Abstract
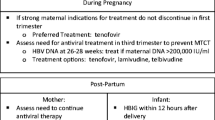
Mother-to-child transmission of hepatitis B virus (HBV) remains a significant contributor to the prevalence of HBV in endemic regions. Universal screening helps identify pregnant women with chronic HBV whose infants need immunoprophylaxis with the HBV vaccine and hepatitis B immunoglobulin, as well as women with active or advanced disease who may require treatment with pregnancy category B antivirals (e.g. tenofovir disoproxil fumarate or telbivudine) in the first trimester. Immunoprophylaxis failure remains high in mothers with high viral load, therefore antiviral therapy in the third trimester with pregnancy category B agents is recommended.

Similar content being viewed by others
References
Chamroonkul N, Piratvisuth T. Hepatitis B during pregnancy in endemic areas: screening, treatment, and prevention of mother-to-child transmission. Paediatr Drugs. 2017;19(3):173–81.
Hepatitis B vaccines: WHO position paper-July 2017. Wkly Epidemiol Rec: 2017;92(27):369–92.
Guidelines for the prevention. care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization; 2015.
Sirilert S, Traisrisilp K, Sirivatanapa P, et al. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int J Gynaecol Obstet. 2014;126(2):106–10.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;1(1):1–98.
Zou H, Chen Y, Duan Z, et al. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HbsAg positive mothers. J Viral Hepat. 2012;19:e18–25.
Yi W, Pan CQ, Hao J, et al. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol. 2014;60(3):523–9.
Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165(9):837–46.
Lin K, Vickery J. Screening for hepatitis B virus infection in pregnant women: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150(12):874–6.
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98.
A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: immunization of infants, children, and adolescents. Atlanta (GA): Centers for Disease Control and Prevention; 2005.
Terrault NA, Bzowej NH, Chang KM, et al. American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–83.
Antiretroviral Pregnancy Registry Steering Committee. The Antiretroviral Pregnancy Registry interim report. 1 January 1989 through 31 January 2017 (Version 15.0). Wilmington (NC): Registry Coordinating Center; 2017.
Viread® (tenofovir disoproxil fumarate): US Prescribing Information. Foster City (CA): Gilead Sciences, Inc.; 2017.
Tyzeka® (telbivudine): US prescribing information; East Hanover (NJ): Novartis; 2013.
Han GR, Cao MK, Zhao W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55(6):1215–21.
Zhang H, Pan CQ, Pang Q, et al. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60(2):468–76.
Epivir-HBV (lamivudine): US prescribing information. Research Triangle Park (NC): GlaxoSmithKline; 2013.
Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324–34.
van Zonneveld M, van Nunen AB, Niesters HG, et al. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–7.
Xu WM, Cui YT, Wang L, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, doubleblind, placebo-controlled study. J Viral Hepat. 2009;16(2):94–103.
Wu Q, Huang H, Sun X, et al. Telbivudine prevents vertical transmission of hepatitis B virus from women with high viral loads: a prospective long-term study. Clin Gastroenterol Hepatol. 2015;13(6):1170–6.
Greenup AJ, Tan PK, Nguyen V, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol. 2014;61(3):502–7.
Chen HL, Lee CN, Chang CH, et al. Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV PreMIT Study. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62(2):375–86.
Author information
Authors and Affiliations
Consortia
Ethics declarations
The article was adapted from Paediatric Drugs 2017;19(3):173–181 [1] by employees of Adis/Springer and was not supported by any external funding.
Rights and permissions
About this article
Cite this article
Adis Medical Writers. Prevent transmission of hepatitis B from mother to child in endemic areas with appropriate screening, antiviral therapy and vaccination. Drugs Ther Perspect 33, 478–483 (2017). https://doi.org/10.1007/s40267-017-0438-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-017-0438-2