Abstract
In recent years, better knowledge of the pathophysiology of inflammatory bowel diseases (IBD) has led to a relevant expansion of the therapeutic arsenal for these conditions. Janus kinase (JAK) inhibitors are a family of small molecules that block one or more of the intracellular tyrosine kinases, including JAK-1, JAK-2, JAK-3 and TYK-2. Tofacitinib, a non-selective small molecule JAK inhibitor, and upadacitinib and filgotinib, which are selective JAK-1 inhibitors, have been approved by the US Food and Drug Administration (FDA) for moderate-to-severe active ulcerative colitis. Compared to biological drugs, JAK inhibitors have a short half-life, rapid onset of action, and no immunogenicity. Both clinical trials and real-world evidence support the use of JAK inhibitors in the treatment of IBD. However, these therapies have been linked with multiple adverse events (AEs) including infection, hypercholesterolemia, venous thromboembolism, major adverse cardiovascular events, and malignancy. While early studies recognized several potential AEs, post-marketing trials have shown that tofacitinib may increase the risk of thromboembolic diseases and major cardiovascular events. The latter are seen in patients aged 50 years or older with cardiovascular risk factors. Hence, the benefits of treatment and risk stratification need to be considered when positioning tofacitinib. Novel JAK inhibitors with a more selective effect on JAK-1 have proven to be effective in both Crohn’s disease and ulcerative colitis, offering a potentially safer and efficacious therapeutic option to patients, including those with previous non-response to other therapies such as biologics. Nevertheless, long-term effectiveness and safety data are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stratifying risks for adverse events when positioning therapies is critical. Younger patients with no cardiovascular risk factors are good candidates for JAK inhibitors. |
Considering that adverse events to JAK inhibitors are dose dependent, the lowest effective dose should be used during the maintenance phase of treatment. |
Even though novel, more selective JAK inhibitors potentially offer a better safety profile, long-term data are needed. |
1 Introduction
In the past 2 decades, the advent of biologic agents that target specific components of the immune response has greatly improved outcomes of patients with inflammatory bowel diseases (IBD). However, patients may not respond (primary non-response) or lose response after experiencing a benefit (secondary non-response) to biologic therapy. Furthermore, some patients develop adverse events (AEs) that often lead to treatment discontinuation [1, 2]. Thus, several new compounds have been in development with the goal to further improve the efficacy, while maintaining or improving the safety profile seen with current drugs. Although most of the approved drugs for IBD are biologics, novel small molecules have been introduced and have been approved or in the late phases of development.
Among those novel therapies, we find multiple small-molecule drugs (SMDs). This “new generation” of SMDs have several potential benefits over biologics. Small-molecule drugs have a molecular weight of less than 1kDa [3]. Due to their metabolism and binding to plasma proteins, SMDs usually have a short serum half-life when compared to biologics [4]. The lack of immunogenicity of SMDs is another benefit over biologics and can potentially provide sustained efficacy and increase drug persistence [4].
Janus kinase (JAK) inhibitors have emerged as a novel strategy to modulate downstream cytokine signaling in immune-mediated diseases. The four members of the JAK family (JAK-1, JAK-2, JAK-3, and tyrosine kinase 2) are part of transmembrane cytokine receptor complexes that are activated upon binding of a ligand, leading to recruitment, phosphorylation, and activation of signal transducers and activators of transcription [5].
The JAK-signal transducer and activator of transcription (STAT) pathway plays an important role in innate immunity, adaptive immunity, and hematopoiesis, participating in cellular processes such as cell growth, survival, differentiation, and migration [6]. Based on this, inhibition of the JAK pathway has been studied for the treatment of numerous autoimmune diseases [7]. Tofacitinib was the first JAK inhibitor to be introduced to market, demonstrating clinical efficacy in rheumatoid arthritis (RA) as monotherapy in patients on non-biologic disease modifying drugs (DMARDs) or inadequate response to biologic treatment [8, 9].
Both ulcerative colitis (UC) and Crohn’s disease (CD) share common pathogenesis mechanisms, including a dysregulated JAK pathway. Hence, targeting the JAK pathway in patients with IBD offers a promising therapeutic option. Three of these JAK inhibitors are already approved for UC and others are currently being trialed in Phase 2 and 3 programs for several indications including CD.
The aim of this review is to assess the available data on the risk of AEs in patients undergoing treatment with JAK inhibitors. We will briefly describe the efficacy in both, clinical trials and real-world studies followed by a review of the evidence on safety that is currently available. An electronic literature search was carried out using PubMed, EMBASE and clinicaltrials.gov looking for randomized controlled articles and case–control studies published up to October 2022. The keywords used were “adverse reaction”,” small molecules”, “Janus kinase inhibitors”, “tofacitinib”, “upadacitinib”, “filgotinib”, “inflammatory bowel disease”, “ulcerative colitis” and “Crohn’s disease” “Real-world”. Reference lists and conference abstracts were also searched to identify additional studies.
2 Efficacy of Janus-Kinase Inhibitors
2.1 Tofacitinib
Tofacitinib is a pan-JAK inhibitor with more action on JAK-1 and -3 and, to a lesser extent, JAK-2 and tyrosine kinase 2 (TYK-2). Initially approved in 2012 for the treatment of moderate-to-severe RA [10], in 2017 for psoriatic arthritis (PsA) [11], in 2018 for UC and more recently was approved for the treatment of active ankylosing spondylitis [12].
This drug has been shown to be effective in inducing and maintaining remission in patients with moderate-to-severe UC and was approved for the treatment of moderate-to-severe UC for patients who had failed standard therapies and/or biologic agents [13].
Tofacitinib was evaluated in a Phase 2 randomized and placebo-controlled trial of patients with moderate-to-severe UC. The primary endpoint, clinical response at Week 8, was achieved by a higher proportion of patients receiving tofacitinib 15 mg twice daily (BID) (38/49, 78%) compared to placebo (20/48, 42%; p < 0.01) [14]. Based on these results, two subsequent Phase 3 double-blinded-placebo controlled induction trials, (the OCTAVE 1 and OCTAVE 2 studies) were performed in 598 and 541 patients, respectively [15]. The patients were randomly assigned to receive 10 mg of tofacitinib BID or placebo for 8 weeks. The rate of clinical remission at Week 8 was significantly higher in the tofacitinib group compared with placebo (OCTAVE 1: 18.5% vs 8.2% [p = 0.01] and OCTAVE 2: 16.6% vs 3.6% [p < 0.01]). Table 1 summarized the data reported for clinical trials for tofacitinib and other JAK inhibitors.
Subsequently, the OCTAVE SUSTAIN trial was performed in 593 patients who achieved clinical response after the induction therapy. Being randomly assigned to receive either tofacitinib as maintenance therapy (5 mg or 10 mg BID) or placebo for 52 weeks. The rate of clinical remission at Week 52 was higher in both tofacitinib groups compared with placebo (5 mg: 34.3% or 10 mg: 40.6%, p < 0.01 vs placebo 11.1%). Mucosal healing was achieved in a higher proportion of patients who received tofacitinib (5 mg BID: 37.4%, 10 mg BID: 45.7% vs placebo 13.1% p < 0.01) [16]. The long-term open-label extension (OLE) of the OCTAVE studies assessed dose de-escalation of tofacitinib 10 mg BID to 5 mg BID in patients who had previously achieved and maintained clinical remission after 52 weeks. In the de-escalation group, 53 of 63 patients (84.1%) maintained clinical response and 47 (74.6%) maintained clinical remission [17].
In patients with moderate-to-severe CD, tofacitinib showed no significantly better clinical remission or response rates when compared to placebo [18]. In another study mostly including patients with previous anti-tumor necrosis factor (TNF) non-response, results were also rather disappointing, with remission rates not reaching a significant difference when compared to placebo [19]. It is plausible that the failure to meet the primary endpoint in the Phase 2 trial and the high rate of placebo response may be a result of high proportion and prolonged taper of corticosteroid use. Another important limitation of the study was a lack of endoscopic central reading [20].
In 2020, Fenster et al conducted a retrospective cohort study to examine the real-world efficacy and safety of the off-label use of tofacitinib in unclassified IBD and CD patients who had been previously treated with biologic therapy. Within the cohort, 48.7% have had non-response to at least two biologic agents [21]. Seventy-six patients were followed for a median of 7.6 months. Clinical response at Week 8/16 was 46.6%; 15.1% had achieved clinical remission. Male sex was associated with increased odds of achieving clinical response (adjusted odds ratio (aOR 5.4; confidence interval [CI], 1.9–15.5, p = 0.002). Another “real-world” cohort from the UK included a large patient population receiving tofacitinib. Among them, 80% had been previously treated with TNF inhibitors. By Week 8, 74% had achieved clinical response and by Week 26, 44% had achieved steroid-free remission. Primary nonresponse was independently associated with higher C-reactive protein (CRP) levels at baseline (p = 0.004) and with younger age (p = 0.014) [22]. Table 2 summarizes the real-world efficacy data that has been reported in the literature [23,24,25,26,27].
Despite the safety signals seen in long-term studies with tofacitinib, novel JAK inhibitors with a higher selectivity towards JAK-1 promise efficacy while potentially offering a better safety profile when compared to non-selective JAK inhibitors. Several compounds are in development; some have completed Phase 3 trials in IBD and two (upadacitinib and filgotinib) have been approved for UC.
2.2 Upadacitinib
In UC, a Phase 2b placebo-controlled trial (U-ACHIEVE) that included a total of 250 patients receiving upadacitinib at different doses (7.5 mg, 15 mg, 30 mg, 45 mg) or placebo showed that at Week 8, those patients receiving 45 mg daily of upadacitinib had better clinical remission rates when compared to placebo (19.6% vs none, respectively [p = 0.002]) [28]. The most common AEs were infections and elevated serum lipoprotein levels. Serious AEs including death, stroke, and venous thromboembolism were rare, although larger clinical trials and registry studies with a longer follow-up are required to confirm the safety of upadacitinib.
In the Phase 3 program, two induction studies (U-ACHIEVE induction [UC1] and U-ACCOMPLISH [UC2]) and a single maintenance study (U-ACHIEVE maintenance) were performed. More patients achieved clinical remission with upadacitinib 45 mg (83 [26%] of 319 patients in UC1 and 114 [34%] of 341 patients in UC2) than in the placebo groups; p < 0.0001. In both induction studies, serious AEs and AEs leading to discontinuation of treatment were less frequent in the upadacitinib 45 mg group than in the placebo group.
In the maintenance study, clinical remission was more commonly achieved on those patients receiving upadacitinib (42% with 15 mg and 52% with 30 mg) versus those receiving placebo (12%; p < 0.001). The proportion of serious AEs was similar than that in the placebo group. The most reported AEs were nasopharyngitis, acne, and UC exacerbation [29].
Upadacitinib is a selective JAK-1 inhibitor. Its efficacy was assessed in patients with moderate-to-severe CD who had failed to respond to or tolerate TNF inhibitors (CELESTE TRIAL) [30]. In a 52-week study, clinical remission rates were higher with upadacitinib 6 mg given BID versus placebo, (27% vs 11%, respectively) [30]. In the CELESTE OLE, 107 patients completed a 30-month follow up. Clinical remission was maintained between Week 0 and Month 30 in all groups (61% with 15 mg; 54% with 30 mg; and 55% of those patients who first received 15 mg and were dose escalated to 30 mg daily). Endoscopic response was similar in all cohorts (68%, 67% and 40%, respectively) [31].
2.3 Filgotinib
Filgotinib is also a selective JAK-1 inhibitor, which has shown effectiveness in IBD. In patients with moderate to severe active UC, a Phase 2b/3, double-blind, randomized, placebo-controlled trial (SELECTION) found that filgotinib 200 mg was well tolerated and had a great efficacy compared to placebo in inducing and maintaining clinical remission [32]. Long-term data from extension trials are pending. The AE profile of filgotinib in patients with IBD in the Phase 3 SELECTION and Phase 2 FITZROY studies was consistent with that of patients with RA and others JAK inhibitors.
Filgotinib received the European license in November 2021 for the treatment of adult patients with moderate-to-severe active UC who have failed or are intolerant to conventional or biologic therapy [33].
It has also shown effectiveness for induction of remission in CD [34]. In a Phase 2 study (FITZROY) that included 128 patients, those receiving filgotinib 200 mg achieved clinical remission at a higher rate when compared to those receiving placebo (47% vs 23%, respectively [p = 0.0077]). This difference was even higher in treatment-naïve patients (60% vs 13%) [35].
Multiple other studies that evaluated the safety and efficacy of filgotinib in CD patients with and without previous exposure to biologics are ongoing and include the DIVERSITY trial (NCT02914561) and DIVERSITY LTE (long-term extension) (NCT02914600. The DIVERGENCE 1 (NCT03046056) and 2 (NCT03077412) trials are specifically evaluating the efficacy of filgotinib in CD patients with perianal fistulizing, which is an important unmet need in clinical practice and may help to position the drug in the treatment algorithm.
2.4 Other Janus Kinase Inhibitor Molecules
Several other novel JAK inhibitors have been in development. Izencitinib (TD-1473) is an orally, non-selective and gut-selective Pan-JAK inhibitor. As expected, considering its gut-specificity, exposure in blood was very low, making it an attractive candidate drug to be used in IBD. Data from a Phase 1 FTIH trial [NCT2657122] study in healthy volunteers found izencitinib to be safe in a daily dose given for 14 days; no serious AEs were seen. Subsequently, a multicenter randomized placebo-controlled Phase 1b trial [NCT 02818686] evaluated three doses (20, 80 and 270 mg) given within a period of 28 days in patients with moderate-to-severe UC. The study showed numerical trends toward higher rates of clinical response, endoscopic response, and decreased CRP levels for all doses when compared to placebo [36]. However, in a Phase 2 trial, patients who received TD-1473 failed to achieve higher rates of remission at Week 8 when compared to placebo [37]. The program was discontinued, leaving an unmet need for development of effective gut-selective JAK inhibitors.
Brepocitinib is a dual oral TYK-2/JAK-1 inhibitor that binds to the active sites in the catalytic domains of TYK-2 and JAK 1 [38]. Two Phase 2 trials are evaluating the efficacy and safety of oral brepocitinib in patients with moderate-to-severe UC (NCT02958865) and CD (NCT0399515). The former having already finished the recruitment process and the latter currently recruiting subjects. These studies will evaluate both endoscopic improvement at Week 12 and safety for up to Week 68. Other compounds selectively blocking the TYK-2 are in early development. Table 3 summarizes the most relevant JAK inhibitors that have been under development [39,40,41,42,43].
3 Safety of Janus Kinase Inhibitors
One of the biggest barriers to initiating immuno-suppressive therapy are safety concerns. It is critical to discuss the safety profile of each drug, the risks and benefits and to create awareness of the implications of these therapies in the shared decision-making process when selecting a therapy with a patient. Janus kinase inhibitors have gained attention due to the report of several AEs seen in post-marketing studies. This has ultimately led to a change in prescription drug labeling for those JAK inhibitors that are currently available in the market. The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) added multiple prescription safety warnings to JAK inhibitors between 2019 and 2022), noting risks of blot clots and heart events and death (Table 4).
3.1 Mortality
Long-term extension (LTE) studies of tofacitinib included patients with RA, PsA, UC and psoriasis. Exposures of between 3 and 9.5 years demonstrated a consistent safety profile over time. A total of 13,567 patients received tofacitinib and were included in the study. All-cause mortality risk was similar across cohorts. The common cause of death within the 28-day risk period was cardiac related. Age-adjusted and sex-adjusted standardized mortality ratios (95% CI) were as follows: 0.2 (0.2–0.3) for RA, 0.2 (0.0–0.4) for PsA, 0.1 (0.0–0.5) for UC and 0.2 (0.1–0.4) for psoriasis [40].
In a recent systematic review and meta-analysis, comprising 66,159 patients with immune-mediated diseases who were exposed to a JAK inhibitor, mortality was not increased when compared to placebo (relative risk [RR]: 0.72; 95% CI 0.40–1.28). Overall mortality among those exposed to JAK inhibitors was 0.37 per 100 person-years [44].
3.2 Infections
In the Phase 2 tofacitinib trial performed in UC patients, the most common infectious AEs were influenza (n = 6) and nasopharyngitis (n = 6). Two patients experienced serious infectious AEs (SAEs). In a 4.4-year follow-up, Sandborn et al, found the incidence rate for serious infections to be 2.0 cases per 100 patient-years (PYs) [47] (95% CI 1.4–2.8). The incidence of serious infections was higher among those using tofacitinib in the induction phase versus placebo, while rates were lower and remained equivalent between treatment groups in the maintenance phase [45].
A worldwide tofacitinib post-marketing surveillance database that analyzed 4426 UC case reports from May 2018 to August 2020 and included 12,103 AEs, of which 1839 were labeled as SAEs [46], the most frequently reported AEs of interest were infections (RR: 3.28 per 100 PY), vascular disorders (1.26 per 100 PY) and respiratory disorders (0.74 per 100 PY). Overall, 16.8% (934) of cases reported an infection of which 292 were serious. This was observed more frequently in UC (3.28 per 100 PY) versus RA patients (2.57 per 100 PY). The most frequently reported infections were nasopharyngitis (134 [14.3%]) and herpes zoster (HZ) (127 [13.5%]). The most common serious infection events in UC were Clostridioides infections (51 [5.4%]), pneumonia (36 [3.8%]) and COVID-19 (12 [1.28%]). These results are consistent with those reported in tofacitinib clinical trials.
Among patients in the OCTAVE trials and the open-labeled extension, there was a clear signal towards a higher risk of developing HZ (5.6% of the study population). This association was dose dependent. In the induction cohort, HZ occurred in 0.6% (6) of patients receiving tofacitinib 10 mg BID, versus 0.4% (1) of patients receiving placebo [14]. In the overall cohort (n = 1157), 92 patients had HZ events at an incidence rate of 3.48 (95% CI 3.48 [2.79–4.30] and a median time to onset of 474 days (range 13–1799 days) [47]. Including all Phase 2/3/open-label extension studies involving patients with UC receiving tofacitinib, 65 (5.6%) patients developed HZ infection; among those, one patient developed encephalitis and 11 had multi-dermatomal involvement. Age ≥ 60 years, lower body weight, and prior TNF inhibitors exposure were identified as risk factor for herpes infections [48].
The risk of HZ has been reported for all JAK inhibitors that are either available or under development [49]. However, the incidence of serious HZ on those patients exposed to filgotinib has been very low, independently of the dose. Herpes zoster events observed after treatment with tofacitinib tended to be noncomplicated and in most cases did not result in permanent discontinuation of therapy nor additional HZ recurrence [50, 51]. For upadacitinib, three cases were reported during the CELEST trial [30]. Data from long-term follow-up registries and real-world data as well as the effect of systematic vaccination on these rates are needed.
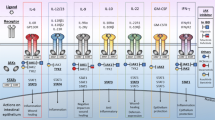
Is important to mention that live vaccines are contraindicated in patients receiving JAK inhibitors and should be administrated at least four weeks before the start of treatment. However, recombinant vaccines can be safely used and are therefore preferred [52]. Figure 1, summarizes indications and schedules for patients initiating JAK inhibitor therapy [53,54,55,56].
An important question is how JAK inhibitors can affect the risk of developing COVID-19 or if they increase the risk of complications when compared to patients off immunosuppression. The SECURE-IBD registry is a global, collaborative registry established in March 2020 to understand COVID-19 outcomes in IBD patients [57]. Among patients with IBD enrolled in the registry, 37 were on treatment with tofacitinib. These patients did not have worse outcomes when compared to other therapies [58]. Furthermore, there were no significant differences between tofacitinib-treated patients and other therapies when looking at hospitalization rates (21.6% vs 23.3%), need for intensive care unit admission (5.4% vs 4.5%) and developing severe COVID-19 (6.2% in both groups) [59]. Is important to note that the number of patients on tofacitinib was low and the analysis was not powered to detect small differences. In a large cohort of patients with IBD and diverse exposure to immunosuppressive agents, the authors found that full vaccination (> 7 days after the second dose against SARS-CoV-2) but not partial vaccination, was significantly associated with a reduced rate of COVID-19 when compared to non-vaccinated patients (80.4%) [60].
In a follow-up publication that included a total of 6077 patients with IBD, RA and psoriasis, a higher risk for hospitalization or death was seen in patients receiving combination therapy with azathioprine/6-mercaptopurine (OR 1.74 [95% CI 1.17–2.58], p = 0.006) and JAK inhibitor monotherapy (OR 1.82; 95% CI, 1.21–2.74, p = 0.004) when compared with patient who received TNF inhibitor monotherapy [61]. In another systematic review and meta-analysis with 18 studies, tofacitinib was not associated with COVID-19-related hospitalization (RR = 0.81, 95% CI 0.49–1.33, p = 0.40) [62].
In an integrated analysis of patients with RA receiving filgotinib for RA, there was a higher incidence of infections when compared to placebo and even though the overall rate was similar, patients receiving the higher dose (200 mg) had a higher rate of serious infections [63].
3.3 Hypercholesterolemia
In the tofacitinib UC trials, concentration of both LDL and HDL cholesterol increased after Week 8 of therapy with a total-to-HDL cholesterol ratio being stable and normalizing after drug discontinuation [64]. Serum lipids should be monitored within 2 months of starting tofacitinib. After long-term tofacitinib use, no significant changes were detected during a 61-week period and 4.4 years of follow-up [65]. The short- and long-term clinical significance of these findings are still unclear. A recent meta-analysis showed that all the JAK inhibitors approved for RA lead to increase in HDL 8.11 mg/dL (95% CI 6.65–9.58, I2 = 82%) and a mean increase of 11.37 mg/dL (95% CI 7.84–14.91, I2 = 88%) in LDL levels from baseline [66].
3.4 Malignancies
Among the tofacitinib UC clinical trials population, 22 patients had malignancies, 11 of which developed non-melanoma skin cancer (NMSC); six had a previous history of NMSC. Among those who developed NMSC, all had prior exposure to a thiopurine and 8 of 11 patients had been previously treated for a NMSC. Through the OCTAVE trials, four deaths were recorded in the overall population, with 3 out of 4 cases being secondary to malignancies (hepatic angiosarcoma, acute myeloid leukemia, and cholangiocarcinoma) [14, 15]. In a recent publication, NMSC events were evaluated from 3 randomized, placebo-controlled studies that included patients with UC. Within the cohort, Cox regression models identified three significant risk factors: prior NMSC (hazard ratio [HR] 9.09; p = 0.0001), anti-TNF failure (3.32; p = 0.0363) and age (HR per 10-year increase: 2.03; p = 0.0004) as significant independent factors associated with the development of NMSC [67]. No patients developed malignancy during the maintenance trials in UC and CD. Furthermore, the risk of malignancies was similar to that observed in patients receiving tofacitinib with RA and psoriasis, even though that risk was comparable to that of patients on other biologics [68]. The ORAL Surveillance study (NCT02092467) was an open-label, randomized noninferiority and safety end-point trial that enrolled patients with active RA who were aged 50 years and had at least one additional cardiovascular (CV) risk factor. Subjects were randomly assigned in a 1:1:1 ratio to receive tofacitinib at dose 5 mg or 10 mg BID or a TNF inhibitor [69]. Through a median follow-up of 4.0 years, the incidence of cancer was higher with tofacitinib (at any dose) (4.2% [n = 122]) versus those patients who received TNF inhibitors (2.9% [n = 42])—HR 1.48 (95% CI 1.04–2.09) [74] In another study, among 5671 patients with RA exposed to tofacitinib, 107 developed malignancies (excluding NMSK); the most common were lung cancer (24 [22.4%]), breast cancer (19 [17.7%]) and lymphoma (10 [9.3%]) [70].
In the upadacitinib UC induction trials, one NMSC was reported in a patient receiving the 24 mg BID dose. All patients had prior exposure to azathioprine. During the maintenance phase, two malignancies were reported: one Hodgkin’s lymphoma (the patient had been previously exposed to three biologics) and one malignant neoplasm of the thymus (with concomitant immunosuppressive therapy and previously exposed to two biologic agents) [29]. No malignancies were reported in the filgotinib trials and long-term studies have reported similar rates versus the overall RA population. Moreover, longer exposure doesn’t seem to increase the risk further, even though registries with larger populations are needed [35]. Long-term real-world and safety registry data will be essential to better determine the risk of malignancies with the newer, selective JAK-1 inhibitors and how they compare to tofacitinib or anti-TNF agents.
3.5 Thrombotic Events
In July 2019, the FDA placed a “black box warning” on tofacitinib alerting of a possible higher risk for developing pulmonary embolism and increased risk of mortality [71]. This led to a change in indication and positioning of JAK inhibitors after anti-TNFs [72]. However, it is important to highlight that the greatest thrombotic risk has been seen in the RA population and when comparing tofacitinib 10 mg with anti-TNF (19 cases in 3884 patients-year vs 3 cases in 3982 PYs, respectively) [73]. As expected, a greater risk was observed in those patients with a history of malignancy, aged > 50 years and those with ≥ 1 CV risk factors. In a post hoc analysis of patients in the OCTAVE clinical trials who received at least one dose of tofacitinib, the overall risk of deep venous thrombosis (DVT) was 0.04 events/100 PYs of exposure (95% CI 0.00–0.23) and 0.16 PY (95% CI 0.04–0.41) for pulmonary embolism (PE). Real-world safety signals seen in patients on tofacitinib are similar to those reported during clinical trials. The incidence rates of SAEs were 10.0 (95% CI 8.9–11.2 per 100 PY of follow-up. Five patients developed HZ infection and two developed venous thrombo-embolism (VTE) (all were receiving 10 mg tofacitinib, BID) [72].
Because these events are relatively rare, large trials with long-term follow-up are needed. An analysis that included 12,410 tofacitinib-treated patients from development programs in RA, PsA, and psoriasis as well as the FDA Adverse Reporting System (FAERS), US Corona registries and the IBD MarketScan database showed that incidence rates of DVT, PE, and arterial thrombotic events were higher in patients with known baseline CV or VTE risk factors when compared to those with no previous history [73]. It is recommended to use the minimum effective dose to maintain remission and avoid its use in patients aged > 50 years with one or more CV risk factor [74, 75].
An important question—should extrapolate these results to other JAK inhibitors with a more selective effect on the JAK-1? Across the upadacitinib rheumatologic trials, 6 venous thromboembolic events were reported over 461 treatment-arm PYs, compared with one event in 366 placebo-arm PYs [74]. No thrombotic events have been reported with upadacitinib in RA, but the drug was only recently introduced to the market (in 2019) and long-term studies in larger populations are warranted [75].
In a Phase 2 study with upadacitinib in patients with UC, a patient on upadacitinib 45 mg developed a PE and DVT 26 days after drug discontinuation due to worsening of UC [30]. In another Phase 2 study in CD, one patient developed a mesenteric vein thrombophlebitis during the induction period (receiving a dose of 3 mg BID). No events of DVT or PE were observed [22]. We need to consider that Phase 2 studies are not powered to look into these types of AEs and further data will be needed.
3.6 Major Adverse Cardiovascular Events
Major adverse cardiovascular events (MACE), defined as any myocardial infarction (MI), cerebrovascular event (stroke), or CV death (defined as death caused by coronary, cerebrovascular, or cardiac events) represent one of the most common comorbidities in patients with RA. In the QUEST-RA study, the prevalence of CV morbidity in patients with RA was 9.3% for any CV event (MI, angina, coronary disease, or stroke) with considerable heterogenicity among countries. The overall prevalence for the whole cohort of lifetime MI was 3.2% and the prevalence of stroke was 1.9% [76]. In a post-marketing report of tofacitinib, 67 cases of MACE were reported. Of those, 45 were labeled as serious (estimated reporting rate: 0.50 per 100 PYs). The most reported MACE was acute MI, angina pectoris and pericarditis [47].
In a post hoc analysis of 2 long-term extension studies and 6 Phase 3 studies over 7 years that included patients with moderate-to-severe RA receiving tofacitinib, 52 cases MACE occurred in 4076 patients over 12,873 PYs of exposure (IR 0.4 per 100 PY). In subsequent multivariable analyses, aged > 49 years, with hypertension, and the total cholesterol to HDL cholesterol ratio remained significantly associated with a risk of developing a MACE [77].
Overall, tofacitinib trials for patients with UC have shown an increased risk of MACE compared to placebo (OR = 5 [CI 95% 1.7–10]) based on 4 cases (MI, acute coronary syndrome, aortic dissection, and hemorrhagic stroke) [66]. Likewise, 3 of 4 patients with a MACE had ≥ 4 predisposing CV factors. An aortic dissection resulted in death of the patient and the other MACE resolved after tofacitinib discontinuation [45]. In upadacitinib, one MACE was reported in induction phase of CD, with no cases seen in the maintenance phase [30].
A post hoc analysis from ORAL Surveillance evaluated the risk of MACE with tofacitinib 5 and 10 mg versus TNF inhibitors in patients with RA. The highest risk was associated with age, > 50 years with at least one additional CV risk. In this patient population the recommendation is to use the lowest effective dose to maintain clinical response after 8 weeks of induction therapy [78].
In RA patients, approximately 2600 patients have been exposed to upadacitinib. Exposure-adjusted event rates of adjudicated MACE were not different across treatment groups and did not increase over time: 1.2 events/100 PY (95% CI 0.2–3.4) in placebo, 0.6 events/100 PY (95% CI 0.4–1.0) in upadacitinib 15 mg and 1.0 events/100 PY (95% CI 0.5–1.6) in upadacitinib 30 mg [79].
Data from filgotinib in RA have not shown a signal towards a higher rate of MACE, even when considering that risk factors in this patient population are relatively high. Long-term registries assessing the incidence of MACE events are warranted [63].
3.7 Pregnancy and Breastfeeding
As with other small molecules, the active metabolite of JAK inhibitors can cross the placenta during the first trimester. As with other novel compounds, there is always a concern for fetal exposure. Preclinical studies with tofacitinib showed that drug exposure at a much higher dose than the therapeutic dose (100 mg/kg/day), can cause fetal malformations (CV and bone malformations) [80].
In interventional studies with tofacitinib in RA and psoriasis, of 1821 female patients of child-bearing age, 47 women became pregnant, including 33 who were on tofacitinib monotherapy, 13 who received combination therapy with methotrexate, and one patient whose therapy was still blinded. No fetal deaths, one case of a congenital malformation (pulmonary valve stenosis) and 7 spontaneous abortions were reported [80].
A prospective registry study by Mahadevan et al described 11 cases of maternal exposure and 14 cases of paternal exposure to tofacitinib. No evidence of fetal or neonatal death was seen and no congenital malformations were reported [81].
The American Gastroenterology Association guidelines recommend avoiding tofacitinib use during lactation and at least in the first trimester of pregnancy, A 1-week washout period should be enough before attempting conception [82]. More data are needed before establishing recommendations for other JAK inhibitors but as of now, they should be avoided during pregnancy.
3.8 Other Adverse Events
In patients on tofacitinib, an initial decrease in hemoglobin, lymphocyte, neutrophil, and platelet counts were reported. However, they tended to be mild and reversible after drug discontinuation. As for filgotinib, there were no differences in hemoglobin levels or platelet counts [35]. In the SELECTION study, throughout Week 52, patients who received upadacitinib had no clinically meaningful changes from baseline in hemoglobin, leukocytes, neutrophils, transaminases, or creatinine concentrations across all treatment arms [30]. The proportion of patients with an abnormal increase of creatine kinase was higher in the filgotinib group versus placebo, with no association with rhabdomyolysis [83].
An open-label long-term extension of the OCTAVE trial followed patients on tofacitinib for up to 7 years. A total of 26 patients were evaluated for drug-induced liver injury; 7 patients administered tofacitinib 5 mg BID and 19 on 10 mg BID [17]. This adverse reaction has not been observed with filgotinib or upadacitinib. However, as with other less common potential AEs, larger, long-term studies are needed.
There have been reports of gastrointestinal perforation in patients receiving JAK inhibitors. From the WHO pharmacovigilance database, and among 126,816 reported AEs to tofacitinib, ruxolitinib and baricitinib, the risk is dose dependent. Gastrointestinal perforation with tofacitinib was greater with higher doses [84]. In patients with UC, two cases of intestinal perforations were reported. Both were in patients who had active IBD, which confounds these observations. Figure 2 summarizes the most common AEs associated with the use of JAK inhibitors and ways in which to monitor for them.
4 Conclusions
As JAK inhibitors make their way into the therapeutic landscape of IBD, more options become available to patients. Currently, tofacitinib, upadacitinib, and filgotinib are approved for treatment of patients with UC, and other JAK inhibitors are undergoing clinical trials for both UC and CD.
Even though the introduction of tofacitinib and other novel JAK inhibitors address an unmet need in the IBD therapeutic arsenal, safety concerns have positioned these drug classes lower in the therapeutic algorithm. While novel, selective JAK inhibitors aim to address these safety concerns, long-term data are needed. While as of now they are indicated in those patients who do not respond to other advanced therapies such as biologics, this represents a large population of patients with IBD in need of more effective therapies. Risk stratification, patient counseling, and adequate monitoring is pivotal. As of now, the recommendation is to use the minimal effective dose to maintain remission and to avoid their use in patients aged ≥ 50 years with one or more CV risk factor. However, we must consider that filgotinib is an effective and safe treatment of both biologic-naïve and biologic-experienced patients with moderate-to-severe UC with no associated risk of thrombosis and HS infections compared to other JAK inhibitors.
As with any other therapy, the risks and benefits should be discussed with each patient and treatment plans should be tailored on a case-by-case basis considering not only their IBD history, but also their complete medical history.
References
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–7. https://doi.org/10.1038/ajg.2008.88.
Qiu Y, Chen BL, Mao R, Zhang SH, He Y, Zeng ZR, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNF alpha dose intensification in Crohn’s disease. J Gastroenterol. 2017;52:535–54. https://doi.org/10.1007/s00535-017-1324-3.
Gilardi D, Gabbiadini R, Alloca M, Correale C, Fiorino G, Furfaro F, et al. PK, PD and interactions: the new scenario with JAK inhibitors and S1P receptor modulators, two classes of small molecules drugs in IBD. Expert Rev Gastroenterol Hepatol. 2020;14(9):797–806. https://doi.org/10.1080/17474124.2020.1785868.
Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2017;66:199–209. https://doi.org/10.1136/312912.
De Vries LCS, Wildenberg ME, De Jonge WJ, D’Haenes GR. The future of Janus kinase inhibitors in inflammatory bowel disease. J Crohns Colitis. 2017;11:885–93. https://doi.org/10.1093/ecco-jcc/jjx003.
Olivera P, Danese S, Peyrin-Biroulet L. JAK inhibition in inflammatory bowel disease. Expert Rev Clin Immunol. 2017;13:693–703. https://doi.org/10.1080/1744666X.2017.1291342.
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate responde inhibitors. N Engl J Med. 2017;377:1525–36. https://doi.org/10.1056/NEJMoa1615977.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, ORAL Solo Investigators, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. https://doi.org/10.1056/NEJMoa1109071.
Strand V, Kremer J, Wallenstein G, Kanit KS, Connell C, Gruben D, et al. Effects of tofacitinib monotherapy op patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307. https://doi.org/10.1186/s13075-015-0825-9.
Drugs.com FDA approves Xeljanz for rheumatoid arthritis. https://www.drugs.com/newdrugs/fda-approvesxeljanz-rheumatoid-arthritis-3558.html. Published November 6, 2012. Accessed 10 Feb 2022.
Drugs.com Pfizer announces FDA approval of Xeljanz (tofacitinib) and Xel- janz XR for the treatment of active psoriatic arthritis. https://www.drugs.com/newdrugs/pfizer-announces-fda-approval-xeljanz-tofacitinib-xeljanz-xr-active-pso-riatic-arthritis-4677.html. Published December 14, 2017. Accessed 10 Feb 2022.
Drugs.com FDA approves Xeljanz for active ankylosing spondylitis. https://www.drugs.com/newdrugs/u-s-fda-approves-pfizer-s-xeljanz-tofacitinib-active-ankylosing-spondylitis-5734.html Published December 14, 2021. Accessed 10 Feb 2022.
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, IBD guidelines eDelphi Consensus Group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106. https://doi.org/10.1007/gutjnl-2019-318484.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell M, for the Study A3921063 investigators, et al. Tofacitinib, an oral janus kinase inhibitor in active ulcerative colitis. N Engl J Med. 2012;367:616–24. https://doi.org/10.1056/NEJMoa1112168.
Panés J, Vermeire S, Lindsay JO, Sands BE, Su C, Friedman G, et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomized controlled induction and maintenance studies. J Crohns Colitis. 2018;12:145–56. https://doi.org/10.1093/ecco-jcc/jjx133.
Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36. https://doi.org/10.1056/NEJMoa1606910.
Sands BE, Armuzzi A, Marshall JK, Lindsay JO, Sandborn WJ, Danese S, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51(2):271–80. https://doi.org/10.1111/apt.15555.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014. https://doi.org/10.1016/j.cgh.2014.01.029.
Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D’Haens G, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomized placebo-controlled trials. Gut. 2017;66:1049–59. https://doi.org/10.1136/gutjnl-2016-312735.
Liu E, Aslam N, Nigam G, Limdi JK. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease—where we are and where we are going. Drugs Context. 2022. https://doi.org/10.7573/dic.2021-11-4.
Fenster M, Alayo QA, Khatiwada A, Wang W, Dimopoulos C, Gutierrez A, et al. Real-world effectiveness and safety of tofacitinib in Crohn’s disease and IBD-U: a multicenter study from the TROPIC Consortium. Clin Gastroenterol Hepatol. 2021;19:2207–9. https://doi.org/10.1016/j.cgh.2020.10.025.
Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicenter UK experience. J Crohns Colitis. 2020;14(10):1385–93. https://doi.org/10.1093/ecco-jcc/jjaa075.
Taxonera C, Olivares D, Alba C. Real-World effectiveness, and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32–40. https://doi.org/10.1093/ibd/izab011.
Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35–42. https://doi.org/10.1093/ecco-jcc/jjaa145.
Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–8. https://doi.org/10.1111/apt.1568933.
Ungaro R, Fenster M, Dimopoulos C, Patel A, Deepak D, Syal G, et al. P344 real-world effectiveness of tofacitinib in ulcerative colitis: a multi-centre study. J Crohns Colitis. 2019;13(Suppl. 1):S274–5. https://doi.org/10.1093/ecco-jcc/jjy222.468.
Avni-Biron I, Shirit A, Koslowsky B, Levartovsky A, Kopylov U, Weisshof R, et al. Short-term effectiveness and safety of tofacitinib in ulcerative colitis-real world data from tertiary medical centers in Israel. Dig Liver Dis. 2022;54:192–7. https://doi.org/10.1016/j.dld.2021.11.009. (PMID: 34887214).
Sanborn WJ, Gosh S, Panes J, Schreiber S, D’Haens G, Tanida S, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139–49. https://doi.org/10.1053/j.gastro.2020.02.030.
Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomized trial. Lancet. 2022;399(10341):21132128. https://doi.org/10.1016/SO140-6736(22)00581-5.
Sandborn WJ, Feagan BG, Loftus EV, Peyrin-Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and safety of Upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158:2123–38. https://doi.org/10.1053/j.gastro.2020.01.047.
D’Haens G, Panès J, Louis E, Lacerda A, Zhou Q, Liu J, et al. Upadacitinib was efficacious and well tolerated over 30 months in patients with Crohn’s disease in the CELEST extension study. Clin Gastroenterol Hepatol. 2021. https://doi.org/10.1016/j.cgh.2021.12.030.
Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomized, placebo-controlled trial. Lancet. 2021;397:2372–84. https://doi.org/10.1016/S0140-6736(21)00666-8.
Drugs.com FDA approves Rinvoq (Upadacitinib) for the treatment of adults with moderately to severely active ulcerative colitis. https://www.drugs.com/newdrugs/rinvoq-upadacitinib-receives-fda-approval-adults-moderately-severely-active-ulcerative-colitis-5800.html. Published March16, 2021. Accessed 1 Sept 2022.
Kim JW, Kim SY. The era of Janus Kinase inhibitors for inflammatory bowel disease treatment. Int J Mol Sci. 2021;22(21):11322. https://doi.org/10.3390/ijms222111322.
Vermeire S, Schreiber S, Petryka R, Kuenbacher T, Hebuterne X, Roblin X, et al. Clinical remission in patients with moderate to severe Crohn’s disease treated with filgotinib (The FITZROY study): results from a phase 2, double blind, randomized, placebo-controlled trial. Lancet. 2017;389:266–75. https://doi.org/10.1016/S0140-6736(16)32537-5.
Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, Woo J, et al. Development of gut-selective pan-janus kinase inhibitor TD-1473 for ulcerative colitis: a translational medicinal programme. J Crohns Colitis. 2020;14:1202–13. https://doi.org/10.1093/ecco-jcc/jjaa049.
Burr NE, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis; systematic review and network meta-analysis. Gut. 2021. https://doi.org/10.1136/gutjnl-2021-326390.
Clinical trials Arena (https://www.clinicaltrialsarena.com/news/theravance-izencitinib-ulcerative-colitis). Theravance’s izencitinib fails in Phase IIb ulcerative colitis trial. August 24, 2021. Accessed 8 Feb 2022.
Danese S, Panaccione R, D’Hanes G, Peyrin-Biroulet L, Scheirber S, Koyabashi S, et al. DOP42 efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in patients with moderately-to-severely active Ulcerative Colitis: 12-week results from the Phase 2 LATTICE-UC study. J Crohns Colitis. 2022;16(1):91–2.
Burmester G, Nash P, Sands BE, Papp L, Jones TV, Tan H. Adverse events of special interest in clinical trials of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and psoriasis with 37066 patient-years of tofacinitb exposure. RMD Open. 2021;7(2):e001595. https://doi.org/10.1136/rmdopen-2021-001595.
Danese S, Peyrin-Biroulet L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm Bowel Dis. 2021;27(12):2023–30. https://doi.org/10.1093/ibd/izab135.
Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, Woo J, et al. Development of gut-selective Pan-Janus kinase inhibitor TD-1473 for ulcerative colitis: a translational medicine programme. J Crohn’s Colitis. 2020;14:1202–13. https://doi.org/10.1093/ecco-jcc/jjaa049.
D’Amico F, Peyrin-Biroulet L, Danese S, Fiorino G. New drugs in the pipeline for the treatment of inflammatory bowel diseases: what is coming? Curr Opin Pharmacol. 2020;55:141–50. https://doi.org/10.1016/j.coph.2020.10.015.
Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158:1554–73. https://doi.org/10.1053/j.gastro.2020.01.001.
Sandborn WJ, Panés J, D’Haens G, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17:1541–50. https://doi.org/10.1016/j.cgh.2018.11.035.
Winthrop K, Loftus EV Jr, Baumgart DC, Reinisch W, Nduaka CI, Lawendy N, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates from ulcerative colitis clinical programme. J Crohns Colitis. 2021;15:914–29. https://doi.org/10.1093/ecco-jcc/jjaa233.
Rubin DT, Modesto I, Vermeire S, Danese S, Ng SC, Kwok K, et al. Worldwide post marketing safety surveillance experience with tofacitinib in ulcerative colitis. Aliment Pharmacol Ther. 2022;55:302–10. https://doi.org/10.1111/apt.16619.Epub2021Oct9.
Winthrop KL, Loftus EV Jr, Baumgart DC, Reinisch W, Nduaka CI, Lawendy N, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of infection rates from the ulcerative colitis clinical programme. J Crohns Colitis. 2021;15(6):914–29. https://doi.org/10.1093/ecco-jcc/jjaa233. (PMID: 33245746).
Winthrop KL, Melmed GY, Vermeire S, Long MD, Chan G, Pedersen RD, et al. Herpes Zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflam Bowel Dis. 2018;24(10):2258–65. https://doi.org/10.1093/ibd/izy131.
Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis. 2018;24:2172–82. https://doi.org/10.1093/ibd/izy150.
Choi W, Ahn SM, Kim YG, Lee CK, Yoo B, Hong S. Safety of Jak inhibitor use in patients with rheumatoid arthritis who developed herpes zoster after receiving JAL inhibitors. Clin Rheumatol. 2022. https://doi.org/10.1007/s10067-022-06096-0.
Syed YY. Recombinant zoster Vaccine (Shingrix®): a review in herpes zoster. Drugs Aging. 2018;35:1031–40. https://doi.org/10.1007/s40266-018-0603-x.
Alexander JL, Kennedy NA, Ibraheim H, Anandabaskaran S, Saifuddin A, Seoane RC, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7(40):342–52. https://doi.org/10.1016/S2468-1253(22)00005-X.
Liu E, Aslam N, Nigam GL. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going. Drugs Context. 2022. https://doi.org/10.7573/dic.2021-11-4. (PIMID 35462642).
Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. ECCO Guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15(6):879–913. https://doi.org/10.1093/ecco-jcc/jjab05.
Calvet X, Carpio D, Rodríguez-Lago I, García-Vicuña R, Barreiro-de-Acosta M, Juanola X, et al. Riesgo de infección asociado a los inhibidores de las quinasas Janus (JAK) y las terapias biológicas en enfermedad inflamatoria intestinal y artritis reumatoide. Estrategias de prevención Gastroenterología y Hepatología. 2021;44:587–98. https://doi.org/10.1016/j.gastre.2021.01.003.
Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonist are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481-49.e3. https://doi.org/10.1053/j.gastro.2020.05.032.
Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70:725–32. https://doi.org/10.1136/gutjnl-2020-322539.
Agrawal M, Brenner EJ, Zhang X, Modesto I, Woolcott J, Ungaro RC, et al. Characteristics and outcomes of IBD patients with COVID-19 on tofacitinib therapy in the SECURE-IBD registry. Inflamm Bowel Dis. 2021;27:585–9. https://doi.org/10.1093/ibd/izaa303.
Khan N, Mahmud N. Effectiveness of SARS-COV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure. Gastroenterology. 2021;161(3):827–36. https://doi.org/10.1053/j.gastro.2021.05.044.
Izadi Z, Brenner EJ, Mahil SK, Dand N, Yiu ZN, Yates M, et al. Association between tumor necrosis factors inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4(10):e2129639. https://doi.org/10.1001/jamanetwork.2021.29639.
Alrashed F, Alasfour H, Shebab M. Impact of biologics and small molecules for inflammatory bowel disease on COVID-19-related hospitalization and mortality: a systematic review and meta-analysis. JGH Open. 2022;60(4):241–50. https://doi.org/10.1002/jgh3.12728.
Winthrop KL, Tanaka Y, Takeuchi T, Kivitz A, Matzkies F, Genovese MC, Jiang D, et al. Integrated safety analysis of Filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 16 years. Ann Rheum Dis. 2022;81:184–92. https://doi.org/10.1136/annrheumdis-2021-221051.
Sandborn WJ, Panés J, Dhaens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17:1541–50. https://doi.org/10.1016/j.cgh.2018.11.035.
Sands BE, Taub PR, Armuzzi A, Friedman GS, Moscariello M, Lawendy N, et al. Tofacinitib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(1):123-132.e3. https://doi.org/10.1016/j.cgh.2019.04.059.
Na L, Zhong-Ping G, Shuang-Qing D, Xiao-Hong Z, Hui L, Xiu-Fang L. Effect of JAK inhibitors on high- and low-density lipoprotein in patients with rheumatoid arthritis: a systematic review and network meta-analysis. Clin Rheumatol. 2022;41(3):677–88. https://doi.org/10.1007/s10067-021-06003-z.
Lichtenstein GR, Rogler G, Ciorba MA, Su C, Chan G, Pedersen RD, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancy (excluding nonmelanoma skin cancer) events across the ulcerative colitis clinical program. Inflam Bowel Dis. 2021;27:816–25. https://doi.org/10.1093/ibd/izaa199.
Sands BE, Long MD, Reinisch W, Panés J, Loftus EV Jr, Nduaka CI, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of nonmelanoma skin cancer rates from the ulcerative colitis clinical program. Inflamm Bowel Dis. 2022;28:234–45. https://doi.org/10.1093/ibd/izab056.
Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–41. https://doi.org/10.1136/nnrheumdis-2014-205847.
Mease P, Charles-Schoeman C, Cohen S, Fallon L, Woolcott J, Yun H, et al. Psoriasis and psoriatic arthritis development programmes and from real world data. Ann Rheum Dis. 2020;79(11):1400–13. https://doi.org/10.1136/annrheumdis-2019-216761.
US Food and Drug Administration; Drug Safety Communications. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. https://www.fda.gov/media/120485/download. Accessed 30 Aug 2022.
European Medicines Agency. Increased risk of blood clots in lungs and death with higher dose of Xeljanz (tofacitinib) for rheumatoid arthritis. https://www.ema.europa.eu/en/documents/press-release/increased-risk-blood-clots-lungs-death-higher-dose-xeljanz-tofacitinib-rheumatoid-arthritis_en.pdf. Accessed 30 July 2022.
Mease P, Charles-Schoeman C, Cohen S, Fallon L, Woolcott J, Yun H, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real world data. Ann Rheum Dis. 2020;79:1400–13. https://doi.org/10.1136/annrheumdis-2019-216761.
Clarke B, Yates M, Adas M, Bechman K, Galloway J. The safety of JAK-1 inhibitors. Reumatology. 2021;60:ii24–30. https://doi.org/10.1093/rheumatology/keaa895.
Naranjo A, Sokka T, Descalzo MA, Calvo-Alén J, Hørslev-Petersen K, Luukkainen RK, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther. 2008;10:R30. https://doi.org/10.1186/ar2383.
Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–26.
Xie W, Xiao S, Huang Y, Sun X, Zhang Z. Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis. 2019. https://doi.org/10.1177/1759720X19895492.
Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2011;80(3):304–11. https://doi.org/10.1136/annrheumdis-2020-218510.
D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danse S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Ther Adv Gastroenterol. 2019;12:1–10. https://doi.org/10.1177/1756284819848631.
Mahadevan U, Robinson C, Bernaslo N, Boland B, Chambers C, Dubinsky M, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American gastroenterological association IBD parenthood project working group. Inflamm Bowel Dis. 2019;25:627–41. https://doi.org/10.1093/IBD/izz037.
Mahadevan U, Dubinsky MC, Su C, Lawendy N, Jones TV, Marren A, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494–500. https://doi.org/10.1093/ibd/izy160.
Mahadevan U, Robinson C, Bernasko N, Boland B, Chambers C, Dubinsky M, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American gastroenterological association IBD parenthood project working group. Inflamm Bowel Dis. 2019;25:627–41. https://doi.org/10.1093/ibd/izz037.
Chiorian MV, Allegretti JR, Sharma PP, Chastek B, Salese L, Bell EJ, et al. Real world characteristics, treatment experiences and corticosteroid utilization of patients treated with tofacitinib for moderate to severe ulcerative colitis. BMC Gastroenterol. 2022;22(1):356. https://doi.org/10.1186/s12876022022987.
Hoisnard L, Lebrun-Vignes B, Maury S, Mahevas M, El Karoui K, Roy L, et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. 2022;12:7140. https://doi.org/10.1038/s41598-022-10777-w.
Sands BE, Sandborn WJ, Feagan BG, Lichtenstein GR, et al. Peficitinib, an Oral Janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohns Colitis. 2018;12(10):1158–69. https://doi.org/10.1093/ecco-jcc/jjy085.
Sandborn WJ, Danese S, Leszczyszyn J, Romatowski J, et al. Oral ritlecitinib and brepocitinib for moderate-to-severe ulcerative colitis: results from a randomized, phase 2b study. Clin Gastroenterol Hepatol. 2023 (In Press).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Conflict of interest
PN has been a consultant for Janssen and Ferring, RQ has been a consultant for Jansen and AJY has been a consultant for Takeda, Pfizer, Bristol Myers Squibb, Arena pharmaceuticals, Prometheus Labs and Procise.
Ethics approval
Not applicable
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed equally to this review with the conception and design of the study, literature review and analysis, drafting and critical revision and editing, and approval of the final version
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Núñez, P., Quera, R. & Yarur, A.J. Safety of Janus Kinase Inhibitors in Inflammatory Bowel Diseases. Drugs 83, 299–314 (2023). https://doi.org/10.1007/s40265-023-01840-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01840-5