Abstract
Triheptanoin (DOJOLVI™), a synthetic medium odd-chain (C7) triglyceride, is an effective and generally well tolerated source of calories and fatty acids for the treatment of paediatric and adult patients with molecularly-confirmed long-chain fatty acid oxidation disorders (LC-FAODs). The beneficial effects of triheptanoin are assumed to be linked to the anaplerotic properties of triheptanoin, which set it apart from the standard medium-chain triglyceride (MCT) oil. In a pivotal, randomized, phase 2 trial comparing triheptanoin to trioctanoin in patients with nonsevere LC-FAODs and normal cardiovascular function at baseline, patients in both treatment groups had similar mean changes from baseline in measures of cardiac function and structure after 4 months’ treatment. In patients with severe LC-FAODs, 78-weeks’ treatment with triheptanoin reduced pretreatment mean annualized event and duration rates for major clinical events in an open-label phase 2 trial (CL201). The therapeutic effect of triheptanoin appeared to persist during longer-term therapy in a long-term extension study (CL202). The most frequently reported adverse events were gastrointestinal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Q&A can be found at https://doi.org/10.6084/m9.figshare.13718008. |
Provides an alternative source of calories and fatty acids to bypass LC-FAOD enzyme deficiencies |
May have the potential to improve cardiac function and structure |
May have the potential to reduce major clinical events |
Generally well tolerated, with gastrointestinal-related adverse events occurring most commonly |
What is the rationale for developing triheptanoin in LC-FAODs?
Long-chain fatty acid oxidation disorders (LC-FAODs) represent a group of rare, life-threatening, inborn errors of metabolism that prevent the body from converting LC-FAs into energy during periods of fasting and physiological stress [1]. LC-FAODs are caused by mutations in genes encoding mitochondrial enzymes involved in the carnitine shuttle (for transport of LC-FAs into the mitochondria) or β-oxidation (for conversion of LC-FAs into energy) and include carnitine palmitoyltransferase (CPT) I or II deficiency, very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency (the most common LC-FOAD), long-chain 3-hydroxy-acyl-CoA dehydrogenase (LCHAD) deficiency and trifunctional protein (TFP) deficiency. Deficiencies in these crucial mitochondrial enzymes lead to partial or incomplete oxidation of LC-FAs. This compromises energy homeostasis and causes the accumulation of potentially toxic fatty acid intermediates in the blood and organs, thereby causing systemic effects [1].
In general, clinical manifestations of LC-FAODs are similar among the different disorders and include recurrent rhabdomyolysis (more common from > 6 years of age), acute or chronic cardiomyopathy (at any age), and hepatic dysfunction, including severe hypoglycaemia (dominates the clinical picture in infants and younger children) and hyperammonaemia, [1]. FAODs are especially fatal in newborns, who typically develop profound cardiomyopathy, liver dysfunction and hypoketotic hypoglycaemia within the first few days or weeks of life [2]. Episodic rhabdomyolysis is commonly the initial presentation from adolescence onwards [2].
LC-FAOD treatment, which varies depending on the type and severity of the individual disorder, typically involves the avoidance of fasting, a diet restricting LC-FAs, supplementation with medium-chain triglyceride (MCT) oil (containing medium-chain, even-carbon FAs that bypass the typical steps of LC-FAO for metabolism) and supplementation with carnitine (if a secondary carnitine deficiency develops) [1]. Despite newborn screening and early intervention, patients with LC-FAODs continue to experience recurrent hospitalizations and high rates of morbidity and mortality due to episodes of metabolic decompensation. This may be due, at least in part, to persistent energy deficiency from the depletion of odd-chain tricarboxylic acid (TCA) cycle intermediates when using MCT oil supplementation [1].
Triheptanoin (DOJOLVI™) is a triglyceride of medium-chain, odd-carbon FAs [3] with anaplerotic properties (i.e. resupplies TCA cycle intermediates) [1] that has been approved in the USA as a source of calories and fatty acids for the treatment of paediatric and adult patients with molecularly-confirmed LC-FAODs [3]. This article provides an overview of the use of oral triheptanoin in this patient population.
How should triheptanoin be used?
The recommended target daily dosage of triheptanoin is up to 35% of a patient’s total prescribed daily caloric intake (DCI), divided into at least 4 doses and administered with meals or snacks and always mixed well with soft food or beverages [3]. First, calculate the patient’s total prescribed DCI. Then, determine the total daily dose of triheptanoin (in mL) using the following equation: patient DCI (kcal) × target % dose of DCI divided by 8.3 kcal/mL (the caloric value of triheptanoin). Round the total daily dose of triheptanoin (mL) to the nearest whole number, and divide into at least 4 approximately equal individual doses. Consider more frequent smaller doses if a patient has difficulty tolerating a quarter of the total daily dosage at one time [3]. Triheptanoin maintained chemical and physical stability when mixed with beverages and foods (including skim milk, applesauce and metabolic formula) for up to 4 h at 25–40 °C and up to 48 h at 2–8 °C and when baked in muffins at 200 °C and kept for 7 days in a stability study [4]. An overview of the use of triheptanoin in the USA is provided in Table 1.
How does triheptanoin work?
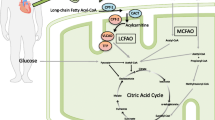
Triheptanoin is an MCT made up of three odd-chain 7-carbon length fatty acids (heptanoate; C7) [3]. Following oral administration, triheptanoin is hydrolyzed extensively by pancreatic lipases in the intestines to heptanoate and glycerol. The systemic absorption of triheptanoin is minimal [3]. Heptanoate then freely diffuses into the mitochondria and is metabolized by small- and medium-chain β-oxidation enzymes [1].
Heptanoate can undergo either one or two cycles of β-oxidation, producing two units of 2-carbon acetyl-coenzyme A (CoA) and one unit of 3-carbon propionyl-CoA, or one unit of acetyl-CoA and one unit of 5-carbon pentanoyl-CoA, respectively [1, 2, 5]. Acetyl-CoA can enter the TCA cycle to produce adenosine triphosphate (ATP), or be utilized for the synthesis of C4 ketone bodies [1, 5]. Propionyl-CoA is further metabolized to succinyl-CoA and succinate, resupplying TCA cycle intermediates (and indirectly supporting gluconeogenesis through increased ATP production) and supporting mitochondrial energy production [1, 5]. In the liver, pentanoyl-CoA can serve as an anaplerotic substrate and generates C5 ketone bodies [β-hydroxypentanoate (BHP) and β-ketopentanoate] that can be utilized by peripheral tissues [2].
What is the pharmacokinetic profile of triheptanoin?
Heptanoate pharmacokinetics are highly variable between patients, and heptanoate exposure increases more than dose-proportionally in the triheptanoin dose range of 0.3–0.4 g/kg [3]. More than one peak concentation is observed following the administration of oral triheptanoin. In healthy adults, the time to first peak concentration was 0.4–1.0 h (median 0.5 h) following a single dose of 0.3 g/kg, 0.4–6.4 h (median 0.8 h) following a single dose of 0.4 g/kg and 0.0–2.4 h (median 1.2 h) following multiple doses of 0.3 g/kg administered four times a day for 2 days (total daily dosage of 1.3 g/kg/day). Heptanoate is 80% bound to plasma proteins, independent of total concentration. Of note, heptanoate increases the unbound fraction of valproic acid ≈ twofold [3].
The half-life of heptanoate cannot be determined due to multiple peak concentrations following oral administration [3]. The mean apparent clearance of heptanoate in healthy subjects given a single dose of triheptanoin 0.3 g/kg or 0.4 g/kg was 6.05 L/h/kg and 4.31 L/h/kg, respectively [3]. In adult patients with LC-FAOD, the apparent clearnace of heptanoate was ≈ 22% lower that estimates in healthy subjects of the same body weight [6]. Heptanoate can be metabolized to BHP and β-hydroxybutyrate in the liver. Heptanoate and BHP are neither CYP nor UGT substrates, and heptanoate does not inhibit cytochrome P450 (CYP)1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4. Triheptanoin and its metabolites are minimally excreted in urine [3].
What is the efficacy of triheptanoin in LC-FAODs?
Triheptanoin provides an alternative source of calories and medium-chain FAs in patients with an LC-FAOD [7], with the potential to improve cardiac function and structure [8] and reduce major clinical events (MCEs) [7, 9]. The efficacy of triheptanoin in patients with an LC-FAOD was initially shown in a retrospective chart review [10] and a series of case reports [11] suggesting that triheptanoin can reduce hospitalization days/year and decrease the frequency of hypoglycaemia events [10], as well as improve cardiac function and stabilize cardiomyopathy [11]. Subsequent phase 2 trials investigated the efficacy of triheptanoin compared with that of trioctanoin in patients with nonsevere LC-FAOD [7, 8] and compared outcomes of a triheptanoin treatment period with a pre-triheptanoin treatment period in patients with severe LC-FAOD [9, 12].
Triheptanoin was effective in treating LC-FAODs in the key randomized, double-blind phase 2 trial, which enrolled patients aged ≥ 7 years with a confirmed diagnosis of an LC-FAOD (carnitine polmitoyltransferase-2, very long-chain acylCoA dehydrogenase, trifunctional protein or long-chain 3-hydroxyacylCoA dehydrogenase deficiencies) that included documentation of at least one significant episode of rhabdomyolysis [8], and stable, nonsevere disease [7]. Exclusion criteria included peripheral neuropathy limiting the ability to walk, history of myocardial infarction, and anaemia. At baseline, patients (n = 32) were aged between 7 and 64 years, all but one patient had normal cardiac function, and all patients were on a diet low in LC-FAs and receiving supplementation with a commercial MCT oil [8].
Patients received instructions to consume 20% of their estimated total energy needs from the prescribed study oil (triheptanoin or trioctanoin) [8]. Patients were contacted weekly during the 4 months of treatment to assess for compliance. Of note, patients consumed less than the prescribed amount of study oil (only ≈15% of total energy from either triheptanoin or trioctanoin). Primary endpoints included cardiac function by echocardiogram, exercise tolerance, total energy expenditure (TEE) and phosphocreatine recovery following acute exercise [8]. Efficacy analyses were performed in the intent-to-treat population [7, 8].
While the relative change in left ventricular (LV) ejection fraction (LVEF) and LV wall mass from baseline differed between the triheptanoin and trioctanoin groups at the 4-month assessment (Table 2), increases in LVEF and reductions in LV wall mass in both treatment groups were within the normal range in patients who had normal cardiac function at baseline. In addition, triheptanoin and trioctanoin recipients achieved the same degree of aerobic work upon treadmill exercise testing (Table 2). The clinical relevance of these changes in patients with baseline normal cardiac ejection fraction is not yet known [8] and both treatment groups were considered to have similar mean changes from baseline in LV ejection fraction and LV wall mass and similar maximal heart rates [3]. There were no between-group differences observed in other primary endpoints (e.g. phosphocreatine recovery following acute exercise, TEE) or secondary endpoints (e.g. body composition, blood biomarkers) in this trial [8].
The effectiveness of triheptanoin in treating LC-FAODs was also investigated in an open-label, single-arm phase 2 study (CL-201), which enrolled patients aged ≥6 months with a confirmed diagnosis of an LC-FAOD with severe disease [based on having any of the following significant clinical manifestations despite management: severe susceptibility to hypoglycaemia, evidence of functional cardiomyopathy, frequent severe major medical episodes, asymptomatic but highly elevated creatine kinase (CK), episodic elevated CK with reported muscle dysfunction, and chronic elevated CK with MCEs] [9, 12]. Patients had to have been on a stable treatment regimen in the 60 days prior to enrolment [12]. Exclusion criteria included patients with carnitine-acylcarnitine translocase and carnitine palmitoyl transferase 1 deficiency LC-FAODs (due to the rarity of these diagnoses as well as the likelihood that the severity of disease would limit full participation in study requirements) and confounding comorbidities. At baseline, the mean age was 12.06 years (range 10 months to 58 years) and the majority (86%) of patients had ongoing severe musculoskeletal disease. Most patients received MCT (93% of patients) and carnitine (76%) as part of their disease management prior to starting treatment with triheptanoin and all were on a low-fat diet high in carbohydrates, [9, 12].
Following a 4-week run-in period, which provided baseline data on patients’ current disease management, patients stopped MCT use (if applicable) and started 24 weeks of treatment with triheptanoin [12] in combination with a low-fat diet and other ongoing treatments such as carnitine (if applicable) [9]. Patients had the option of continuing treatment in a 54-week extension period, for a total treatment period of 78 weeks [9, 12]. The dose of triheptanoin was titrated to a target dose of 25–35% of total DCI and was administered orally or via gastrostomy tube at least four times a day, mixed with food or formula [12]. The overall mean dose of triheptanoin was 30% of DCI through 24 weeks [12] and 27.5% of DCI through 78 weeks of treatment [9]. Patients provided a 3-day diet diary of all foods and liquids consumed, at run-in, baseline, and subsequent visits [9].
The key objectives of the study were to evaluate the impact of triheptanoin on the acute clinical pathophysiology associated with LC-FAODs following 24 weeks of treatment [12], and the impact of triheptanoin on MCEs associated with LC-FAODs over 78 weeks of treatment [9]. The effects observed in the initial 24-week treatment period were compared with baseline values from screening or baseline evaluations, or from the 4-week run-in period [12]. MCEs were inclusive of emergency room visits, hospitalizations and emergency home or clinic interventions due to hypoglycaemia, rhabdomyolysis or cardiomyopathy [7, 9]. MCEs captured retrospectively from the medical records of all enrolled patients for the 78-week period prior to triheptanoin treatment were compared with those occurring in the 78 weeks following triheptanoin initiation [9]. There were statistically significant changes from baseline in some efficacy parameters, but the study was not powered for a specific endpoint (because of the small sample size for each one) [12].
At 24 weeks (the prespecified interim data cut-off), treatment with triheptanoin was associated with positive changes in self-reported health-related quality of life in adult patients [12]. Both the physical component summary score and the mental component summary score on the Medical Outcomes Study 12-item Short Form Version 2 (SF-12v2) increased significantly from baseline to week 24 (Table 2). In addition, the energy expenditure index (ratio of heart rate per meter walked in a 12-meter walk test) decreased significantly from baseline to week 24 (Table 2) [12].
At 78 weeks (final analysis), treatment with triheptanoin was associated with a reduction in the rate and duration of MCEs [9]. Both the mean annualized event rate and mean annualized duration rate of MCEs decreased by approximately 50% with triheptanoin treatment (Table 2). In terms of hospitalizations, which accounted for the majority of MCEs, both the mean annualized hospitalization event rate and the mean annualized hospitalization duration rate were significantly lower following triheptanoin initiation (Table 2). In addition, health-related quality of life (as measured by SF-10 in paediatric patients and SF-12v2in adult patients with assessment scores converted to converted to EQ-5D utility values) was substantially improved from baseline during triheptanoin treatment [13].
A retrospective observational study in an Austrian cohort of 8 paediatric patients with LC-FAOD treated with triheptanoin found that total hospitalization days/year decreased from a mean 27.1 days /year in the year prior to initiating triheptanoin treatment to a mean 8.2 days/year in the year after starting treatment [14].
Are the benefits of triheptanoin sustained over the longer term?
Following the completion of study CL201, 24 patients entered an open-label, long-term, phase 2 extension study (CL202) [15]. Patients transitioned into the extension study at the same dose of triheptanoin that they were taking in CL201, and the dose was titrated per investigator discretion (the target dose was 25–35% of total DCI). At a cut-off date of June 1, 2018, the mean study duration of triheptanoin therapy was 23.1 months (excluding CL201) in rollover patients. Of note, the study also included a triheptanoin-naive treatment arm and rollover patients from investigator-sponsored trials/expanded access programs. Results compare the 18-month pre-triheptanoin period with the 36-month triheptanoin period (18 months each from CL201 and CL202) [15].
The therapeutic effect of triheptanoin appeared to be sustained during longer-term therapy [15]. From the 18-month pre-triheptanoin period to the 36-month triheptanoin period, the mean annualized total MCE rate decreased 45% (from 1.76 events/year to 0.96 events/year; p = 0.0319), and the mean annualized hospitalization event rates decreased by 46.9% (from 1.43 to 0.76 events/year; p = 0.0429). In addition, the improvements with triheptanoin in the physical health summary score seen in CL201 were maintained in CL202 [15].
What is the tolerability profile of triheptanoin?
Triheptanoin was generally well tolerated when used to treat LC-FAODs [7]. In a pooled analysis (n = 79) of paediatric and adult patients with an LC-FAOD who received triheptanoin at a daily dosage range of 12–41% DCI for a mean duration of 23 months, the most commonly reported adverse events (AEs) were gastrointestinal (GI)-related AEs [3]. GI AEs included abdominal pain (GI pain, upper abdominal pain, or abdominal discomfort, distension or pain) [60%], diarrhoea (44%), vomiting (44%) and nausea (14%). The median time to onset of a first occurrence of a GI AE was 7.3 weeks. GI AEs led to triheptanoin dose reductions in 35% of patients in Study CL201 and 12% of patients in Study CL202 [3].
What is the current clinical position of triheptanoin?
Triheptanoin is the first FDA-approved treatment for LC-FAODs in the USA [16]. Commercially available MCT oils are made of a combination of octanoate (C8), decanoate (C10) and some dodecanoate (C12) FAs esterified to a glycerol backbone, and the proportions of the individual FAs vary from lot to lot [1]. In addition, prescription forms of MCT oil are pure trioctanoyl glycerol. These medium-chain, even-carbon FAs have limited effectiveness due to the depletion of odd-chain TCA cycle intermediates. Triheptanoin, as an anaplerotic compound, addresses this problem [1].
Triheptanoin was effective in terms of being a source of calories and fatty acids for the treatment of patients with confirmed LC-FAODs [7]. The similarity in mean changes from baseline in measures of cardiac function and structure, phosphocreatine recovery following acute exercise, TEE, body composition and blood biomarkers after 4 months’ treatment observed in the phase 2 trial comparing triheptanoin with trioctanoin in patients with nonsevere LC-FAOD [8] support using triheptanoin as an alternative treatment for LC-FAOD [7]. Although Study CL201 in patients with severe LC-FAOD was not powered for a specific endpoint, statistically significant changes associated with triheptanoin treatment included a decrease in mean annualized MCE event rates and mean annualized MCE duration rates from pre-triheptanoin rates [9]. Data such as these show that triheptanoin has the potential to reduce the disease burden of LC-FAODs, as well as the direct medical costs associated with symptomatic events [17]. More robust, longer-term data and additional studies are needed before definitive statements can be made.
Change history
21 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40267-021-00833-2
References
Vockley J. Long-chain fatty acid oxidation disorders and current management strategies. Am J Manag Care. 2020;26(Suppl 7):S147–54.
Merritt JL, Norris M, Kanungo S. Fatty acid oxidation disorders. Ann Transl Med. 2018;6(24):473.
Ultragenyx Pharmaceutical Inc. DOJOLVI™ (triheptanoin) oral liquid: US prescribing information. 2020. https://www.dojolvi.com/. Accessed 5 Feb 2021.
Geary Hook D, Marsden D, Gillingham MB, et al. Triheptanoin (UX007) stability in foods, formulas, and emulsion [abstract]. In: INFORM The International Network for Fatty Acid Oxidation Research and Management virtual meeting 2020.
Wehbe Z, Tucci S. Therapeutic potential of triheptanoin in metabolic and neurodegenerative diseases. J Inherit Metab Dis. 2020;43(3):385–91.
Gosselin NH, Lee SK, Jomphe C, et al. Population pharmacokinetics of heptanoate in healthy volunteers and patients with long-chain fatty acid oxidation disorders (LC-FAOD) treated with triheptanoin [abstract no. WED-060]. In: American Conference on Pharmacometrics. 2020.
US Food and Drug Administration. Drug approval package: DOJOLVI (Application No 213687) integrated review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213687Orig1s000TOC.cfm. Accessed 5 Feb 2021.
Gillingham MB, Heitner SB, Martin J, et al. Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial. J Inherit Metab Dis. 2017;40(6):831–43.
Vockley J, Burton B, Berry GT, et al. Results from a 78-week, single-arm, open-label phase 2 study to evaluate UX007 in pediatric and adult patients with severe long-chain fatty acid oxidation disorders (LC-FAOD). J Inherit Metab Dis. 2019;42(1):169–77.
Vockley J, Marsden D, McCracken E, et al. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment: a retrospective chart review. Mol Genet Metab. 2015;116(1–2):53–60.
Vockley J, Charrow J, Ganesh J, et al. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol Genet Metab. 2016;119(3):223–31.
Vockley J, Burton B, Berry GT, et al. UX007 for the treatment of long chain-fatty acid oxidation disorders: safety and efficacy in children and adults following 24 weeks of treatment. Mol Genet Metab. 2017;120(4):370–7.
Kruger E, Marsden D, Bensimon A, et al. Quality of life of patients with long-chain fatty oxidation disorders before and during treatment with triheptanoin [abstract no. 531 + poster]. In: Health Technology Assessment International (HTAi) Annual Meeting. 2020.
Zoggeler T, Stock K, Jorg-Streller M, et al. Long-term experience with triheptanoin in 12 Austrian patients with long-chain fatty acid oxidation disorders. Orphanet J Rare Dis. 2021;16(1):28.
Vockley J, Burton B, Berry G, et al. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: results from an open-label, long-term extension study. J Inherit Metab Dis. 2021;44(1):253–63.
Ultragenyx Pharmaceutical Inc. Ultragenyx announces U.S. commercial launch of Dojolvi™ (triheptanoin), the first FDA-approved therapy for the treatment of long-chain fatty acid oxidation disorders [media release]. 22 July 2020. https://www.ultragenyx.com/.
Pannier A. Long-chain fatty acid oxidation disorders: managed care and specialty pharmacy implications. Am J Manag Care. 2020;26(Suppl 7):S155–61.
Acknowledgements
The manuscript was reviewed by: M. Amraee, Islamic Azad University of Medical Sciences, Tehran, Iran; A. Zandi, Shahid Beheshti University of Medical Sciences School of Medicine, Tehran, Iran; A. Singh, All India Institute of Medical Sciences Raipur, Raipur, Chhattisgarh, India. During the peer review process, Ultragenyx Pharmaceutical Inc., the marketing-authorization holder of triheptanoin was offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflicts of interest
ES Kim, a contracted employee of Adis International Ltd/Springer Nature and SJ Keam, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, consent to participate, consent to publish, availability of data and material, code availability
Not applicable.
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, E.S., Keam, S.J. Triheptanoin in the management of long-chain fatty acid oxidation disorders: a profile of its use. Drugs Ther Perspect 37, 187–193 (2021). https://doi.org/10.1007/s40267-021-00816-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00816-3