC, JX, LY, and XN; writing—original draft preparation: XC, JX, LY, XN; writing—review and editing: all authors; funding acquisition: EM; resources: EM; supervision: EM. All authors approved the version of the submitted manuscript and agree to be accountable for all aspects of the work.
Additional information
Erica Iantuono: Former employee of Genentech, Inc.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pratz, K.W., Chai, X., Xie, J. et al. Cost-Effectiveness Analysis of Venetoclax in Combination with Azacitidine Versus Azacitidine Monotherapy in Patients with Acute Myeloid Leukemia Who are Ineligible for Intensive Chemotherapy: From a US Third Party Payer Perspective. PharmacoEconomics 40, 777–790 (2022). https://doi.org/10.1007/s40273-022-01145-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01145-7