Abstract
Background and Objectives
Increasing longevity and advances in treatment have increased the cancer burden in the elderly, resulting in complex follow-up care needs; however, in China, little is known about the follow-up care preferences of these patients. This study quantified older cancer patients’ preferences for follow-up care and examined the trade-offs they are willing to make to accept an alternative follow-up model.
Methods
A discrete choice experiment was conducted among inpatients aged over 60 years with breast, prostate, or colorectal cancer, at two large tertiary hospitals in Nantong, China. Preference weights for follow-up care were estimated using mixed logit analysis. Subgroup analysis and latent class analysis were used to explore preference heterogeneity.
Results
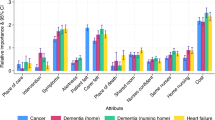
Complete results were obtained from 422 patients (144 with breast cancer, 133 with prostate cancer, 145 with colorectal cancer), with a mean age of 70.81 years. Older cancer patients stated a preference for follow-up by specialists over primary healthcare (PHC) providers (\(\beta\)= −1.18, 95% confidence interval −1.40 to −0.97). The provider of follow-up care services was the most valued attribute among patients with breast cancer (relative importance [RI] 37.17%), while remote contact services were prioritized by patients with prostate (RI 43.50%) and colorectal cancer (RI 33.01%). The uptake rate of an alternative care model integrating PHC increased compared with the baseline setting when patients were provided with preferred services (continuity of care, individualized care plans, and remote contact services).
Conclusion
To encourage older cancer patients to use PHC-integrated follow-up care, alternative follow-up care models need to be based on patients’ preferences before introducing them as a routine option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Specialist-led follow-up care does not meet the increasing care needs of patients; this study provides policy makers with insights for developing alternative follow-up care models for older cancer patients. |
Follow-up care by a specialist was much preferred over follow-up in primary healthcare (PHC). |
Acceptability of PHC-integrated follow-up care models can be improved through implementing patients’ preferred services, such as providing continuity of care, individualized care plans, and remote contact services. |
1 Introduction
The aim of follow-up clinical care for cancer focuses on the detection of recurrence and metastases after treatment [1]. With an ageing population leading to increasing numbers of cancer patients and advances in treatment leading to improved survival, follow-up for cancer patients has evolved to also address the physical and psychosocial issues caused by cancer and its treatment. This often involves coordination between multiple healthcare providers [2]. In China, the over 60-year-olds account for around 56% of all cancer cases, and the burden of long-term care is rising [3]. To date, the specialist-led model of care remains the dominant model for cancer care and follow-up in most countries, including China. However, elderly patients living with cancer often experience complex comorbidities requiring continuity of care between providers for optimal management [4]. Within the health system, specialist oncology departments are often overburdened by the need to manage active treatment while also delivering ongoing follow-up care [5]. Therefore, alternative models of follow-up are needed to improve the quality of care.
Several studies in different countries and settings have explored alternative follow-up care models for cancer patients. These include the use of specialist nurses, general practitioners, and shared care models that involve the joint participation of oncology and primary health care [4, 6, 7]. Meta-analyses of randomized controlled trials of such follow-up pathways, compared with conventional specialist-led ones, have shown encouraging results, with overall improvements in health-related quality of life, time to detect recurrence, psychological health, and cost saving [7,8,9,10].
In China, there is no system for gatekeeping, therefore patients can access the health system at any point, from primary healthcare (PHC) centers through to tertiary hospitals [11]. While PHC centers account for more than 96% of healthcare facilities in China, limited resources mean that standards of care are often distrusted and services are underutilized by patients [12]. The Healthy China 2030 strategy has highlighted the importance of enhancing the role of PHC in the management of chronic diseases [13]. One of the key roles identified is the coordination of care for older adults who present with chronic conditions and require long-term care, to relieve the burden on tertiary hospitals [14]. Efforts have been made to provide integrated care for cancer patients, in some areas, through collaboration between tertiary hospitals and lower-level healthcare facilities, including in PHC. Collaboration and coordination are enabled through the Cancer Medical Consortium, which shares medical records and facilitates two-way referrals; however, this is still in the early stages and focuses on screening and diagnosis [15]. Its potential for follow-up care for cancer patients has yet to be realized [15]. Lack of referral and coordinated follow-up systems, compromise continuity of care and result in overall substandard poor quality healthcare delivery [16, 17].
Older patients with cancer form a particularly vulnerable group because of chronic disease, comorbidities, and functional and cognitive decline, as well as a higher level of symptom burden from cancer and its treatment, which may last many years and may be poorly tolerated [18]. Older patients may also be less likely to comply with prescribed medication and to attend for follow-up, especially when at inconvenient locations [19, 20]. The combination of these factors can result in serious adverse outcomes. There has been very little research in China to explore the best ways of managing elderly cancer patients throughout their illness. In particular, there is virtually no research on older patients with cancer preferences for follow-up and their extended cancer care needs after treatment.
The discrete choice experiment (DCE) is a novel method aimed at weighing the strength of different characteristics (attributes) of a service. It is increasingly used to quantify respondents’ stated preferences for healthcare by making trade-offs between different attributes to inform healthcare policy [21]. The DCE method has been used to design patient-centered healthcare across the cancer care continuum, for example in screening [22], treatment [23], and palliative care [24]. Several studies have assessed preferences in cancer follow-up care [25,26,27,28,29,30,31,32] but none have focused on older patients with cancer in China. In particular, there has been no assessment of the preferences of older cancer patients in PHC follow-up care. This is of note because in 2021 the National Health Commission specifically recommended the use of PHC for cancer follow-up in line with the new Chinese integrated care policy [33].
We performed this DCE study among patients aged over 60 years with either breast, prostate or colorectal cancer, in Nantong City, Jiangsu Province. The objectives were to (1) quantify older cancer patients’ follow-up preferences and explore their preference heterogeneity; and (2) determine the trade-offs patients are willing to make to accept alternative follow-up involving PHC. The results of this study aimed to inform the details of the development of forthcoming PHC follow-up interventions and to promote the engagement of older cancer patients in making choices for cancer follow-up care.
2 Methods
This study was developed in accordance with, and followed, the Professional Society for Health Economics and Outcomes Research (ISPOR) checklist for DCE in health [34, 35], and the outcome reporting follows the guidelines from the International Academy of Health Preference Research [36, 37].
2.1 Identification of Attributes and Levels
The study design was informed by published studies on both the follow-up of cancer patients and interviews with patients and specialists. The PubMed, Embase, and Google Scholar databases were searched using the keywords: ‘cancer’, ‘aftercare’, ‘follow-up’, ‘prefer’, ‘prioritize’, and ‘discrete choice’, with our search yielding eight studies [25,26,27,28,29,30,31,32]. We then conducted semi-structured interviews with 19 patients aged over 60 years (10 with breast cancer, 3 with prostate cancer, and 6 with colorectal cancer), exploring their experiences, expectations on their follow-up care, and those aspects of follow-up care they valued. The interviews lasted for 40 min on average. The characteristics of the 19 patients are presented in Online Resource Table S1. We then characterized and selected the most commonly mentioned attributes. Prioritized attributes included follow-up providers, continuity of care, communication outside clinic visits (remote contact), personalized follow-up plans, additional services, frequency of follow-up, and cost. These attributes mostly matched a previous study conducted in China [26]. Levels of additional services were adjusted according to our qualitative study. To ensure relevance for the Chinese setting, we discussed these potential attributes with six specialists (two were specialized in each of the three cancer types), and removed both the frequency of follow-up and cost. The reasons were that healthcare providers’ recommendations have a greater role in determining the frequency of follow-up than patient preference; regarding costs, informants argued that the cost of follow-up care varies widely in real practice depending on the actual care and treatment received.
We conducted a pilot study among six patients, adopting think-aloud techniques to check the understanding, plausibility and appropriateness of attribute-level combinations. This pilot only resulted in minor changes to the wording of the Introduction, as well as the attributes and levels explanations in the questionnaire, therefore further confirming that the attributes and levels are understandable to participants. During this process, we found that older patients tend to share decision making with their caregivers, usually family members, hence we added questions related to response style (patient self-report, patient and caregiver joint answer, and caregiver alone). Basic information about involved caregivers was also documented. The final five attributes included are shown in Table 1.
2.2 Study Design
The full factorial design with three two-level and two four-level attributes yielded 23 \(\times\) 42=128 possible scenarios. Therefore, to make the questionnaire easier to complete, we used a D-efficient design using the idefix package in R software, with simulation of 500 random draws from a normal distribution [38]. We generated 16 choice sets and randomly divided these paired questions into two blocks with eight choice tasks each. Each participant was randomly assigned a block of questions. Since all cancer patients are recommended to receive routine follow-up, we did not include the opt-out option in order to capture respondents’ maximum preference information [39]. Figure 1 presents the example choice set. The test-retest internal validity test was conducted by repeating choice task 2 as choice task 9 (Online Resource 2). We opted not to exclude participants who did not pass the test-retest internal validity, to minimize potential selection bias and maintain statistical efficiency [40,41,42,43].
2.3 Sample Size
The sample size was calculated according to Orme’s equation [N>500 c/(t \(\times\) a)], where c, t, and a represent the largest number of levels for any of the attributes, the number of choice tasks, and the number of alternatives, respectively [44]; this suggested that the minimum sample size was 125. To allow for 20% of invalid questionnaires and for subgroup analysis of different cancer types, we aimed to recruit at least 160 participants for each cancer type.
2.4 Study Sample
Participants were inpatients from two large tertiary hospitals (Nantong Cancer Hospital and the Affiliated Hospital of Nantong University) in Nantong, a city with the largest proportion of elderly people in China. We recruited patients using purposive sampling. Patients were approached face-to-face by trained interviewers in inpatient wards and all questionnaires were administered by researchers. Inclusion criteria were (1) patients with any one of the selected cancers: colorectal, breast, or prostate cancer; (2) aged ≥60 years; (3) recipient of any primary treatment, i.e. surgery, chemotherapy, or radiotherapy; (4) diagnosed with cancer stage I, II, or III (not terminally ill); and (5) without any cognitive impairment or communication barriers.
For the DCE, patients were asked to imagine the follow-up care they expected to receive and then to choose their preferred alternative in every paired choice according to their utility. To help with understanding, examples and visual aids were used by the interviewer. A choice set figure from an existing free website was amended. For better illustration and clarity, each choice set included pertinent images alongside each level of attributes, while also aiming to increase respondents’ engagement (Fig. 1).
For respondents who felt unable to make their own choices, caregivers were sometimes involved. In these cases (n = 132), we documented the response style and basic information of the caregivers (all were family members, except two paid caregivers). Data were collected from August 2022 to January 2023. Ethical approval was obtained from the Ethics Committee of Zhejiang University School of Public Health (ZGL-202106-01) and all participants provided informed consent.
2.5 Statistical Analysis
The characteristics of respondents were described, along with group differences by cancer type using analysis of variance (ANOVA) and Chi-square where appropriate. In the DCE, each alternative corresponds to a value (utility), and individuals are assumed to make choices between paired alternatives based on their highest utility [45]. To quantify the preference weight of attributes and levels, and address the preference variation across the respondents, a mixed logit model was constructed to estimate the weights of each attribute. In line with previous relevant studies [28, 30], the random parameters of each level were defined as normally distributed, and the model was fitted using 500 Holton draws.
To explore the influence of preference heterogeneity, we stratified participants by cancer type using mixed logit models. Then, to test the heterogeneity caused by respondent characteristics, we included a range of interaction terms of prespecified demographic variables (age, sex, education, residence, comorbidity status, and diagnosis interval less than or more than 3 months) with each attribute accordingly, and interaction terms were set with a fixed effect. To investigate preference heterogeneity, latent class analysis (LCA) was conducted. To generate the optimal number of classes, we repeated the latent class analyses by setting the number of classes from two until the model reached the convergent limit, and the number of classes was determined by using the Bayesian information criterion (BIC) and Akaike information criterion (AIC). In addition, we explored the demographic difference between different classes according to an individual’s posterior probability of being in each class [46].
The relative importance (RI) of each attribute among all cancer patients and different cancer types was calculated by dividing the difference between the largest and smallest preference weights of attributes and dividing by the sum of all weights [47]. Furthermore, to test the utility of interested scenarios, we calculated the uptake probability by changing the level of attributes. We took the combination of all referent categories of each attribute (follow-up by specialist and no continuity of care, personalized follow-up care plan, with availability of communication outside the clinic visits, and additional support services provided) as a baseline care to make simulations. The current follow-up care model is specialist dominated and other attributes were varied in real practice. In all follow-up care scenarios, we calculated the relative probability (uptake change) of choosing the simulated alternative over baseline care based on the logit probability estimation equation [48, 49] and the attribute coefficient vector yielded from the mixed logit model (details in the Online Resource Method).
Statistical analyses were performed using R 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) and Stata 15.1 (StataCorp LLC, College Station, TX, USA). All tests were two-sided with a significant p-value of <0.05.
3 Results
3.1 Participants’ Demographic Characteristics
After removing participants younger than 60 years of age (n = 4) and incomplete responses (n = 23), a total of 422 questionnaires were analysed. There were 144 breast cancer patients, 133 with prostate cancer, and 145 with colorectal cancer. Of these, 77.5% (327/422) of participants passed the test-retest internal validity test. Characteristics of all patients and involved caregivers are shown in Table 2 and Online Resource Table S2, respectively. The mean (standard deviation [SD]) age of the sample was 70.81 years (6.49), 52.1% were male, 15.9% had no formal education, 77.7% were from rural areas, and more than 95% of patients had basic health insurance. Most patients (73.6%) had been diagnosed in the previous 6 months (73.6%). Among the three cancer types, patients with breast cancer tended to have lower education attainment and higher rates of self-reported chronic disease. As shown in Online Resource Table S3, patients who were female, older, and less well-educated, as well as rural residents, tended to share decision making with their caregivers.
3.2 Preference Weight
The results of the mixed logit model are shown in Table 3. Patients were mostly against using PHC providers during the follow-up (\(\beta\) = −1.18, 95% confidence interval [CI] −1.40 to −0.97, p < 0.001). Receiving care from the same care providers and individualized care plans was regarded as important, with positive coefficients reaching 0.43 (p < 0.001) and 0.44 (p < 0.001), respectively. Results indicated that regular communications from healthcare providers combined with available counseling were preferred by respondents (\(\beta\) = 1.17, 95% CI 0.95–1.39, p < 0.001); however, levels of ‘patient-initiated counseling’ (p = 0.112) and ‘regular calls’ (p = 0.118) were not valued. Additional services were not a significant attribute. Since the SD for each level of attribute reached statistical significance (p ≤ 0.001), except for lifestyle instructions, it indicated a wide range of heterogeneity in preference.
3.3 Preference Heterogeneity
Preference weights stratified by cancer types are shown in Online Resource Table S4. A similar pattern of preference for follow-up provided by specialists, with continuity of care and individualized care plans, was observed across all three cancer types. However, although patients all preferred remote contact comprising regular calls and counseling, patients with colorectal cancer showed a significant negative preference for self-initiated calls (\(\beta\) = −0.49, p < 0.001). Patients with breast cancer exhibited a stronger preference for medication instructions (\(\beta\)= 0.34, p = 0.007), while those with colorectal cancer had a higher utility for psychological support (\(\beta\) = 0.31, p = 0.019). The preference weights for all cancers combined and separately are shown in Fig. 2. The most important attribute for patients with breast cancer (RI 37.17%) was the type of follow-up provider; however, remote contact services were slightly more important for prostate cancer patients (RI 43.50%) and patients with colorectal cancer than follow-up care providers. (RI 33.01%). No qualitative difference was identified between patients diagnosed less than 3 months and more than 3 months previously (Online Resource Table S5). In addition, we conducted a subgroup analysis based on whether the patient or caregiver was the respondent. The results showed no notable difference between groups (Online Resource Table S6). Informed by model fit indicators, we finally generated two latent classes among all respondents. However, after comparing the demographics between the two groups, we did not identify any noticeable group differences (Online Resource Table S7 and Table S8).
In terms of interaction effects between the demographics and six attributes, patients who reported chronic disease (\(\beta\) = 0.17, p = 0.037) and live in the city (\(\beta\) = 0.23, p = 0.012) were more likely to prefer additional services for medication instructions (Online Resource Table S9).
3.4 Probability of Uptake of Primary Healthcare-Integrated Follow-Up Care
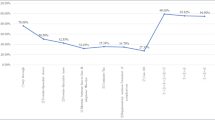
We simulated the scenarios by changing the levels of attributes (Fig. 3). Scenarios without specialist-led follow-up had a 34.5% lower probability of being chosen; however, providing remote calls and counseling services almost offset the uptake probability loss. If an offer of continuity of care and an individualized follow-up plan was made, the probability of choosing PHC-involved care increased to 13%. This shows that providing a combination of services comprising continuity of care, an individualized care plan, remote contact incorporating regular calls and counseling, and additional services (medication instructions or psychology support) could increase the acceptability of being followed-up by PHC providers by up to 14.3% compared with the baseline care scenario.
Uptake rate under different follow-up services. The baseline care scenario was set as following-up by the specialist, and no continuity of care, personalized follow-up care plan, availability of communication outside the clinic visits, and additional self-management support provided. The x-axis labels denote the scenario with the specific level(s) change to the corresponding attribute(s) compared with baseline care. For example, (1)+(2)+(3)+(6)+(7) denotes providing a combination of services, comprising continuity of care, an individualized care plan, remote contact incorporating regular calls and counseling, and additional services (medication instructions) could increase the acceptability of being followed up by PHC providers by up to 14.3% compared with the baseline care scenario. PHC primary healthcare
4 Discussion
To our knowledge, this is the first DCE to explore preferences for follow-up care among older Chinese patients with breast, prostate or colorectal cancer. The relatively large sample size allowed for subgroup analyses to explore preference heterogeneity. Furthermore, the study involved patients from two large hospitals, thereby enhancing the diversity of the patients. The results provide insights for the development of age-appropriate follow-up strategies. We found that specialist-led follow-up with remote contact, including both counseling and regular calls, was preferred, followed by continuity of care and the availability of a personalized follow-up plan. Furthermore, we assessed the patients’ acceptance of alternative follow-up care models by integrating PHC within the new Chinese integrated care policy. Although studied patients with cancer presented high attachment to the specialist-led follow-up model, there is potential to increase the acceptance of an alternative care model by introducing the positively valued strategies.
In line with previous studies [25, 26, 29], our findings revealed that the overwhelming majority of patients did not favor PHC-based follow-up. A recent study of Chinese patients with gastric cancer across the age range suggested low acceptance of PHC-led follow-up care [26]. This may be the effect of status quo bias, since at present PHC is rarely used for post-treatment care [50, 51]. These are exacerbated for serious diseases such as cancer, even though the common symptoms experienced by cancer patients after discharge, such as fatigue and pain, do not usually require specialist treatment [52, 53]. Despite progress in integrated care, the link between hospital and PHC centers remains weak in most settings, and sharing of patient records and referrals between levels are very inconsistent [13].
Previous studies have also identified barriers to integrating PHC in cancer follow-up care. These include poor experience of PHC providers leading to lack of patient trust, and inadequate communication between oncologists and PHC providers [54,55,56]. These findings may explain why patients showed less preference for PHC follow-up care. However, PHC is integrated into cancer care in many countries, showing that adjustments can be made according to attribute-based preferences to encourage older patients with cancer to use PHC-integrated follow-up care. Our results suggest that providing continuity of care, individualized care plans, and remote contact services could partially compensate for the utility loss of moving to a PHC follow-up model of care. Under the circumstances of the integrated care system in China, shared care management between sectors is now feasible. As suggested by emerging randomized controlled trials, patients with cancer who received follow-up care from PHC providers with continuity of care intervention achieved at least equal health-related outcomes and reduced cost compared to usual care [57,58,59,60]. However, the provision of follow-up care training for PHC providers, adequate handover information, and effective cross-sector collaboration have yet to be fully established and require further investigation [15].
Remote contact mode was allocated the most weight among other attributes for all three cancers. This finding was also found in a Netherlands study in breast cancer patients [29]. This likely relates to advances in postoperative care that have shortened the hospital stay, with many patients now discharged before full recovery [61]. Remote follow-up is therefore an essential approach to reassure patients, improve care, and reduce in-person visits [62]. A recent survey in 13 cancer hospitals in Sichuan province, China, revealed that 93% of patients across the age range experienced varying degrees of post-discharge symptoms, and one-third of them lacked the knowledge and confidence to deal with them [52]. Older patients with cancer are even more vulnerable after discharge. Thus, appropriate remote follow-up is very important. In current practice in China, remote follow-up is mostly inadequate and relies on patients themselves initiating it. This may affect the speed of recovery.
Personalized care plans and continuity of care were the third and fourth most important attributes valued by patients. Preference for continuity of care has been reported consistently [25, 26]. Our results also showed that respondents had distinct preference patterns for almost all levels of attributes, emphasizing the need for customized follow-up plans to improve the health outcomes of patients and increase the efficiency of healthcare systems [63].
It is of note that the attribute ‘additional service’ was rated as the least important. The findings differ from previous studies, where, for example, receiving an information pack about cancer and treatment as well as diet and lifestyle were valued as important attributes to increase patient utility [25, 26, 64]. This may be because older patients reported more difficulties in processing information [65]. Furthermore, patients’ need for information varies greatly in content and quantity during the illness [64]. This suggests a need to identify patients’ preference heterogeneity for additional services.
Preference patterns among different cancer types occurred mainly in additional services. Notably, subgroup analysis supported the inclusion of medication instructions (including medication reconciliation and adherence) for those with chronic disease. Comorbidities involve polypharmacy, which may mediate the cancer treatment effect [66] and complicate self-management [67]. Thus, specific follow-up care protocols for those with comorbidities are imperative. Since subgroup analyses were exploratory and not adjusted for multiple comparisons, the results need to be interpreted cautiously.
This study has several limitations. First, we approached participants in inpatient wards, where most patients were newly diagnosed, and thus patients may feel dependent on specialists. However, the subgroup analyses and testing for interaction effects of diagnosis interval on preferences found no significant group differences. Given the potential change in care preference over time for patients, additional research targeting different patient groups over the course of the illness deserves study. Second, although we selected attributes appropriate to the China context, the complexity of the real world cannot be fully captured by involved attributes. In the current study, to avoid participants’ confusion, we did not include cost as an attribute. Future study needs to carefully consider the wording of the cost attribute (such as forms and frequency of payment) as well as levels of cost attribute expected. This will enable the measurement of willingness to pay and offer deeper insights into resource allocation and service delivery. Third, the DCE is a method aiming to examine stated preference, which may differ from actual preference. Fourth, we used purposive sampling to recruit participants, and patients had the right to refuse, which may limit the generalizability.
5 Conclusion
Our study revealed a strong preference of older patients with cancer for specialist-led follow-up, involving regular contact with the specialist on demand. Although the utility of providing follow-up by PHC providers could partially be compensated by other attributes, preference for it is low. The study identified distinct patterns in patient choice, suggesting the need to develop personalized care to meet patients’ needs.
References
Jefford M, Rowland J, Grunfeld E, Richards M, Maher J, Glaser A. Implementing improved post-treatment care for cancer survivors in England, with reflections from Australia, Canada and the USA. Br J Cancer. 2013;108(1):14–20.
Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19–29.
Ju W, Zheng R, Zhang S, Zeng H, Sun K, Wang S, et al. Cancer statistics in Chinese older people, 2022: current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea. Sci China Life Sci. 2023;66(5):1079–91.
Jefford M, Howell D, Li Q, Lisy K, Maher J, Alfano CM, et al. Improved models of care for cancer survivors. The Lancet. 2022;399(10334):1551–60.
Alfano CM, Jefford M, Maher J, Birken SA, Mayer DK. Building personalized cancer follow-up care pathways in the United States: lessons learned from implementation in England, Northern Ireland, and Australia. Am Soc Clin Oncol Educ Book. 2019;39:625–39.
Nekhlyudov L, O’Malley DM, Hudson SV. Integrating primary care providers in the care of cancer survivors: gaps in evidence and future opportunities. Lancet Oncol. 2017;18(1):e30–8.
Chan RJ, Crawford-Williams F, Crichton M, Joseph R, Hart NH, Milley K, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J Cancer Surviv. 2023;17(1):197–221.
Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2016;2016(5): CD001768.
Høeg BL, Bidstrup PE, Karlsen RV, Friberg AS, Albieri V, Dalton SO, et al. Follow-up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database Syst Rev. 2019;2019(11): CD012425.
Lewis RA, Neal RD, Williams NH, France B, Hendry M, Russell D, et al. Follow-up of cancer in primary care versus secondary care: systematic review. Br J Gen Pract. 2009;59(564):e234–47.
World Health Organization. Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva: World Health Organization; 2017.
Zhang A, Nikoloski Z, Albala SA, Yip W, Xu J, Mossialos E. Patient choice of health care providers in China: primary care facilities versus hospitals. Health Syst Reform. 2020;6(1): e1846844.
Li X, Lu J, Hu S, Cheng KK, De Maeseneer J, Meng Q, et al. The primary health-care system in China. The Lancet. 2017;390(10112):2584–94.
Hu L, Glavin YW, Yan R, Pei C, Yan M, Zhang Y, et al. Integrating Health and Care in China: lessons learned and future outlook. Int J Integr Care. 2021;21(4):18.
Cai M, Liu E, Tao H, Qian Z, Fu QJ, Lin X, et al. Does a medical consortium influence health outcomes of hospitalized cancer patients? An integrated care model in Shanxi, China. Int J Integrated Care. 2018. https://doi.org/10.5334/ijic.3588.
Shen X, Diao M, Lu M, Feng R, Zhang P, Jiang T, et al. Pathways and cost-effectiveness of routine lung cancer inpatient care in rural Anhui, China: a retrospective cohort study protocol. BMJ Open. 2018;8(2): e018519.
Wu D, Lam TP. Underuse of primary care in China: the scale, causes, and solutions. J Am Board Family Med. 2016;29(2):240–7.
Shahrokni A, Kim SJ, Bosl GJ, Korc-Grodzicki B. How we care for an older patient with cancer. J Oncol Pract. 2017;13(2):95–102.
Ruddy KJ, Herrin J, Sangaralingham L, Freedman RA, Jemal A, Haddad TC, et al. Follow-up care for breast cancer survivors. J Natl Cancer Inst. 2020;112(1):111–3.
Puts MTE, Tu HA, Tourangeau A, Howell D, Fitch M, Springall E, et al. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol. 2014;25(3):564–77.
Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
Hall R, Medina-Lara A, Hamilton W, Spencer AE. Attributes used for cancer screening discrete choice experiments: a systematic review. Patient. 2022;15(3):269–85.
Kowal M, Douglas F, Jayne D, Meads D. Patient choice in colorectal cancer treatment—a systematic review and narrative synthesis of attribute-based stated preference studies. Colorectal Dis. 2022;24(11):1295–307.
Leng A, Maitland E, Wang S, Nicholas S, Lan K, Wang J. Preferences for end-of-life care among patients with terminal cancer in China. JAMA Netw Open. 2022;5(4): e228788-e.
Murchie P, Norwood PF, Pietrucin-Materek M, Porteous T, Hannaford PC, Ryan M. Determining cancer survivors’ preferences to inform new models of follow-up care. Br J Cancer. 2016;115(12):1495–503.
Li HQ, Yuan H, Wan GY, Xue H, Zhang XY. Preferences of gastric cancer survivors for follow-up care-a multicenter discrete choice experiment study. Support Care Cancer. 2022;30(2):1221–9.
Wong SF, Norman R, Dunning TL, Ashley DM, Khasraw M, Hayes TM, et al. A discrete choice experiment to examine the preferences of patients with cancer and their willingness to pay for different types of health care appointments. J Natl Compr Canc Netw. 2016;14(3):311–9.
McFerran E, Boeri M, Kee F. Patient preferences in surveillance: findings from a discrete choice experiment in the “my follow-up” study. Value Health. 2020;23(10):1373–83.
Kimman ML, Dellaert BG, Boersma LJ, Lambin P, Dirksen CD. Follow-up after treatment for breast cancer: one strategy fits all? An investigation of patient preferences using a discrete choice experiment. Acta Oncol. 2010;49(3):328–37.
Bessen T, Chen G, Street J, Eliott J, Karnon J, Keefe D, et al. What sort of follow-up services would Australian breast cancer survivors prefer if we could no longer offer long-term specialist-based care? A discrete choice experiment. Br J Cancer. 2014;110(4):859–67.
Koel C. Patient preferences for follow-up in breast cancer. Enschede: University of Twente; 2016.
Damery S, Biswas M, Billingham L, Barton P, Al-Janabi H, Grimer R. Patient preferences for clinical follow-up after primary treatment for soft tissue sarcoma: a cross-sectional survey and discrete choice experiment. Eur J Surg Oncol. 2014;40(12):1655–61.
Circular on Issuing the Opinions on Accelerating the Development of Rehabilitation Medical: National Health Commission of People’s Republic of China; 2021. Available at: http://www.gov.cn/zhengce/zhengceku/2021-06/17/content_5618767.htm.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health. 2011;14(4):403–13.
Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value in Health. 2013;16(1):3–13.
Mühlbacher AC, de Bekker-Grob EW, Rivero-Arias O, Levitan B, Vass C. How to present a decision object in health preference research: attributes and levels, the decision model, and the descriptive framework. Patient. 2024. https://doi.org/10.1007/s40271-024-00673-y. (Epub 10 Feb 2024).
Marshall DA, Veldwijk J, Janssen EM, Reed SD. Stated-preference survey design and testing in health applications. The Patient. 2024. https://doi.org/10.1007/s40271-023-00671-6. (Epub 31 Jan 2024).
Traets F, Gil Sánchez D, Vandebroek M. Idefix: efficient designs for discrete choice experiments. J Stat Software. 2020. https://doi.org/10.18637/jss.v096.i03.
Veldwijk J, Lambooij MS, de Bekker-Grob EW, Smit HA, de Wit GA. The effect of including an opt-out option in discrete choice experiments. PLoS One. 2014;9(11): e111805.
Johnson FR, Yang J-C, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–60.
Seo J, Heidenreich S, Aldalooj E, Poon JL, Spaepen E, Eby EL, et al. Patients’ preferences for connected insulin pens: a discrete choice experiment among patients with type 1 and type 2 diabetes. The Patient. 2023;16(2):127–38.
Scherz N, Bachmann-Mettler I, Chmiel C, Senn O, Boss N, Bardheci K, et al. Case management to increase quality of life after cancer treatment: a randomized controlled trial. BMC Cancer. 2017;17(1):223.
Lancsar E, Louviere J. Deleting ‘irrational’responses from discrete choice experiments: a case of investigating or imposing preferences? Health Econ. 2006;15(8):797–811.
de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–84.
Ryan M, Gerard K, Amaya-Amaya M. Using discrete choice experiments to value health and health care. Springer Science & Business Media; 2007.
Yoo HI. lclogit2: an enhanced command to fit latent class conditional logit models. Stand Genomic Sci. 2020;20(2):405–25.
Lancsar E, Louviere J, Flynn T. Several methods to investigate relative attribute impact in stated preference experiments. Soc Sci Med. 2007;64(8):1738–53.
World Health Organization. How to conduct a discrete choice experiment for health workforce recruitment and retention in remote and rural areas: a user guide with case studies. Geneva: World Health Organization; 2012.
Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics. 2017;35:697–716.
Li L, Zhu L, Zhou X, Zeng G, Huang H, Gan Y, et al. Patients’ trust and associated factors among primary care institutions in China: a cross-sectional study. BMC Prim Care. 2022;23(1):109.
Teng L, Li Y. Analysis on the willingness and influencing factors of choosing primary healthcare institutions among patients with chronic conditions in China: a cross-sectional study. BMJ Open. 2022;12(3): e054783.
Dai S, Liu X, Chen X, Bie J, Du C, Miao J, et al. Current status of out-of-hospital management of cancer patients and awareness of internet medical treatment: a questionnaire survey. Front Public Health. 2021;9: 756271.
Shi Y. Chinese perspective of the role of primary care in cancer control. Lancet Oncol. 2015;16(12):1227–8.
Stephens C, Klemanski D, Lustberg MB, Noonan AM, Brill S, Krok-Schoen JL. Primary care physician’s confidence and coordination regarding the survivorship care for older breast cancer survivors. Support Care Cancer. 2021;29(1):223–30.
Wollersheim BM, van Asselt KM, Pos FJ, Akdemir E, Crouse S, van der Poel HG, et al. Specialist versus primary care prostate cancer follow-up: a process evaluation of a randomized controlled trial. Cancers (Basel). 2022;14(13):3166.
Lawrence RA, McLoone JK, Wakefield CE, Cohn RJ. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med. 2016;31(10):1222–36.
Aubin M, Vézina L, Verreault R, Simard S, Hudon É, Desbiens JF, et al. Continuity of cancer care and collaboration between family physicians and oncologists: results of a randomized clinical trial. Ann Fam Med. 2021;19(2):117–25.
Jefford M, Emery JD, Martin AJ, Lourenco RDA, Lisy K, Grunfeld E, et al. SCORE: a randomised controlled trial evaluating shared care (general practitioner and oncologist) follow-up compared to usual oncologist follow-up for survivors of colorectal cancer. EClinicalMedicine. 2023;66: 102346.
Nielsen J, Palshof T, Mainz J, Jensen A, Olesen F. Randomised controlled trial of a shared care programme for newly referred cancer patients: bridging the gap between general practice and hospital. BMJ Qual Saf. 2003;12(4):263–72.
Fethney J, Kim B, Boustany C, McKenzie H, Hayes L, Cox K, et al. Evaluating a shared care pathway intervention for people receiving chemotherapy to reduce post-treatment unplanned hospital presentations: a randomised controlled trial. Support Care Cancer. 2024;32(1):77.
Jonker LT, Plas M, de Bock GH, Buskens E, van Leeuwen BL, Lahr MMH. Remote home monitoring of older surgical cancer patients: perspective on study implementation and feasibility. Ann Surg Oncol. 2021;28(1):67–78.
Rochette C, Michallet AS, Malartre-Sapienza S, Rodier S. Telephone follow-up of oncology patients: the contribution of the nurse specialist for a Service-Dominant Logic in hospital. BMC Health Serv Res. 2021;21(1):580.
Alfano CM, Mayer DK, Bhatia S, Maher J, Scott JM, Nekhlyudov L, et al. Implementing personalized pathways for cancer follow-up care in the United States: proceedings from an American Cancer Society-American Society of Clinical Oncology summit. CA Cancer J Clin. 2019;69(3):234–47.
Leydon GM, Boulton M, Moynihan C, Jones A, Mossman J, Boudioni M, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ. 2000;320(7239):909–13.
Posma ER, van Weert JC, Jansen J, Bensing JM. Older cancer patients’ information and support needs surrounding treatment: an evaluation through the eyes of patients, relatives and professionals. BMC Nurs. 2009;8:1.
Williams GR, Mackenzie A, Magnuson A, Olin R, Chapman A, Mohile S, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–57.
Karuturi MS, Holmes HM, Lei X, Johnson M, Barcenas CH, Cantor SB, et al. Potentially inappropriate medication use in older patients with breast and colorectal cancer. Cancer. 2018;124(14):3000–7.
Acknowledgments
The authors would like to thank the patients and their families for their participation, and the interviewers for assisting with data collection in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest/Competing Interests
Jiawei Geng, Ran Li, Xinyu Wang, Rongfang Xu, Jibin Liu, Haiyan Jiang, Gaoren Wang, and Therese Hesketh declare they have no competing interests relevant to the contents of this manuscript.
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 72150710552).
Ethics Approval
This study was performed following the principles and standards in the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Zhejiang University School of Public Health (ZGL-202106-01).
Consent to Participate
Informed consent was obtained from all participants, or their representatives, in this study.
Author Contributions
JWG, RL, and TH conceptualized the project. JWG, RL, XYW, RFX, and HYJ acquired, analyzed and interpreted the data. JWG performed the data analysis and wrote the first draft. All authors critically revised the manuscript for important intellectual content. RL and TH obtained funding. JBL and GRW administrated the study and provided material support. GRW and TH supervised the study. All authors read and approved the final version of this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Geng, J., Li, R., Wang, X. et al. Eliciting Older Cancer Patients’ Preferences for Follow-Up Care to Inform a Primary Healthcare Follow-Up Model in China: A Discrete Choice Experiment. Patient (2024). https://doi.org/10.1007/s40271-024-00697-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40271-024-00697-4