Abstract
Objectives
The aim of this study was to assess the cost effectiveness of midostaurin + cytarabine + daunorubicin (midostaurin arm) versus placebo + cytarabine + daunorubicin (placebo arm) in the treatment of adult patients with newly diagnosed FLT3-mutated acute myeloid leukemia (AML) who are eligible for standard cytarabine + daunorubicin chemotherapy, from a US third-party payer perspective.
Methods
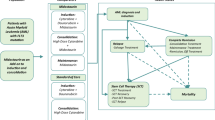
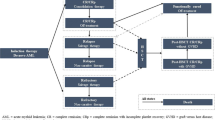
A lifetime partitioned survival model with four health states (active disease, complete remission [CR], relapse, and death) was constructed. Efficacy inputs (time to CR or death, time to relapse or death, and overall survival) were estimated using data from the RATIFY trial (NCT00651261). Costs (inflated to 2016 US dollars) included treatment, drug monitoring, stem cell transplantation (SCT), adverse events costs, and medical costs associated with health states. Incremental costs per quality-adjusted life-year (QALY) and life-year (LY) gained were estimated. Deterministic (DSA) and probabilistic sensitivity analyses (and PSA) were performed to assess model robustness.
Results
In the base case, patients in the midostaurin arm incurred higher total direct costs over a lifetime compared with the placebo arm ($4,043,470 vs. $3,959,741), resulting in an incremental cost of $83,729; however, the midostaurin arm had better effectiveness, with 1.59 more LYs and 1.37 more QALYs. These led to a base-case incremental cost-effectiveness ratio (ICER) of $52,596 per LY, or $61,167 per QALY. Results were robust in the DSA. In the PSA, the probability of the midostaurin arm being cost-effective compared with the placebo arm was 65.9%, at a willingness to pay of $150,000/QALY.
Conclusions
This analysis suggests that midostaurin is a cost-effective treatment for adult patients with newly diagnosed FLT3-mutated AML, from a US third-party payer perspective.





Similar content being viewed by others
References
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
American Cancer Society. What are the key statistics about acute myeloid leukemia? Available at: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html. Accessed 22 Sep 2018.
National Cancer Institute. Cancer stat facts: acute myeloid leukemia (AML). 2017. Available at: https://seer.cancer.gov/statfacts/html/amyl.html. Accessed Sep 2017.
Sotak M, Marin M, Coombs J, Teitelbaum A, editors. The burden of illness (BOI) of FLT3-mutated acute myeloid leukemia in the United States. In: 2012 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 17th Annual International Meeting; Washington, DC.
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–78.
Szer J. The prevalent predicament of relapsed acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2012;2012:43–8.
Gallogly MM, Lazarus HM. Midostaurin: an emerging treatment for acute myeloid leukemia patients. J Blood Med. 2016;7:73–83.
Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89.
Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–80.
Fathi AT, Chen YB. Treatment of FLT3-ITD acute myeloid leukemia. Am J Blood Res. 2011;1(2):175–89.
Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematol Am Soc Hematol Educ Program. 2013;2013:220–6.
Garcia JS, Stone RM. The development of FLT3 inhibitors in acute myeloid leukemia. Hematol Oncol Clin N Am. 2017;31(4):663–80.
Kim ES. Midostaurin: first global approval. Drugs. 2017;77(11):1251–9.
Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;26(129):3403–6.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64.
FDA. RYDAPT® (midostaurin): highlights of prescribing information. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207997s000lbl.pdf. Accessed Oct 2017.
Woods B, Sideris E, Palmer S, Latimer N. Nice DSU Technical Support Document 19: Partitioned survival analysis for decision modelling in health care: a critical review. 2017. Available at: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/06/Partitioned-Survival-Analysis-final-report.pdf. Accessed Sep 2017.
Stein EM, Bonifacio G, Latremouille-Viau D, Guerin A, Shi S, Gagnon-Sanschagrin P, et al. Treatment patterns, healthcare resource utilization, and costs in patients with acute myeloid leukemia in commercially insured and Medicare populations. J Med Econ. 2018;21(6):556–63.
Verma D, Kantarjian H, Faderl S, O’Brien S, Pierce S, Vu K, et al. Late relapses in acute myeloid leukemia: analysis of characteristics and outcome. Leuk Lymphoma. 2010;51(5):778–82.
Cheng MJ, Hourigan CS, Smith TJ. Adult acute myeloid leukemia long-term survivors. J Leuk (Los Angel). 2014;2(2):1–15 (pii: 26855).
Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63(7):1–63.
Stein EM, Yang M, Guerin A, Gao W, Galebach P, Xiang CQ, et al. Assessing utility values for treatment-related health states of acute myeloid leukemia in the United States. Health Qual Life Outcomes. 2018;16(1):193.
Forsythe A, Brandt PS, Dolph M, Patel S, Rabe APJ, Tremblay G. Systematic review of health state utility values for acute myeloid leukemia. Clinicoecon Outcomes Res. 2018;10:83–92.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology for acute myeloid leukemia, V.1.2017. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed Mar 2017.
Truven Health Analytics. RED BOOK Online. 2017. Available at: http://www.micromedexsolutions.com/micromedex2/librarian/. Accessed Sep 2017.
Centers for Medicare & Medicaid Services. Physician Fee Schedule. Available at: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed May 2016.
Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule. 2016. Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/clinlab.html. Accessed May 2016.
Agency for Healthcare Research and Quality. HCUP. Nationwide Inpatient Sample (NIS). 2013. Available at: http://hcupnet.ahrq.gov/. Accessed 26 May 2016.
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–66.
Bureau of Labor Statistics. Consumer Price Index Medical Care Component. Available at: http://data.bls.gov/pdq/SurveyOutputServlet. Accessed Sep 2016.
Joshi N, Hensen M, Patel S, Xu W, Lasch K, Stolk E. Health state utilities for acute myeloid leukaemia: a time trade-off study. Pharmacoeconomics. https://doi.org/10.1007/s40273-018-0704-8 (epub 23 Aug 2018).
Institute for Clinical and Economic Review. Overview of the ICER value assessment framework and update for 2017–2019. Available at: https://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-update-FINAL-062217.pdf. Accessed 22 June 2017.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Bae YH, Mullins CD. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Spec Pharm. 2014;20(11):1086–92.
Institute for Clinical and Economic Review (ICER). Final value assessment framework for 2017–2019. 2017. Available at: https://icer-review.org/final-vaf-2017-2019/. Accessed Sep 2017.
Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14(3):476–9.
Acknowledgements
Medical writing assistance was provided by Cinzia Metallo, PhD, an employee of Analysis Group, and was ultimately paid for by Novartis Pharmaceuticals Corporation. The authors would like to thank Praveen Gunda, an employee of Novartis Healthcare Pvt. Ltd, for his technical insights.
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception and design of the study, analysis and interpretation of the data, drafting of the manuscript, or revising the manuscript critically for intellectual content. All authors approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Conflict of interest
Eytan Stein has received consultancy fees and/or research funding from Novartis, Agios Pharmaceuticals, Celgene Corporation, Pfizer, GlaxoSmithKline, Seattle Genetics, and Constellation Pharma. Umakanth Vudumula is an employee of Novartis Healthcare Private Limited. Briana Ndife, Gaetano Bonifacio, and George Joseph are employees of Novartis Pharmaceuticals Corporation and own stock/stock options in the company. Jipan Xie and Annie Guerin are employees of Analysis Group, which has received consultancy fees from Novartis Pharmaceuticals Corporation for this study. Subrata Bhattacharya was an employee of Novartis Healthcare Pvt. Ltd at the time this study was conducted. Nanxin Li and Emilie Duchesneau were employees of Analysis Group at the time this study was conducted.
Ethical approval
This study did not involve human participants, therefore no formal consent was required.
Data availability
Model inputs are described within the article. The model was developed in Microsoft Excel and is not publicly available, but is available from the authors upon reasonable request, with authorization from Novartis Pharmaceuticals Corporation and receipt of a signed confidentiality agreement.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stein, E., Xie, J., Duchesneau, E. et al. Cost Effectiveness of Midostaurin in the Treatment of Newly Diagnosed FLT3-Mutated Acute Myeloid Leukemia in the United States. PharmacoEconomics 37, 239–253 (2019). https://doi.org/10.1007/s40273-018-0732-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0732-4