Abstract
Glucocorticoids (GCs) are essential and effective medications commonly prescribed to patients with autoimmune disorders and inflammatory diseases. However, they often adversely affect bone health, including a rapid bone mineral density reduction and an increased bone fracture rate. An estimated 30% of long-term GC users develop secondary osteoporosis [glucocorticoid-induced osteoporosis (GIO)], whereas the measurement and prevention for GC-treated patients are sometimes clinically overlooked in clinical practice. Fortunately, many guidelines for GIO-related fracture risk assessment have been established, and several new drugs that benefit primary osteoporosis patients may also serve as potential GIO therapeutic options. Because of the broad application of GCs in clinical practice and the growing prevalence of GIO, increasing with the dose and time of GC administration, awareness of GIO development is crucial for implementing preventive therapy promptly and effectively. In this paper, we discuss the pathomechanisms of GIO and bone health problems depending on the method and route of GC administration in various groups of patients. Various treatment regimens are broadly analyzed, highlighting the most crucial aspects and new therapeutic options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One-third of long-term glucocorticoid (GC) users will develop secondary osteoporosis. Fractures, which might be asymptomatic, are the most serious consequence of glucocorticoid-induced osteoporosis (GIO), so quick diagnosis and prompt treatment are important. |
Since osteoporosis may develop even when low-dose or short-term GC therapy is used, each patient treated with corticosteroids, regardless of sex, age, corticosteroid dosage, treatment duration, or administration method, should be carefully assessed for the risk of fractures. |
The first 3 months of GC treatment are critical for deficiency and bone loss. Thus, adequate prevention of GIO should be immediately implemented (lifestyle modification and calcium and vitamin D supplementation). Bisphosphonates are the first-line treatment for GIO, but other drugs should also be considered. |
Introduction
Glucocorticoids (GCs) are considered safe and are widely used for treating various inflammatory-associated disorders and autoimmune diseases due to their anti-inflammatory and immunosuppressive properties [1]. It is reported that an estimated 1–2% of the general population receives long-term (more than 3 months) GC treatment [2]. GCs can be administered in various ways, including orally, through inhalation, topically, intra-articularly, intravenously (IV), and intramuscularly (IM) [3].
Glucocorticoid-induced osteoporosis (GIO) is the most common cause of secondary osteoporosis, and iatrogenic bone fractures may occur in up to 50% of patients treated with glucocorticosteroids for a long time [4]. The frequency of fractures is higher in females and increases with advanced age, higher doses, and longer treatment duration [5]. Even a low dose of prednisone (2.5–7.5 mg daily) or a short treatment (less than 30 days) can cause bone loss. In addition, the risk of fractures rapidly increases after the first dose of glucocorticoids, and the risk of low-energy fractures increases with the chronic use of these medications [6]. It is essential to recognize that attributing all instances of increased fracture risk solely to steroid usage would be an oversimplification [7]. This is because the concurrent presence of an underlying condition (e.g., rheumatoid arthritis), which necessitated the prescription of steroids, could itself contribute to the reduction of bone density and fracture risk.
The pathogenesis of GIO is associated with GCs’ direct effect on osteoblasts, osteocytes, and osteoclasts and indirect regulation via hormonal change. The decreased number of osteoblasts via apoptosis and inhibited differentiation reduced bone formation and skeletal structure formation. Impaired osteocyte viability leads to loss of bone structure and quality, leading to bone fragility [8]. Trabecular bone is more sensitive to GCs’ effect than cortical bone. With trabecular bone located in long bones and vertebral bodies, fractures in these skeletal locations occur in 30–35% of patients receiving chronic GC therapy, with vertebral fractures accounting for up to 37%. Fractures in the long bones involve the metaphyseal area, where trabecular bones are abundant [9, 10]. These changes occur as early as the first 3–6 months of initiating glucocorticoids; thus, preventing osteoporosis development during GC management is of primary importance. Unfortunately, many patients continue to underestimate the susceptibility to fractures, leading to a lack of proper preventive treatment. With this in mind, this review describes the main pathomechanisms underlying the progression of glucocorticoid-induced osteoporosis (GIO), discusses potential complications, and examines strategies for preventing and effectively managing this significant skeletal disease.
Methods
A systematic literature review has analyzed recent data on steroids’ influence on bone health. Databases such as PubMed or Embase were used to search for articles published from 2000 (coverage dates: 1 January 2000–5 April 2024AU). The search terms were “glucocorticoid-induced osteoporosis,” “glucocorticoid exposure,” and “glucocorticoid therapy.” These phrases were combined with other keywords, including “epidemiology,” “pathogenesis,” “mechanisms of action (fracture risk),” “bone health”/“bone mineral density” (“BMD”), “diagnosis,” and “prevention/treatment strategies,” to make the search more specific for finding relevant data about GIO. Corresponding English-only articles were chosen, consisting of original studies, meta-analyses, and narrative and systematic reviews, and examined criteria for including this review were studied in relation to the risk of bone destruction due to glucocorticoid use, considering different ages and sexes with varied doses and ways of administration. The selection process followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Each reviewer independently screened every article, first by title and then by abstract. Eligibility of articles in full-text format was confirmed by two reviewers (C.W. and E.I.), and any disagreements were resolved through conversation.
The important things to look at, called key inclusion criteria, were studies that explored the prevalence of GIO, its etiology, its diagnosis, and its treatment. Reviewers examined the findings and methods used in these studies and their quality to provide a comprehensive summary of GIO. Additionally, the patient’s characteristics, such as sex and age, and the duration and dosage of glucocorticoid treatment were considered. The literature search aimed to include recent research that was available during the analysis period. In general, this systematic review attempted to offer a recent and thorough analysis of GIO. It involved different types of studies and tackled many facets of this condition.
Epidemiology of GIO
Although the risk of primary osteoporosis is mainly correlated with advanced age and female sex, younger patients receiving systemic glucocorticoids are susceptible to secondary osteoporosis and the related increased risk of fracture [11, 12]. Available data indicate that approximately 1–3% of the population uses steroid therapy. Due to the increasing use of GCs in the treatment of many diseases, including endocrine, rheumatological, and pulmonary diseases (e.g., asthma and chronic obstructive pulmonary disease), GIO is the most common cause of secondary osteoporosis. The dose of steroids and the duration of treatment are the main factors contributing to its development [12]. Patients taking glucocorticoids most often experience spine fractures, and the risk is 160–185% higher than in healthy subjects without GC therapy (the second most common fracture is hip fracture) [13]. Risk factors influencing vertebral fractures are mainly high doses of GCs, co-occurring hypogonadism, and intravenous GC bolus therapy [14].
There is conflicting evidence regarding the difference in long-term outcomes of osteoporosis in patients who inhaled glucocorticoids or taken them orally or parenterally (intravenously); however, duration of use, cumulative dose, and age of onset could all negatively impact bone health [15,16,17,18,19,20,21,22,23] (Table 1).
The most common demographic for osteoporotic fractures is post-menopausal women, and the risk of GIO development has been estimated at 40% [24]. For postmenopausal women treated with GC therapy, the risk of osteoporosis is four-fold compared with those not taking GCs [25]. Additionally, more than 37% of postmenopausal glucocorticoid users experience asymptomatic vertebral fractures [26]. Similarly, premenopausal women taking GC therapy are characterized by a decreased BMD in the spine, hip, and femur compared with healthy age-matched controls [27]. Male steroid users also have a much higher incidence of fractures, but the risk is not as high as in postmenopausal women [28].
GIO is characterized by vertebral fractures that usually occur within 3–6 months of initiating GC therapy. However, patients taking daily steroids at doses as low as 2.5–7.5 mg of prednisolone or equivalent still experience increased bone loss induced by these drugs, resulting in an increased risk of fractures (summarized in Table 2) [29,30,31,32,33,34]. It has been shown that patients receiving glucocorticoids for more than 3 months in 1 year have a 1.25-fold higher risk of major fracture compared with patients who have never used glucocorticoids, and the risk of hip fracture increases 1.6-fold [35]. Occasional use of oral corticosteroids in high dosages (≥ 15 mg/day and cumulative exposure ≤ 1 g/day) may cause a slight increase in the risk of osteoporotic fracture. However, several dose courses of the glucocorticoids mentioned above significantly increase the risk of fractures [36].
Corticosteroid treatment is frequently administered in managing autoimmune diseases, especially connective tissue diseases (CTDs), including rheumatoid arthritis or systemic lupus erythematosus. CTDs are three times more common in women and commonly develop in the third and fourth decades of life. Similar to women, most men with CTDs also require corticosteroid treatment; however, Norwegian data show that males receiving corticosteroid treatment receive less prevention for GIO (only 4% of males compared with 10% of the female population) [37].
While most of the studies on GIO focus on adults, it is also observed in the pediatric population. Nearly 1.2% of children receive at least one oral GC therapy within 1 year, mainly for severe asthma management. Children who have been prescribed more than four short cycles of oral GCs are at increased humeral fracture risk [38]. Vertebral fractures are the hallmark of GIO in both adult and pediatric populations. However, the growing skeleton is a dynamic structure with the capacity for BMD reclamation and vertebral body reshaping. Thus, GC-treated children may spontaneously recover from osteoporosis without medical intervention, but recovery from GIO depends on adequate residual linear growth [39]. Without prompt intervention, GC use could still result in irreversible vertebral deformity, meaning pediatric patients with less residual growth should be adequately treated [40].
Glucocorticoids in clinical practice
Systemic treatment with GCs is typically administered orally, intravenously, or intramuscularly. As with any other pharmaceutical drug, GCs can be administered at various doses, from as low as 7.5 mg/day to as high as 100 mg/day. Pulsatile administration is an alternative method involving the infusion of 250–1000 mg/day of prednisone for 1–3 days [41]. GCs are widely prescribed to patients with autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, severe skin problems, and inflammatory bowel diseases [42,43,44]. Chronic use of systemic GCs, even at physiologic dose, is associated with significant adverse effects, including physical appearance change (moon face, buffalo hump, visceral fat deposition, and muscle atrophy), hypothalamic–pituitary–adrenal axis suppression, gastrointestinal bleeding, glaucoma, and cataract [45]. There is also increasing evidence of several side effects, albeit the short-term use of GCs, for instance, the enhanced risk of sepsis, venous thromboembolism, reduced bone density, and fracture [46].
Other methods of GC administration can also cause a decrease in BMD and enhance the fracture risk. For example, topical use of GCs is a standard treatment of psoriasis and atopic dermatitis, which is considered relatively safe and usually causes only localized side effects such as skin atrophy and telangiectasias [47]. However, a cohort study of 723,251 adults in the USA shows that every doubled dose of topical GCs can increase 3% of osteoporosis and major osteoporotic fracture risk [21].
Inhaled corticosteroids (ICS) used in managing obstructive airway disease such as asthma are another everyday exposure to GCs, which can cause side effects [48]. The 2019 Global Initiative for Asthma (GINA) guidelines recommend that all asthmatic patients be treated with inhaled corticosteroids daily or as required [49]. Patients with severe asthma, which comprise 5–10% of all asthma individuals, require high doses and chronic use of ICS. Thus, they are particularly susceptible to systemic adverse effects [50]. A study by Sasagawa et al. demonstrated that female asthmatic patients who use ICS for more than 6 months experience considerable deterioration in bone status, as measured by osteo-sono-assessment index (OSI) analysis. This risk is comparable to that observed with low-dose systemic glucocorticoid use [51].
Unfortunately, intraarticular corticosteroid injection (IACS) therapy is used in the growing hip and knee osteoarthritis population [52]. The benefit of IACS for osteoarthritis is currently under debate, primarily due to its association with increased risk of osteonecrosis without inducing GIOs. Existing evidence shows that the adverse effects of IACS include accelerated osteoarthritis progression, subchondral insufficiency fracture, osteonecrosis of the femoral head and condyles, and rapid destruction of joints with bone loss [53]. Along with the risk of osteoporosis and fracture, common adverse effects of GCs include arterial hypertension, diabetes mellitus, lowered immunity, and gastric ulcers, which should also be taken into consideration during treatment planning [54].
Pathophysiology of GIO
GCs were first discovered in the 1940s as adrenal cortex extracts and have since become one of the most widely prescribed and effective treatments to control inflammatory and autoimmune diseases [55]. The molecular mechanism of GCs to control inflammation is based on binding GCs to the glucocorticoid receptor (GR) in the cytoplasm, which subsequently forms the GC-GR complex and exhibits two mechanisms: transactivation and transrepression. The process of transactivation upregulates the expression of an anti-inflammatory protein-coding gene, including annexin-A1 (ANXA1), secretory leukoprotease inhibitor (SLPI), and mitogen-activated protein kinase phosphatase-1 (MKP-1). In turn, transrepression is the downregulation of pro-inflammatory transcription factor activity, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1) [56,57,58]. The administration of GCs thereby inhibits inflammatory cytokine secretion and changes in cell function and survival.
GCs profoundly affect the immune system, suppressing T cell generation and proliferation. They can also directly or indirectly affect numerous other cells and tissue throughout the human body, including endothelial cells, fibroblasts, osteoblasts, osteoclasts, and muscular and adipose tissue, attributed to the inevitable side effects of GC use [59].
Direct effects of glucocorticoid on bone cells
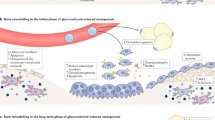
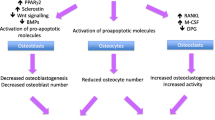
The interplay of GCs between osteoblasts, osteoclasts, and osteocytes accounts for the pathogenesis of GIO (Fig. 1). GCs exert their function on osteoblasts, which synthesize bone matrix and regulate the mineralization of the bone formation via various signaling pathways [60]. GC inhibition of the canonical Wnt pathway, also known as the Wnt/β-catenin pathway, drives bone marrow mesenchymal stem cells (MSCs) toward differentiating into adipocytes and decreases the possibility of osteogenesis [61,62,63]. GCs exert their inhibitory effects on the Wnt pathway through a combination of mechanisms, including direct inhibition of Wnt ligands, interference with β-catenin translocation, upregulation of Wnt antagonists, and induction of osteoblast apoptosis [63]. Additionally, GCs are found to suppress the phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) pathway activation and induce osteoblast apoptosis [64].
Osteocytes, which play a crucial role in orchestrating bone formation and bone resorption in response to mechanical strain, are also targeted by GCs in GIO. Osteocytes are capable of detecting microdamages of bone and subsequently repair them. Under the influence of GCs, osteocytes lose the ability to perform such crucial tasks [10]. Moreover, GCs promote osteocyte apoptosis by disrupting the lacunar–canalicular network. This network contains oxygen and nutrients critical to maintaining osteocytes’ functions and vitality [65]. Unfortunately, inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are enhanced by GCs and activate Fas and the Fas Ligand (FasL) apoptotic pathway, leading to increased osteocyte apoptosis [66, 67]. Osteocytes also play a crucial role in enhancing VEGF levels via hypoxia-inducible factor-α (HIF-α), promoting angiogenesis, which is important for bone hydration and strength. Under the influence of GCs, osteocyte production of VEGF is reduced, resulting in bone dehydration and decreased bone strength [10].
In addition, the administration of GCs increases the differentiation of osteoclasts and decreases osteoclasts’ apoptosis by mediating the receptor activator of nuclear factor kappa-B ligand (RANKL)–receptor activator of nuclear factor kappa-B (RANK)–osteoprotegerin (OPG) system. GCs reduce the level of osteoprotegerin (OPG), an inhibitor of the binding of receptor activator of nuclear factor kappa-B ligand (RANKL) to its receptor RANK. The increased RANKL-to-OPG ratio due to GC therapy matures osteoclasts and promotes bone resorption and subsequent bone loss [68,69,70]. In addition, GCs enhanced the production of macrophage colony-stimulating factor (M-CSF), which induces the expression of RANK and downstream osteoclastogenesis [71, 72].
Indirect effects of glucocorticoid on bone metabolism
The dysregulation of bone metabolism associated with GCs is another factor that contributes to bone loss. GCs enhance renal excretion of calcium and impair intestinal calcium reabsorption, potentially leading to secondary hyperparathyroidism [73,74,75]. Additionally, GCs appear to interact synergistically with D3 and parathyroid hormone (PTH) to potentiate the development of osteoclasts [76].
Estrogen and androgen are steroid hormones with bone-protective effects that increase osteoclasts’ apoptosis and prolong osteoblasts’ lifespan [77, 78]. Hence, the inhibition of estrogen and androgen synthesis and secretion by GCs gives rise to a reduced circulating concentration of these hormones and, thereby, has a detrimental impact on bone health [79].
The growth hormone (GH) and insulin-like growth factor (IGF) axis (Fig. 2), which play a significant role in bone metabolism, are also affected by the chronic administration of GCs [80]. In humans, GH/IGF levels peak during pubertal growth, promoting the proliferation of mesenchymal stem cells while increasing osteoblast mineralization and differentiation [81]. GCs are demonstrated to suppress the transcription of IGF-1 and the synthesis of IGF-binding proteins and, thus, decrease bone density [79, 82].
Influence of GCs on osteoblast and osteoclast function. GH growth hormone, IGF-1 insulin-like growth factor-1, M-CSF macrophage colony-stimulating factor, PPARγ peroxisome proliferator-activated receptor γ, RANKL receptor activator of nuclear factor kappa beta, WNT signaling wingless-related integration site signaling, OPG osteoprotegerin
Diagnosis of GIO in the primary care setting
As with any other medical condition, a thorough medical history is critical to diagnosing GIO. Patients treated with long-term GCs such as postmenopausal women, transplant recipients, and patients with autoimmune diseases are highly susceptible to GIO. Determining the sex and age of the patient is crucial due to the higher incidence of GIO in older women. Taking a history of traumatic fractures is important in estimating the onset of GIO. Patients with a family history of osteoporosis and fractures are at an increased risk of bone pathologies. Furthermore, lifestyle choices such as calcium and alcohol intake, smoking, and physical activity are all factors that play a role in the onset and prognosis of GIO. During clinical evaluation, it is essential to identify cushingoid clinical features in patients undergoing long-term GC therapy. Such clinical features include truncal obesity, skin atrophy, symptoms of vertebral compression, and myopathy [83]. In addition to the patient’s medical history and clinical examination, laboratory evaluation is the next step in diagnosing GIO. Initially, a complete blood cell count, markers of liver and renal function, and serum electrophoresis should be evaluated. Hormone profiles such as thyroid, testosterone, estradiol, luteinizing hormone, and prolactin are recommended for evaluation. Serum calcium, phosphate, 25-OH-vitamin D, alkaline phosphatase, and PTH evaluations are crucial to determining the state and pathology of fractures [83].
Furthermore, markers of bone turnover are vital diagnostic tools of GIO. Active osteoblasts release bone formation markers during osteoblastogenesis stages and reveal the status of osteoblast function. In patients diagnosed with GIO, bone formation markers are relatively decreased. These bone formation markers include osteocalcin, bone-specific alkaline phosphatase, procollagen type I C-terminal propeptide, and procollagen type 1 N-terminal propeptide. Bone resorption markers, however, are collagen degradation products released during the bone breakdown. Higher levels of bone resorption markers are present in bone fracture disorders. These bone resorption markers include hydroxyproline, hydroxylysine, pyridinoline, bone sialoprotein, osteopontin, and cathepsin K, among others [83, 84].
It is important to note that measuring the BMD is the gold standard for diagnosing GIO. BMD provides data regarding bone strength, mass, density, and overall bone health status in osteoporotic patients.
Diagnosis of GIO through BMD scanning
BMD is considered to be the standard measure for the diagnosis of primary and secondary osteoporosis as well as the assessment of fracture risk. According to the 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis, initiating a fracture risk assessment within 6 months of chronic BC therapy for pediatric and adult patients is recommended [85]. The current worldwide calculation of fracture risk for adults > 40 years old is based on the FRAX tool (https://frax.shef.ac.uk/FRAX/tool.aspx?country=9), which evaluates the 10-year probability of hip and major osteoporotic fractures. The FRAX assessment includes factors such as sex, age, body mass index, previous fracture, parental history of hip fracture, and secondary causes of osteoporosis. These secondary causes include GC treatment, smoking, alcohol intake, type 1 diabetes, rheumatoid arthritis, chronic liver disease, and chronic renal failure without dialysis. When BMD testing is available, the FRAX score can be calculated with an optional T-score from the femoral BMD test [densitometry via dual-energy X-ray absorptiometry (DXA)]. If the GC dosage is ≥ 7.5 mg/day, the hip fracture probability should be raised by 20% and the likelihood of a major fracture by 15% [86]. Primary or secondary osteoporosis treatment should be considered for patients with low BMD (a T-score of − 1.0 and − 2.5) or a 10-year risk of a major osteoporosis-related fracture of > 20%, as assessed with the FRAX [87].
The fracture risk should be regularly assessed using densitometry after or during a high dose of GC treatment every 2–3 years [88]. For adults under 40 years with previous fracture history, a BMD test is recommended to assess the initial fracture risk, and patients should be monitored by BMD testing combined with other BMD-independent clinical risk judgments [85, 88]. The method of BMD measurement recommended by the World Health Organization (WHO) and the International Osteoporosis Foundation (IOF) is dual-energy X-ray absorptiometry (DEXA/DXA) [3, 89]. In children who start GC therapy, it is recommended to perform an X-ray of the lateral spine to detect vertebral fractures at the beginning of steroid treatment and after 1 year. However, routine lateral spine X-rays and BMD checks with DXA are not recommended on a routine basis in pediatric patients treated with inhaled corticosteroids at doses below 800 mcg/day unless they have other risk factors (e.g., malnutrition, cystic fibrosis, celiac disease, food allergies, chronic rheumatic diseases, prematurity, etc.) [90].
Management and treatment of GIO
General measures and lifestyle modification
The prevention of GIO involves several clinical and therapeutic options. Firstly, studies suggest that steroid-induced osteoporosis can be reversed in young people when exogenous steroids are discontinued. This study showed increased bone density during the recovery period from exogenous steroids. Such reports should be further studied in older adults due to the reduced capacity to recover bone mass. Clinicians should consider either discontinuing GCs or prescribing at the lowest possible dosage for the shortest duration when there is a substantial risk of bone loss or fractures due to prolonged GC therapy. Both BMD measurements and fracture risk criteria can guide this decision [91, 92]. Secondly, adults receiving an equivalent prednisone dose of more than 2.5 mg/day for 3 months should be well informed about optimizing calcium intake of 1000–1200 mg/day and vitamin D supplementation of 600–800 IU/day [93]. It is important to note that, considering the high prevalence of vitamin D deficiency worldwide, patients with preexisting low serum vitamin D levels undergoing GC therapy should supplement higher doses of vitamins at or above 2000 IU/day in the first three months of beginning GC therapy. The first 3 months are a critical time of deficiency and bone loss, and no detrimental effects regarding the risk of calcium balance or toxicity have been seen with even increasing vitamin D dosages up to 10,000 IU in patients at high risk [94, 95]. Additional lifestyle modifications to reduce the risk of bone fractures include well-balanced nutrients, smoking cessation, and regular weight-bearing activities [93, 96].
Once diagnosis of GIO is made, the initial step of management and treatment includes lifestyle patient education and modification. Adequate intake of nutritional sources of calcium (milk, yogurt, cheese, broccoli, and canned fish) and vitamin D (fatty fish, cereals, eggs, mushrooms, and milk) is recommended to reduce the risk of fractures. A nutritious diet rich in minerals, vitamin supplementation, and sufficient sun exposure are critical for maintaining good bone health. Cessation of smoking and alcohol abuse is strongly recommended for the prevention of primary and secondary osteoporosis. Alcohol intake of more than three drinks a day is associated with decreased bone health [84]. Furthermore, weight-bearing exercises help reduce fracture risks by increasing peak bone mass. These weight-bearing exercises are essential for all ages and include everyday walking, jogging, stair climbing, and dancing. Other moderate-to-intense physical activities such as aerobic, balance, and strength training have been reported to reduce injurious falls by enhancing muscle strength and overall coordination [84].
Vitamin D and calcium supplementation
Vitamin D and calcium prevent GC-related bone loss in the lumbar spine and forearm [97]. A report has shown that vitamin D insufficiency may affect the patient’s response to osteoporosis therapy, particularly with teriparatide. This study suggested that vitamin D deficiency might impair teriparatide response by inducing secondary hyperparathyroidism [98]. Furthermore, a recent study on a novel active vitamin D3 analog, eldecalcitol (ED-71), demonstrated bone mass protection by promoting new bone formation and increasing osteoblast activity while inhibiting osteoclasts [99]. ED-71 has been shown to have a longer half-life. It binds more efficiently to vitamin D-binding proteins, promoting mini bone formation, which has been shown to elevate bone mineral density more significantly than typical vitamin D forms such as calcitriol and alfacalcidol. However, GC-treated patients with vitamin D deficiency should intake 2000–6000 IU/day to prevent iatrogenic complications. The daily vitamin D supplementary dose for the general adult population is 800–2000 IU/day. GC-treated patients with vitamin D deficiency are recommended to intake 2000–6000 IU/day vitamin D as a supplement to prevent iatrogenic complications [100]. In addition, vitamin D may strengthen muscles and improve balance, reducing fall and fracture risk [9]. Vitamin D supplementation is generally safe since the toxicity is rare. The expectation is required only for patients with a predisposed risk of hypercalciuria and hypercalcemia, such as sarcoidosis [101]. Therefore, serum calcium is initially assessed 2–4 weeks following the start of therapy and frequently every 2–3 months after that [75]. Most guidelines recommend supplementation with calcium 1000–1200 mg/day depending on the age [9]. Combined calcium supplementation with vitamin D is better than solely calcium supplementation. However, studies have shown the combined supplementation’s shortcomings in reversing bone loss and fracture incidence in patients treated with high-dose GCs, indicating the importance of other superior osteoporotic drugs such as bisphosphonates, teriparatide, and denosumab [75].
Bisphosphonates
All GIO guidelines indicate that bisphosphonates (e.g., alendronate, risedronate, ibandronate, and zoledronate) should be first-line treatment. Bisphosphonates are used as a mainstay in GIO treatment because these drugs are generally safe, inexpensive, and widely used in osteoporosis treatment. They effectively inhibit osteoclastic bone resorption and bone formation simultaneously [102,103,104]. Despite the differences between individual medications, several randomized clinical control trials proved that bisphosphonates significantly increase lumbar spinal and femoral BMD. A study reported a fracture incidence rate reduced by 59% when bisphosphonates were initiated within 90 days of starting chronic GC therapy [9, 105]. Moreover, their adverse effects are rarely observed (e.g., secondary fractures and osteonecrosis of the jaw bone) [106,107,108,109]. The American College of Rheumatology guideline recommends that adults at moderate to high risk of fracture be treated with oral bisphosphonates in combination with calcium and vitamin D supplementation [9, 110]. A better efficacy is observed if bisphosphonates are given with vitamin D in GIO patients [111]. The administration of oral bisphosphonates can be daily, weekly, or monthly (e.g., ibandronate, alendronate, and risedronate). Intravenous forms are also available (e.g., ibandronate and zoledronate) [112]. Many studies have demonstrated the efficacy of bisphosphonates in reducing fracture risk in GIO. For example, alendronate diminishes vertebral fracture risk, especially in high-dosage GC patients [113,114,115]. Similarly, ibandronate decreases vertebral bone loss and reduces vertebral fracture risk [116, 117]. The effectiveness of bisphosphonates is also related to the administration route. For example, annual intravenous zoledronic acid management is more effective in increasing BMD than oral risedronate [118, 119].
Despite bisphosphonates being first-line treatment, the VERO study demonstrates discrepancies between medications for preventing osteoporosis-related fractures (analysis of the efficacy of subcutaneous teriparatide in a dosage of 20 µg daily or risedronate in a dosage of 35 mg weekly versus subcutaneous placebo). Subcutaneous teriparatide demonstrated a 60% lower fracture risk than those treated with risedronate [120].
Using bisphosphonates to prevent and treat GIO, especially during short-term GC therapy, is a strong recommendation based on study evidence. However, there is a paucity of data regarding head-to-head comparison among different bisphosphonates, and there still needs to be more analysis on the long-term safety of these medications in patients chronically using steroids. Thus, before prescribing these drugs, it is essential to determine the risk of possible atypical femoral fractures [121]. An atypical femoral fracture is a transverse fracture of the femoral diaphysis, defined by clinical criteria and radiographic appearance. Studies show that bisphosphonate use exacerbates the incidence of atypical femoral fracture, which increases with the duration of use, especially after 3 years. Other noteworthy risk factors for atypical femur fracture include Asian ancestry, shorter height, higher weight, and GC use for more than 1 year. If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies such as plain radiography are recommended. If radiography is inconclusive, bone scan or magnetic resonance imaging (MRI) should be considered [122, 123]. With these newly recognized side effects of bisphosphonates, including osteonecrosis of the jaw, it is recommended to consider 2–3 years of drug holiday after 5 years of oral and 3 years of IV bisphosphonate therapy. Drug holidays can effectively reduce the risk of atypical fractures and osteonecrosis of the jaw in long-term users of bisphosphonates [84, 124,125,126]. Esophagitis, hypocalcemia, and musculoskeletal pain are among other potential side effects observed in patients taking oral bisphosphonates [124,125,126]. Poor patient compliance due to possible side effects and inconvenient dosing regimens, poor oral absorption, and poor tolerability were seen in approximately 25% of patients; clinicians are recommended to consider alternative osteoporotic drugs that have proven to be effective in increasing BMD such as anabolic agents [127, 128].
Analog of parathyroid hormone and PTH-related protein
Teriparatide, a recombinant human parathyroid hormone (PTH), is another effective treatment option for GIO patients and is commonly considered a second-line GIO drug. It is also an alternative option for patients with high-risk osteoporosis on long-term bisphosphonates therapy. The drug is given subcutaneously at a dosage of 20 μg daily. Teriparatide significantly reduces the incidence of vertebral fractures and non-vertebral fractures (excluding the femoral neck) in the postmenopausal period. This drug is therefore recommended for the treatment of osteoporosis in postmenopausal women and men at increased risk of fractures. This drug is particularly recommended in GIO treatment in women and men with an increased risk of fractures [108]. In teriparatide therapy, serum amino-terminal propeptide (PINP) is considered an essential marker for monitoring patients receiving treatment. In bone, collagen type I is synthesized in the form of procollagen. The procollagen type I contains amino-terminal propeptide (PINP) and carboxy-terminal propeptide, which are cleaved by proteinases as collagen type I is formed. Both propeptides are found in serum; their concentrations reflect the collagen type I synthesis rate. PINP is considered the most sensitive bone marker and is an essential marker for monitoring patients receiving teriparatide [98].
In contrast to antiresorptive agents, PTH has an anabolic effect in improving osteoblastogenesis and increasing bone remodeling rate [129]. A clinical trial comparing the use of teriparatide and alendronate in GC populations, including men and both premenopausal and postmenopausal women, indicated an increased BMD of the lumbar spine in patients with teriparatide treatment compared with those using alendronate [130]. A meta-analysis based on the evaluation of five randomized controlled trials showed a 0.13-fold reduction in the risk of new vertebral fracture in GIO patients on teriparatide management (RR = 0.13, 95% CI: 0.05–0.34, p<0.00001) compared with those treated with alendronate [131]. Thus, transitioning to an antiresorptive agent after teriparatide treatment for GIO helps maintain the positive effects on bone density and structure while ensuring a balanced bone remodeling process. This approach offers a comprehensive long-term bone health management strategy in individuals with GIO. Combined therapy of alendronate and teriparatide is not recommended due to decreased osteocalcin (bone formation marker) caused by alendronate therapy. Decreased levels of osteocalcin will reduce the role of teriparatide in promoting bone formation [111].
For long-term bisphosphonate users without adequate clinical response or with the appearance of adverse effects, switching to teriparatide or denosumab (a RANKL inhibitor) is recommended as the next therapy step. Teriparatide therapy has shown to be more effective than oral bisphosphonates in increasing lumbar and hip BMD, as well as preventing vertebral fractures in GIO patients [75, 127]. The study of Hirooka et al. has shown similar results in increasing BMD of the lumbar spine and hip when using teriparatide or denosumab. However, a significant increase in femoral neck BMD from baseline has been observed only in the teriparatide group after 24 months of treatment [132]. In another study, the further switch from teriparatide to denosumab indicated better maintenance of BMD in the group that continued teriparatide treatment [133]. Thus, teriparatide might have some advantages over denosumab and be a good alternative for treating GIO patients with prior bisphosphonate treatment.
Discontinuation of teriparatide results in rapid bone loss, and alternative agents such as denosumab and bisphosphonates are recommended to maintain bone density. Teriparatide side effects include transient hypotension, leg cramps, and nausea. Teriparatide treatment is restricted to 2 years in response to the high incidence of osteosarcoma seen in rodent studies. However, this increase was not translated into humans after 15 years of observation post-marketing. Therefore, its use for over 2 years can now be considered for patients whose fracture risks remain high. Its use is still contraindicated in settings of increased risk of osteosarcoma [134].
Abaloparatide is a novel synthetic peptide analog of parathyroid hormone-related protein (PTHrP) recently approved by the Food and Drug Administratio (FDA) for the treatment of osteoporosis in postmenopausal women. It has successfully increased spinal, femoral, and hip BMD in postmenopausal women with osteoporosis. Abaloparatide has been shown to decrease new vertebral fractures by 86% and non-vertebral fractures by about 43% in postmenopausal women receiving treatment for 18 months [135]. An extension study revealed significant improvement in the reduction of bone fractures in patients supplementary treated with alendronate for 6–24 months after the 18-month treatment period with abaloparatide [91]. Other studies showed an increased acetabular BMD and bone strength with 18-month treatment with abaloparatide, providing better posture stability in postmenopausal women with osteoporosis [84, 92]. Similarly, with teriparatide, discontinuation of treatment results in rapid bone loss, and therefore, treatment should be maintained by denosumab or bisphosphonates. Side effects of abaloparatide include muscle weakness, nausea or vomiting, and constipation. The use of this drug is contraindicated in patients with an increased risk of osteosarcoma, and it is generally not recommended to use it for more than 2 years [136].
RANKL inhibitors
Since RANKL increases osteoclast differentiation and activation, using RANKL inhibitors benefits GIO patients. Denosumab is a human monoclonal RNAKL antibody at a subcutaneous dose of 60 mg every 6 months. A 2-year randomized clinical control trial shows a superior effect of denosumab in increasing spinal and hip BMD compared with risedronate use with similar adverse effects in both treatment groups [137]. For chronic GC users, denosumab also significantly increases spinal BMD compared with alendronate treatment [138]. However, it should be noted that discontinuing denosumab therapy may increase the risk of multiple vertebral fractures and bone loss. In such cases, bisphosphonates should be prescribed 6–7 months after the last dose of denosumab [139, 140]. It should also be pointed out that oral bisphosphonates and teriparatide are preferable for childbearing-age women over denosumab use due to the lack of safety evidence and the potential fetal risk during pregnancy [85].
Sex hormones
Hormone replacement therapy (HRT), which includes estrogen, selective estrogen receptor modulators (SERMS), and testosterone, has a protective impact on the skeleton and has been indicated for the prevention of postmenopausal osteoporosis and males with primary osteoporosis [141, 142]. The increased spinal BMD and reduced fracture risk in the group of GC users have also been seen in limited studies and may benefit GC-treated patients with concurrent gonadal insufficiency [143, 144]. Studies showed oral hormone therapy (HT) reduced spine fractures by 35% and non-vertebral skeleton fractures by 22% [145]. Oral HT is not the first line for the treatment of osteoporosis due to severe potential side effects, which include breast cancer, endometrial hyperplasia/carcinoma, deep vein thrombosis, pulmonary emboli, stroke, and myocardial infarction.
Raloxifene is a SERM indicated for the treatment or prevention of osteoporosis in postmenopausal women. Currently, 60 mg daily raloxifene is only recommended for postmenopausal women when oral bisphosphonate and other alternatives are not appropriate or available [85]. Raloxifene has been shown to reduce the incidence of vertebral fractures by 30–40% in patients with prior vertebral fractures and by 55% in patients without prior vertebral fractures [146]. Due to its protective effect against breast cancer, raloxifene is also used for the treatment or prevention of breast cancer. Its side effects include an increased risk of deep vein thrombosis and hot flashes. A combination of SERM and estrogen products (bazedoxifene/conjugated estrogen) provides an alternative therapy for the treatment of osteoporosis with reduced estrogen side effects. This combination drug significantly increases lumbar spine BMD and total hip BMD [146, 147].
Calcitonin
Calcitonin is an FDA-approved intranasal spray synthetic hormone used to treat osteoporosis in postmenopausal women at least 5 years beyond menopause. It decreases osteoclast activity and has been shown to suppress bone resorption and increase BMD. However, calcitonin has proven less effective in increasing BMD than bisphosphonates or denosumab [84]. Therefore, it is not a first-line agent for the treatment of osteoporosis. Common side effects include hypocalcemia, nausea, and allergic reactions.
Potential future novel GIO treatment drugs
Another promising alternative to GIO treatment is the FDA-approved romosozumab monoclonal antibody that binds to sclerostin. Sclerostin secretion by osteocytes is enhanced during GC therapy, and it inhibits Wnt signaling, suppressing the development and function of osteoblasts [148]. A significantly lower fracture risk in postmenopausal women treated with romosozumab compared with placebo and bisphosphonate alone, respectively, has been demonstrated in two large clinical trials [149, 150]. A randomized controlled trial regarding the efficacy of romosozumab versus denosumab patients on chronic GC use is also ongoing [151]. Other anti-sclerostin antibodies, namely blosozumab and setrusumab, are currently in clinical trials. A recent study showed that anti-sclerostin antibodies improved hip and femoral neck BMD compared with alendronate and teriparatide; hence, it could be a promising alternative for treating GIO [84]. Romosozumab has been shown to have several side effects, including myocardial infarction, stroke, and hypersensitivity reactions [152].
Conclusion
Glucocorticoids are effective anti-inflammatory drugs and are the mainstay of treatment of many rheumatic and autoimmune diseases, but they are also an important cause of secondary osteoporosis. The increasing number of patients on prolonged GC therapy highlights the importance of understanding GIO pathophysiology and management. Unfortunately, the long-term use of GCs increases the risk of fragility fracture at a much higher bone mineral density (BMD) than postmenopausal osteoporosis, indicating an additional deleterious effect of GCs on bone quality. Fracture is the most formidable outcome of GIO, yet understanding the mechanisms, preventive methods, and treatments underlying GIO may aid general practitioners in recognizing and managing this preventable health concern.
Patients treated with GCs, albeit with consideration to sex, age, GC dosage, duration, and administration methods, should be carefully evaluated with the fracture risk assessment. Since the number of previous fractures, GC dose-dependent effect, and the complexity of multiple osteoporosis-related comorbidities are not considered by FRAX, finding a more comprehensive evaluation tool is necessary. Such a tool is particularly needed for adult patients under 40 years or pediatric GC users. Moreover, dedicated GIO assessment guidelines remain an unmet medical need. While bisphosphonates are currently the first-line treatment of GIO, the increasing number of studies and the growing availability of anabolic agents may shed light on treatment for high-risk GIO patients and be taken as an alternative first-line option.
References
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–47.
Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65:294–8.
Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467–75.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28.
Compston J. Glucocorticoid-induced osteoporosis: an update. Endocrine. 2018;61:7–16.
Amiche MA, Albaum JM, Tadrous M, et al. Fracture risk in oral glucocorticoid users: a Bayesian meta-regression leveraging control arms of osteoporosis clinical trials. Osteoporos Int. 2016;27:1709–18.
Chakrabarti K, McCune WJ. Glucocorticoid-induced osteoporosis in premenopausal women: management for the rheumatologist. Curr Opin Rheumatol. 2023;35(3):161–9.
Chiodini I, Falchetti A, Merlotti D, et al. Updates in epidemiology, pathophysiology and management strategies of glucocorticoid-induced osteoporosis. Expert Rev Endocrinol Metab. 2020;15:283–98.
Rife E, Abdalla JE, de la Rocha JL, et al. Glucocorticoid-induced osteoporosis: are we practicing prevention? SN Compr Clin Med. 2023;5:57.
Jha SS. Glucocorticoid-induced osteoporosis (GIOP). Indian J Orthop. 2023;57(Suppl 1):181–91. https://doi.org/10.1007/s43465-023-01037-8. (Erratum.In:IndianJOrthop.2023Dec11;58(2):222).
van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–87.
Florez H, Hernandez-Rodriguez J, Carrasco JL, et al. Vertebral fracture risk in glucocorticoid-induced osteoporosis: the role of hypogonadism and corticosteroid boluses. RMD Open. 2020;6:e001355.
Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton. Subcell Biochem. 2019;91:453–76.
Balasubramanian A, Wade SW, Adler RA, et al. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos Int. 2016;27:3239–49.
Soen S, Kaku M, Okubo N, et al. Fracture risk associated with glucocorticoid-induced osteoporosis in Japan. J Bone Miner Metab. 2022;40:636–47.
Chee C, Sellahewa L, Pappachan JM. Inhaled corticosteroids and bone health. Open Respir Med J. 2014;8:85–92.
Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269–70.
Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–90.
Sun X, Wang Y, Zhang M, et al. Intraarticular injection of dexamethasone promotes bone erosion in collagen-induced arthritis in mice through up-regulation of RANKL expression. Inflammopharmacology. 2019;27:503–9.
Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708.
Egeberg A, Schwarz P, Harslof T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275–82.
Yamamoto T, Schneider R, Iwamoto Y, et al. Rapid destruction of the femoral head after a single intraarticular injection of corticosteroid into the hip joint. J Rheumatol. 2006;33:1701–4.
Thompson AR, Ensrud ER. Rapid onset of femoral head osteonecrosis after a single intra-articular hip joint injection of corticosteroid. Am J Phys Med Rehabil. 2020;99:e54–5.
Management of Osteoporosis in Postmenopausal Women: The Position Statement of The North American Menopause Society Editorial P. Management of osteoporosis in postmenopausal women: the position statement of The North American Menopause Society. Menopause. 2021;2021(28):973–97.
Villa P, Moruzzi MC, Lassandro AP, et al. Glucocorticoid therapy as a significant risk factor for osteoporosis and fractures in an Italian postmenopausal population. Gynecol Endocrinol. 2013;29:678–82.
Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in postmenopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39:253–9.
Fassio A, Idolazzi L, Jaber MA, et al. The negative bone effects of the disease and of chronic corticosteroid treatment in premenopausal women affected by rheumatoid arthritis. Reumatismo. 2016;68:65–71.
Adler RA, Hochberg MC. Glucocorticoid-induced osteoporosis in men. J Endocrinol Investig. 2011;34:481–4.
van Staa TP, Leufkens HG, Abenhaim L, et al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford). 2000;39:1383–9.
Abtahi S, Driessen JHM, Burden AM, et al. Low-dose oral glucocorticoid therapy and risk of osteoporotic fractures in patients with rheumatoid arthritis: a cohort study using the Clinical Practice Research Datalink. Rheumatology (Oxford). 2022;61:1448–58.
Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000.
Dovio A, Perazzolo L, Osella G, et al. Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab. 2004;89:4923–8.
McKenzie R, Reynolds JC, O’Fallon A, et al. Decreased bone mineral density during low dose glucocorticoid administration in a randomized, placebo controlled trial. J Rheumatol. 2000;27:2222–6.
Tobias JH, Sasi MR, Greenwood R, et al. Rapid hip bone loss in active Crohn’s disease patients receiving short-term corticosteroid therapy. Aliment Pharmacol Ther. 2004;20:951–7.
Majumdar SR, Morin SN, Lix LM, et al. Influence of recency and duration of glucocorticoid use on bone mineral density and risk of fractures: population-based cohort study. Osteoporos Int. 2013;24:2493–8.
De Vries F, Bracke M, Leufkens HG, et al. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–14.
Hoff M, Skurtveit S, Meyer HE, et al. Anti-osteoporosis drug use: too little, too much, or just right? The HUNT study, Norway. Osteoporos Int. 2018;29:1875–85.
van Staa TP, Cooper C, Leufkens HG, et al. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–8.
Ward LM. Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol (Lausanne). 2020;11:576.
Ward LM, Ma J, Robinson ME, et al. Osteoporotic fractures and vertebral body reshaping in children with glucocorticoid-treated rheumatic disorders. J Clin Endocrinol Metab. 2021;106:e5195–207.
Gensler LS. Glucocorticoids: complications to anticipate and prevent. Neurohospitalist. 2013;3:92–7.
Kaneko K, Chen H, Kaufman M, et al. Glucocorticoid-induced osteonecrosis in systemic lupus erythematosus patients. Clin Transl Med. 2021;11: e526.
Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170:ITC1–16.
Bruscoli S, Febo M, Riccardi C, et al. Glucocorticoid therapy in inflammatory bowel disease: mechanisms and clinical practice. Front Immunol. 2021;12: 691480.
Scherholz ML, Schlesinger N, Androulakis IP. Chronopharmacology of glucocorticoids. Adv Drug Deliv Rev. 2019;151–152:245–61.
Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63:655–70.
Stacey SK, McEleney M. Topical corticosteroids: choice and application. Am Fam Physician. 2021;103:337–43.
Heffler E, Madeira LNG, Ferrando M, et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018;6:776–81.
Mauer Y, Taliercio RM. Managing adult asthma: the 2019 GINA guidelines. Cleve Clin J Med. 2020;87:569–75.
Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73.
Sasagawa M, Hasegawa T, Kazama JJ, et al. Assessment of bone status in inhaled corticosteroid user asthmatic patients with an ultrasound measurement method. Allergol Int. 2011;60:459–65.
Guermazi A, Neogi T, Katz JN, et al. Intra-articular corticosteroid injections for the treatment of hip and knee osteoarthritis-related pain: considerations and controversies with a focus on imaging-radiology scientific expert panel. Radiology. 2020;297:503–12.
Kompel AJ, Roemer FW, Murakami AM, et al. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293:656–63.
Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–65.
Rhen T, Cidlowski JA. Anti-inflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23.
Ingawale DK, Mandlik SK. New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol Immunotoxicol. 2020;42:59–73.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13.
Necela BM, Cidlowski JA. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc Am Thorac Soc. 2004;1:239–46.
Alan IS. Side effects of glucocorticoids. sine loco: IntechOpen; 2018.
Leder BZ, Wein MN. SpringerLink. Osteoporosis: Pathophysiology and Clinical Management. 3rd edn. Cham: Springer International Publishing: Imprint: Humana; 2020.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. https://doi.org/10.1038/nm.3074. (Epub 2013 Feb 6).
Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3:141–7.
Colaianni G, Brunetti G, Faienza MF, et al. Osteoporosis and obesity: role of Wnt pathway in human and murine models. World J Orthop. 2014;5:242–6.
Pan JM, Wu LG, Cai JW, et al. Dexamethasone suppresses osteogenesis of osteoblast via the PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal Transduct Res. 2019;39:80–6.
Ru JY, Wang YF. Osteocyte apoptosis: the roles and key molecular mechanisms in resorption-related bone diseases. Cell Death Dis. 2020;11:846.
Wang T, Yu X, He C. Pro-inflammatory cytokines: cellular and molecular drug targets for glucocorticoid-induced-osteoporosis via osteocyte. Curr Drug Targets. 2019;20:1–15.
Kogianni G, Mann V, Ebetino F, et al. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–95.
Swanson C, Lorentzon M, Conaway HH, et al. Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology. 2006;147:3613–22.
Tobeiha M, Moghadasian MH, Amin N, et al. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int. 2020;2020:6910312.
Chen M, Fu W, Xu H, et al. Pathogenic mechanisms of glucocorticoid-induced osteoporosis. Cytokine Growth Factor Rev. 2023;70:54–66. https://doi.org/10.1016/j.cytogfr.2023.03.002. (Epub 2023 Mar 5).
Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–43.
Falchi M, Varricchio L, Martelli F, et al. Dexamethasone targeted directly to macrophages induces macrophage niches that promote erythroid expansion. Haematologica. 2015;100(2):178–87. https://doi.org/10.3324/haematol.2014.114405. (Epub 2014 Dec 22).
Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-97.
Liao F, Zhu Z, Xiao C, et al. Hydrogen sulfide inhibits calcium and phosphorus loss after fracture by negatively regulating glucocorticoid/glucocorticoid receptor alpha. Life Sci. 2021;274: 119363.
Anastasilaki E, Paccou J, Gkastaris K, et al. Glucocorticoid-induced osteoporosis: an overview with focus on its prevention and management. Hormones (Athens). 2023;22(4):611–22.
Conaway HH, Henning P, Lie A, et al. Glucocorticoids employ the monomeric glucocorticoid receptor to potentiate vitamin D3 and parathyroid hormone-induced osteoclastogenesis. FASEB J. 2019;33:14394–409.
Huber DM, Bendixen AC, Pathrose P, et al. Androgens suppress osteoclast formation induced by RANKL and macrophage-colony stimulating factor. Endocrinology. 2001;142:3800–8.
Srivastava S, Toraldo G, Weitzmann MN, et al. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-kappa B ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–40.
Patschan D, Loddenkemper K, Buttgereit F. Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone. 2001;29:498–505.
Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. 2013;9:265–76.
Dixit M, Poudel SB, Yakar S. Effects of GH/IGF axis on bone and cartilage. Mol Cell Endocrinol. 2021;519: 111052.
Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15:1781–9.
Ilias, I. An overview of glucocorticoid-induced osteoporosis. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK278968/. Accessed 19 Mar 2022.
Anish RJ, Nair A. Osteoporosis management-current and future perspectives—a systemic review. J Orthop. 2024;2(53):101–13.
Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095–110.
Kanis JA, Johansson H, Oden A, et al. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22:809–16.
Unnanuntana A, Gladnick BP, Donnelly E, et al. The assessment of fracture risk. J Bone Jt Surg Am. 2010;92:743–53.
Gregson CL, Armstrong DJ, Bowden J, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2022;17:58.
Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–202.
Galindo-Zavala R, Bou-Torrent R, Magallares-Lopez B, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. 2020;18:20. https://doi.org/10.1186/s12969-020-0411-9.
Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin Proc. 2017;92:200–10.
Sheth NP, Winzenrieth R, Humbert L, et al. Abaloparatide increases bone mineral density in regions corresponding to gruen zones 1, 2, 6, and 7 in postmenopausal women with osteoporosis. J Clin Densitom. 2023;26(3): 101397.
Whittier X, Saag KG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin N Am. 2016;42(177–189):x.
Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498–513.
Davidson ZE, Walker KZ, Truby H. Clinical review: do glucocorticosteroids alter vitamin D status? A systematic review with meta-analyses of observational studies. J Clin Endocrinol Metab. 2012;97:738–44.
Messina OD, Vidal LF, Wilman MV, et al. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res. 2021;33:793–804.
Homik J, Suarez-Almazor ME, Shea B, et al. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database Syst Rev. 2000;1998:CD000952.
Kong M, Gao C, Luan X, et al. Analyzing the factors associated with efficacy among teriparatide treatment in postmenopausal women with osteoporosis. BMC Musculoskelet Disord. 2024;25(1):109. https://doi.org/10.1186/s12891-024-07227-1.
Rong X, Kou Y, Zhang Y, et al. ED-71 Prevents glucocorticoid-induced osteoporosis by regulating osteoblast differentiation via notch and Wnt/beta-catenin pathways. Drug Des Dev Ther. 2022;16:3929–46.
Pludowski P, Kos-Kudla B, Walczak M, et al. Guidelines for preventing and treating vitamin D deficiency: a 2023 update in Poland. Nutrients. 2023;15:695.
Ilias I, Milionis C, Zoumakis E. An overview of glucocorticoid-induced osteoporosis. In: Feingold KR, et al. Endotext. South Dartmouth (MA); 2000.
Lems WF, Saag K. Bisphosphonates and glucocorticoid-induced osteoporosis: cons. Endocrine. 2015;49:628–34.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45.
Hsu E, Nanes M. Advances in treatment of glucocorticoid-induced osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2017;24:411–7.
Overman RA, Gourlay ML, Deal CL, et al. Fracture rate associated with quality metric-based anti-osteoporosis treatment in glucocorticoid-induced osteoporosis. Osteoporos Int. 2015;26(5):1515–24. https://doi.org/10.1007/s00198-014-3022-9. (Epub 2015 Jan 20).
Kan SL, Yuan ZF, Li Y, et al. Alendronate prevents glucocorticoid-induced osteoporosis in patients with rheumatic diseases: a meta-analysis. Medicine (Baltimore). 2016;95: e3990.
Fujieda Y, Horita T, Nishimoto N, et al. Efficacy and safety of sodium risedronate for glucocorticoid-induced osteoporosis with rheumatoid arthritis (RISOTTO study): a multicentre, double-blind, randomized, placebo-controlled trial. Mod Rheumatol. 2021;31:593–9.
Curtis JR, Saag KG. Prevention and treatment of glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2007;5:14–21.
Ward LM, Choudhury A, Alos N, et al. Zoledronic acid vs placebo in pediatric glucocorticoid-induced osteoporosis: a randomized, double-blind, phase 3 trial. J Clin Endocrinol Metab. 2021;106:e5222–35.
Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–37. https://doi.org/10.1002/art.40137. (Epub 2017 Jun 6. Erratum in: Arthritis Rheumatol. 2017 Nov;69(11):2246).
Liang H, Zhao J, Tian T. Pharmacological interventions for glucocorticoid-induced osteoporosis: an umbrella review. Horm Metab Res. 2023;55(8):511–9. https://doi.org/10.1055/a-2112-1596. (Epub 2023 Jun 19).
Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. 2019;30:1145–56.
Axelsson KF, Nilsson AG, Wedel H, et al. Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA. 2017;318:146–55.
Qiu M, Ding L, Zhang M, et al. Meta-regression analysis of the efficacy of alendronate for prevention of glucocorticoid-induced fractures. Medicine (Baltimore). 2020;99: e22690.
Wang YK, Zhang YM, Qin SQ, et al. Effects of alendronate for treatment of glucocorticoid-induced osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97: e12691.
Hakala M, Kroger H, Valleala H, et al. Once-monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: a 12-month, randomized, double-blind, placebo-controlled trial. Scand J Rheumatol. 2012;41:260–6.
Ringe JD, Dorst A, Faber H, et al. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative study. Osteoporos Int. 2003;14:801–7.
Reid DM, Devogelaer JP, Saag K, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373:1253–63.
Roux C, Reid DM, Devogelaer JP, et al. Post hoc analysis of a single IV infusion of zoledronic acid versus daily oral risedronate on lumbar spine bone mineral density in different subgroups with glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23:1083–90.
Body JJ, Marin F, Kendler DL, et al. Efficacy of teriparatide compared with risedronate on FRAX((R))-defined major osteoporotic fractures: results of the VERO clinical trial. Osteoporos Int. 2020;31:1935–42.
Teitelbaum SL, Seton MP, Saag KG. Should bisphosphonates be used for long-term treatment of glucocorticoid-induced osteoporosis? Arthritis Rheum. 2011;63:325–8.
Silverman S, Kupperman E, Bukata S. Bisphosphonate-related atypical femoral fracture: managing a rare but serious complication. Cleve Clin J Med. 2018;85:885–93.
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743–53.
Bauer DC, Abrahamsen B. bisphosphonate drug holidays in primary care: when and what to do next? Curr Osteoporos Rep. 2021;19(2):182–88. https://doi.org/10.1007/s11914-021-00660-4.
Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–65. https://doi.org/10.1210/jc.2009-1947.
Eastell R, Rosen CJ, Black DM et al. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* clinical practice guideline. J Clin Endocrinol & Metab. 2019;(104):1595–622. https://doi.org/10.1210/jc.2019-00221.
Yuan C, Liang Y, Zhu K, et al. Clinical efficacy of denosumab, teriparatide, and oral bisphosphonates in the prevention of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18(1):447.
Dong L, Jiang L, Xu Z, et al. Denosumab, teriparatide and bisphosphonates for glucocorticoid-induced osteoporosis: a Bayesian network meta-analysis. Front Pharmacol. 2024;19(15):1336075.
Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703.
Langdahl BL, Marin F, Shane E, et al. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int. 2009;20:2095–104.
Liu ZM, Zhang M, Zong Y, et al. The efficiency and safety of alendronate versus teriparatide for treatment glucocorticoid-induced osteoporosis: a meta-analysis and systematic review of randomized controlled trials. PLoS One. 2022;17: e0267706.
Hirooka Y, Nozaki Y, Inoue A, et al. Effects of denosumab versus teriparatide in glucocorticoid-induced osteoporosis patients with prior bisphosphonate treatment. Bone Rep. 2020;13: 100293.
Hirooka Y, Nozaki Y, Okuda S, et al. Four-year teriparatide followed by denosumab vs. continuous denosumab in glucocorticoid-induced osteoporosis patients with prior bisphosphonate treatment. Front Endocrinol (Lausanne). 2021;12: 753185.
Kendler DL, Marin F, Zerbini CAF et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy. RCT. 2018;(391):230–40. https://doi.org/10.1016/S0140-6736(17)32137-2.
Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33.
Neer RM, Arnaud CD, Zanchetta JR et al. Effect of Parathyroid Hormone (1-34) on Fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001(344):1434–41. https://doi.org/10.1056/NEJM200105103441904.
Saag KG, Pannacciulli N, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol. 2019;71:1174–84.
Mok CC, Ho LY, Leung SMT, et al. Denosumab versus alendronate in long-term glucocorticoid users: a 12-month randomized controlled trial. Bone. 2021;146: 115902.
Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–7.
Rheumatology ACo. Guideline summary to 2022 Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis (GIOP). 2023.
Gosset A, Pouilles JM, Tremollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2021;35: 101551.
Vescini F, Chiodini I, Falchetti A, et al. Management of osteoporosis in men: a narrative review. Int J Mol Sci. 2021;22:13640.
Mok CC, Ying KY, To CH, et al. Raloxifene for prevention of glucocorticoid-induced bone loss: a 12-month randomised double-blinded placebo-controlled trial. Ann Rheum Dis. 2011;70:778–84.
Reid IR, Wattie DJ, Evans MC, et al. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996;156:1173–7.
Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab, 2019(104):1623–30.
LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis, Osteoporos Int. 2022(33):2049–102. https://doi.org/10.1007/s00198-021-05900-y.
Gennari L, Merlotti D, Nuti R Selective estrogen receptor modulator (SERM) for the treatment of osteoporosis in postmenopausal women: focus on lasofoxifene, Clin Interv Aging. 2010(5):9–29. https://doi.org/10.2147/CIA.S6083.
Thiele S, Hannemann A, Winzer M, et al. Regulation of sclerostin in glucocorticoid-induced osteoporosis (GIO) in mice and humans. Endocr Connect. 2019;8:923–34.
Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43.
Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27.
Romosozumab Versus Denosumab for Osteoporosis in Long-term Glucocorticoid Users: an Open Randomized Controlled Trial. https://clinicaltrials.gov/ct2/show/NCT04091243?term=romosozumab&cond=glucocorticoid+induced+osteoporosis&draw=2&rank=1. Accessed 10 Nov 2023.
Cosman F, Crittenden DB, Adachi JD et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med, 2016(375):1532–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of interest
Chueh Hsuan Hsu, Chueh Lin Hsu, Ashley Langley, Caroline Wojcik, Elysee Iraganje, and Bogna Grygiel-Gorniak have no conflicts of interest that are directly relevant to the contents of this study.
Ethics approval
Not applicable.
Availability of data and material
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors’ contribution
C.H.H. and C.L.H.: conception and design of the study, acquisition and analysis of data, drafting of the manuscript and figures, project administration, and validation. A.L., C.W., and E.I.: validation, acquisition and analysis of data, and drafting of the manuscript. B.G.G.: conception and design of the study, supervision, validation, and project administration. All authors read and approved the final version.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hsu, C.H., Hsu, C.L., Langley, A. et al. Glucocorticoid-induced osteoporosis—from molecular mechanism to clinical practice. Drugs Ther Perspect (2024). https://doi.org/10.1007/s40267-024-01079-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40267-024-01079-4