Abstract
Introduction
School-based preventive chemotherapy (Deworming) with praziquantel and albendazole to control and eliminate schistosomiasis and soil-transmitted helminths as public health problems is recommended by the World Health Organization (WHO). Safety monitoring during mass drug administration (MDA) is imperative but data from sub-Saharan Africa are scarce.
Objective
The aim of this active safety surveillance study was to identify the incidence, type, severity, and risk factors for adverse events (AEs) following mass administration of praziquantel and albendazole.
Methods
Overall, 8037 school children aged 5–15 years in Rwanda were enrolled. Baseline sociodemographic, medical history and any pre-existing clinical symptoms were recorded. Participants received a single dose of praziquantel and albendazole during MDA. AEs were actively monitored on days 1, 2, and 7 post MDA.
Results
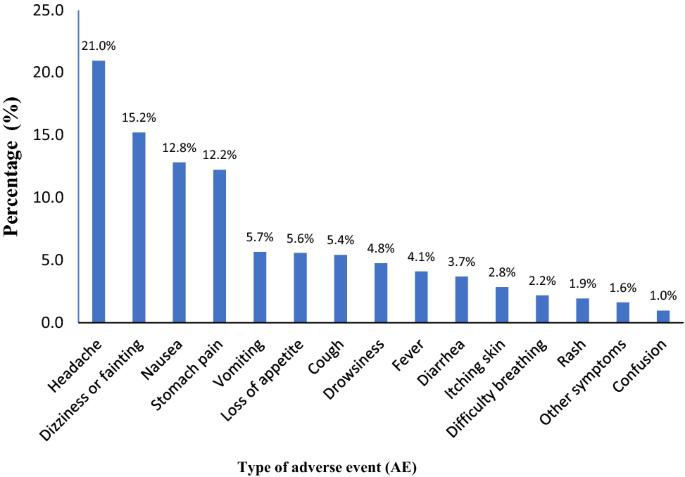
Overall, 3196 AEs were reported by 1658 children; 91.3%, 8.4%, and 0.3% of the AEs were mild, moderate, and severe, respectively, and most resolved within 3 days. Headache (21%), dizziness or fainting (15.2 %), nausea (12.8%) and stomach pain (12.2%) were the most common AEs. The overall cumulative incidence of experiencing at least one type of AE was 20.6% (95% confidence interval [CI] 19.7–21.5%), being significantly higher (p < 0.001) in children with pre-MDA clinical events (27.5%, 95% CI 25.4–29.6%) than those without (18.7%, 95% CI 17.7–19.7%). Females, older age, having pre-MDA events, types of food taken before MDA and taking two or more praziquantel tablets were significant predictors of AEs.
Conclusions
Praziquantel and albendazole MDA is safe and well-tolerated; however, one in five children experience transient mild to moderate, and in few cases severe, AEs. The incidence of AEs varies significantly between sex and age groups. Pharmacovigilance in the MDA program is recommended for timely detection and management of AEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our active safety surveillance study indicates that about one-fifth of children who receive mass praziquantel and albendazole experience transient mild to moderate adverse events (AEs). Headache, dizziness, nausea, and stomach ache were the most common AEs observed. |
Being female, older age, receiving two or more tablets of praziquantel, having pre-existing clinical symptoms, and type of food taken before drug administration are independent significant predictors of AEs following mass drug administration. |
Although mass praziquantel and albendazole coadministration to children is generally safe and tolerable, safety monitoring is recommended for timely detection and management of AEs and to ensure public confidence in mass drug administration programs. |
1 Introduction
Schistosomiasis and soil-transmitted helminths (STH) are among the most prevalent neglected tropical diseases (NTDs) and public health problems that impose a great burden in many parts of the world especially in sub-Saharan Africa (SSA) [1, 2]. Globally, over 1.5 billion people, i.e. approximately 24% of the world’s population, are infected with STH. SSA is one of the regions with a high burden of STH infections, and more than 90% of all schistosomiasis cases are from SSA [1,2,3,4]. Although NTDs are both preventable and treatable, they cause significant morbidity and mortality, especially among children [5,6,7,8]. Schistosomiasis and STH are both endemic in Rwanda, especially along Lake Kivu where a prevalence of up to 69.5% and 77%, respectively, is reported [4, 9].
The World Health Organization (WHO) has introduced a global intervention strategy to control and eliminate these NTDs from being public health problems [10]. The primary intervention strategy recommended by the WHO is preventive chemotherapy (deworming), a large-scale periodical mass administration of praziquantel and albendazole to all at-risk population groups irrespective of disease status [11]. Children are at a higher risk of getting these infections and are therefore the main target groups for praziquantel and albendazole mass drug administration (MDA) [12]. In Rwanda, the annual MDA of praziquantel and biannual MDA of albendazole started in 2008 and several rounds have been conducted since then. More than 1 million school-aged children and 98,017 adults received praziquantel preventive chemotherapy for schistosomiasis and over 5 million preschool and school-aged children received mass albendazole preventive chemotherapy for STH in 2019 [13].
In most SSA countries, schistosomiasis and STH are coendemic, hence praziquantel and albendazole are coadministered during MDA. Although coadministration of the two drugs targeting the two NTDs is cost effective, several factors, including risk for drug interaction and overlapping toxicities, underscores the need for safety monitoring [14, 15]. Safety monitoring is essential to detect the number, type, and severity of AEs and identify risk factors associated with mass albendazole and praziquantel administration-related AEs for timely interventions, thereby minimizing harm and sustaining public confidence in the MDA [16].
The WHO recommends ensuring safety in public health programs implementing large-scale MDA of anthelminthics drugs, including praziquantel and albendazole [17]. MDA of praziquantel and albendazole is delivered to all at-risk populations without prior screening or diagnosis, and hence the infection status and/or intensity is unknown. Individuals with heavy infection intensity experience more AEs compared to those with light to moderate infection, partly due to immunologic reactions as a result of parasites’ treatment-induced death [18,19,20], therefore it is important to monitor public drug safety during MDA.
Every year, millions of children receive periodic MDA of praziquantel and albendazole in SSA, including Rwanda, but safety data from large-scale active cohort event monitoring (CEM) studies are scarce partly due to lack of fully functional pharmacovigilance systems [14, 21, 22]. The NTD programs prioritize MDA coverage and AEs are often managed and contained at the sites they happened for fear of rumors, which may negatively affect public confidence, and are not reported to the National Medicines Regulatory Authorities (NMRAs) [14, 22]. The underreporting of AEs during MDA campaigns makes it challenging to accurately estimate the risks of medicine-related harm and inform healthcare policy and practice [14]. The WHO recommends active CEM for safety surveillance of medicines used in public health interventions, particularly in areas where passive reporting is challenging [23].
Few studies that assessed the safety profiles of albendazole and praziquantel in different settings reported transient AEs such as abdominal pain, nausea, vomiting, diarrhea, headache, dizziness, and fainting [18, 20, 24,25,26]. Safety surveillance studies in various target populations are important since local factors such as comorbidities, nutritional status, genetic and environment differences affect variability in drug responses [27, 28]. Despite multiple rounds of MDA, the safety profile of praziquantel and albendazole MDA has not been investigated in Rwanda. Thus, we conducted a large-scale school-based active safety surveillance study to identify the incidence, type, severity, risk factors for AEs, and tolerability of single-dose mass administration of praziquantel and albendazole among school children in Rwanda.
2 Methods
2.1 Study Design, Population, and Setting
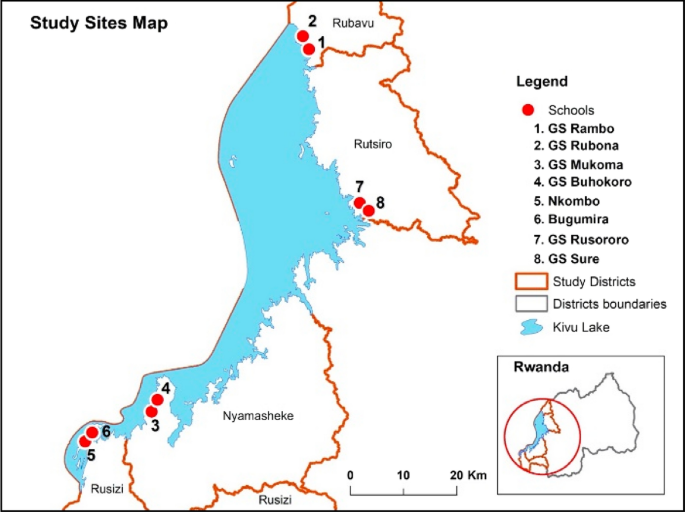
This was a prospective, observational CEM study assessing the AEs following MDA of praziquantel and albendazole. The study participants were children attending eight schools in four districts of the Western Province of Rwanda in April 2019. The public did not participate in the design of study. The study was conducted in four districts located around the belt of Lake Kivu, namely Rubavu, Rutsiro, Nyamasheke, and Rusizi in the Western Province (Fig. 1). The districts were selected using a purposive sampling method based on (1) schistosomiasis and STH prevalence data from previous studies [4, 9]; and (2) inclusion in the national NTD program for distribution of praziquantel and albendazole MDA in 2019. Within each district, two schools were selected based on three criteria: (1) proximity to the lakes; (2) the number of school children attending; and (3) previous schistosomiasis and STH prevalence data. A sample proportion of each school to contribute to the whole study sample was based on each student population size. A total of 8037 children aged 5–15 years from the eight selected schools were enrolled in the safety monitoring study.
Map of Rwanda showing the study districts and selected schools [4]
The target sample size for our study was 10,000, which was guided based on event frequency according to the WHO’s estimation. The sample size to detect at least three events at a frequency of 1 per 3333 with 95% confidence (95% probability) is 10,000 [29]. Our sample size of 8037 will detect at least three events at a frequency of 1 per 2679 with 95% confidence.
2.2 Study Enrollment and Data Collection
Before starting the study, awareness creation and sensitization meetings were held with education offices at the district and the Head of district hospitals, health centers, school teachers, school administrators, and parents/guardians. The awareness meetings were held to provide information about the study, including the study objective, significance, procedures, and type of data to collect. The ultimate intention of the awareness meetings was to get permission from the community authorities and school administrators to conduct the study in the selected schools and obtain oral and written informed consent from parents/guardians.
School children aged between 5 and 15 years attending the selected eight schools whose parents or guardians gave informed consent and provided assent to participate were enrolled. They consented to the collection of personal data, including sociodemographic, comorbidities, concomitant medications, nutritional status using anthropometric data, and any medical, clinical, or physical event before and after the MDA. Children whose parents or guardians were not willing to provide informed consent and or dissent were not included in the study; however, they remained able to participate in the national MDA and received preventive chemotherapy deployed under Rwanda's NTD program, but no safety data were collected from them.
Data including sociodemographic, breakfast status, type of meal, concomitant medication, chronic medical condition, and pre-MDA symptoms were collected at baseline using a case record form before receiving MDA through in-person interviews. After receiving MDA, study participants were followed to document treatment-associated AEs on days 1, 2, and 7 post MDA through in-person interviews. Data collection was performed by trained data collectors and were entered in the electronic database using tablets. A data manager reviewed submitted data daily to cross-check and rectify for errors. The study coordinator assigned to each school supervised the study enrolment, data collection, and data entry into the database. For anthropometric measurements, children’s body weight was measured in kilograms (kg) and height was measured in centimeters (cm), and converted to body mass index (BMI)-for-age Z score (BAZ) and height-for-age Z score (HAZ), respectively, using WHO Anthro-Plus software for school-age children [30]. Children whose HAZ and BAZ scores were < 2 standard deviations were considered stunted and wasted/thin, respectively.
2.3 Exposure and Outcome Variables
2.3.1 Exposure Definition and Measurement
The main exposure of this study was receiving single-dose albendazole and praziquantel oral administration as preventive chemotherapy provided through MDA under the national NTD program. Study participants received praziquantel according to height for children (≥ 94 cm dose pole, designed to deliver a dose of at least 40 mg/kg) and albendazole 400 mg following the national and WHO MDA guidelines [31, 32]. The Rwanda Ministry of Health NTD public health program provided and administered praziquantel and albendazole as preventive chemotherapy to prevent transmission of schistosomiasis and STH. MDA was administered to all children attending the eight schools as scheduled by the Rwanda Ministry of Health. The study team had no role in the MDA planning, and providing or administering the drugs.
2.3.2 Outcome Definition and Measurement
The primary study outcome was the incidence of MDA-associated AEs (post-MDA AEs), defined as any event that was not reported before albendazole and praziquantel administration but occurred after drug exposure. The secondary outcomes were the type and severity of AEs. Prior to MDA, all participants were interviewed for any pre-existing clinical symptoms (pre-MDA event), such as fever, loss of appetite, dizziness or fainting, confusion, drowsiness, headache, cough, difficulty in breathing, nausea, vomiting, diarrhea, stomach pain, itching, rash, and any other symptoms. Study participants were actively and prospectively monitored for any AEs on days 1, 2, and 7 post MDA. Between days 3 and 6, participants only reported if they experienced any AEs.
Events reported by each study participant before and after MDA were cross-checked and verified to differentiate pre-existing clinical symptoms from treatment-associated AEs following praziquantel and albendazole MDA. Events reported after MDA (post MDA) were considered as MDA-associated AEs if the same type of events were not reported before drug intake (pre-MDA). In addition, any event reported on days 2–7 was also considered an MDA-associated AE if the study participant experienced that event on any of the follow-up days but did not experience the same symptom pre MDA and on preceding days.
All reported AEs were graded based on five levels of severity grading using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [33] as follows.
-
Grade 1—Mild: Asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
-
Grade 2—Moderate: Minimal, local, or non-invasive intervention indicated; limiting age-appropriate instrumental activities of daily living (ADL).
-
Grade 3—Severe or medically significant but not immediately life-threatening: hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL.
-
Grade 4—Life-threatening consequences: urgent intervention indicated.
-
Grade 5—Death related to AE
2.4 Statistical Analysis
All data collected in the electronic database were imported into STATA 13 (StataCorp LLC, College Station, TX, USA) for cleaning and analysis. The outcome variable was categorized as a dichotomous variable (having any AE or not), irrespective of the type of AE. Associations between a categorical dependent variable and independent categorical variables were analyzed using the Chi-square test. Predictors of AEs were analyzed by a univariate followed by multivariate binomial logistic regression analysis. Biologically plausible predictor variables with a p value ≤0.2 in the univariate analyses were entered into the final multivariate model for analysis. We used log transformation to change coefficients into incidence risk ratios (IRR) for interpretation without changing their estimations. A p value < 0.05 was considered statistically significant.
2.5 Ethical Consideration
This study was approved by the Rwandan National Ethics Committee (Review Approval Notice No. 0064/RNEC/2019) and the National Health Research Committee of the Ministry of Health, Rwanda (NHRC/2018/PROT/042). Before initiating the study, awareness creation and sensitization meetings were held with education offices at the district and the Head of district hospitals, health centers, school teachers, school administrators, and parents/guardians. Prior to enrolment, participants and their parents or legal guardians received information about the study. For participants ≤ 12 years of age, verbal and written informed consent was obtained from their parent or guardian, and for participants > 12 years of age, verbal and written informed consent was obtained from the parent or guardian and assent was obtained from the study participant.
3 Results
3.1 Characteristics of Study Participants
The study enrolled 8037 school children between 5 and 15 years of age who received albendazole and praziquantel during the MDA campaign in 2019. The sociodemographic and baseline characteristics of study participants is presented in Table 1. Of the total 8037 children, 4224 (52.6%) were male and 5112 (63.6%) were between 10 and 15 years of age. The study participants were recruited from eight schools in four districts located along the shores of Lake Kivu in the Western Province of Rwanda. The anthropometric measurements indicated that 2805 children (34.9%) were stunted and 833 (10.4%) were wasted. Of the total study participants, 3301 (41.1%) received one praziquantel tablet, 2415 (30%) received two to three praziquantel tablets, and 2321 (28.9%) received more than three praziquantel tablets. Single-dose albendazole 400 mg was coadministered to all study participants.
3.2 Incidence of Mass Drug Administration (MDA)-Associated Adverse Events (AEs)
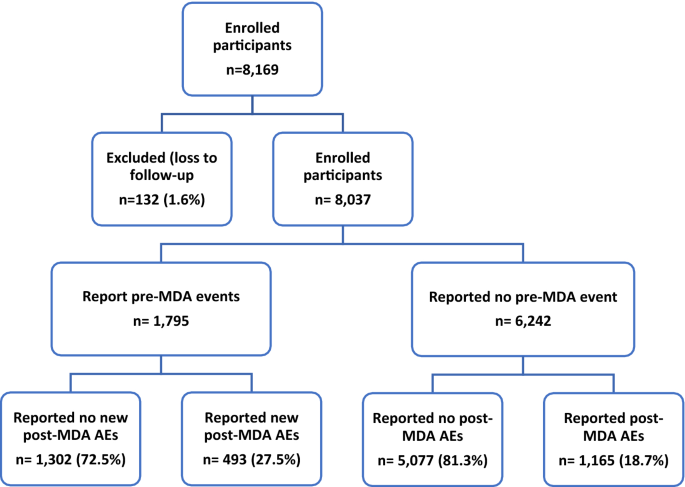
The study participants were followed prospectively for 7 days to record all suspected AEs following the MDA. Baseline assessment of self-reported symptoms through interviews were recorded before drug administration. A total of 1795 children (22.3%) of 8037 participants reported at least one type of clinical symptom before taking MDA (pre-MDA event).
A total of 3196 AEs were reported by 1658 children. The overall cumulative incidence of experiencing at least one type of AE among school children was 20.6% (95% confidence interval [CI] 19.7–21.5%, n = 1658) during the 7-day follow-up. The cumulative incidence of experiencing at least one type of MDA-associated AE among those who reported a pre-MDA event (27.5%, 95% CI 25.4–29.6%) was significantly higher (p < 0.0001) than those who did not report pre-MDA events (18.7%, 95% CI 17.7–19.7%). The study flow chart and findings from the pre-MDA (baseline) and incidence of post-MDA safety monitoring are presented in Fig. 2.
3.3 Proportion of Various Types of AEs
Overall, 3196 AEs were reported during the 7 days of active surveillance. Headache (21%), dizziness or fainting (15.2%), nausea (12.8%), and stomach pain (12.2%) were the most common AEs following praziquantel and albendazole MDA among school children. The least reported AEs were confusion (1%), other symptoms (1.6%), and rash (1.9%). The proportion of AEs reported during a 7-day follow-up period stratified by type of AE is presented in Fig. 3.
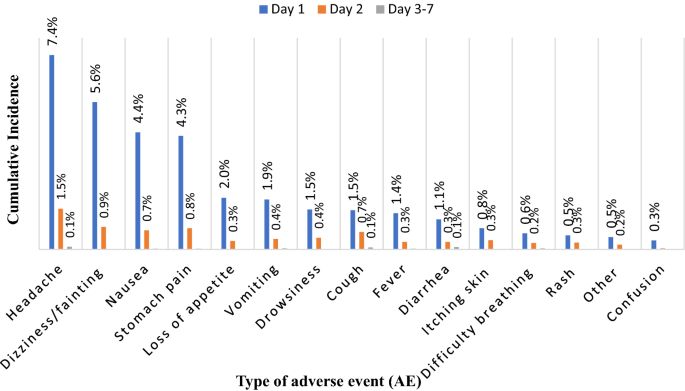
During the 7 days of AE monitoring, the highest number of AEs were observed on day 1 [2588 AEs (81%)] followed by day 2 [570 AEs (17.8%)], and 38 AEs were reported during days 3–7 post MDA. Most AEs reported on day 1 were headache [544 (7.4%)], dizziness or fainting [419 (5.6%)], nausea [352 (4.4%)], and stomach pain [327 (4.3%)]. On day 2, like day 1, headache [118 (1.5%)] and dizziness or fainting [66 (0.9%)] were the most frequently reported AEs. Between days 3 and 7, headache 8 (0.1%) and diarrhea 7 (0.1%) were the most reported AEs, as presented in Fig. 4.
3.4 Severity Grading of AEs
The severity of the reported AEs (3196) was graded as mild, moderate, or severe, following the CTCAE version 5.0 [33]. None of the participants reported potentially life-threatening or disabling AEs or death. The incidences of mild, moderate, and severe AEs were 91.3% (number of AEs = 2919), 8.4% (number of AEs = 267), and 0.3% (number of AEs = 10) respectively. The total number of AEs reported as severe included one case of headache, seven cases of cough, and two cases of stomach pain, as described in Table 2.
3.5 Factors Associated with Post-MDA AEs
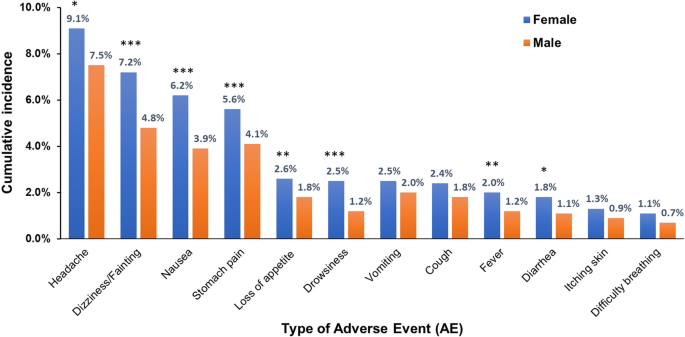
The incidence and associations of reported AEs post MDA are presented in Table 3. Sex was significantly associated with the occurrence of AEs (p < 0.001), and the incidence of AEs was significantly higher among female participants (23.2%) than male participants (17.7%) (p < 0.001). Stratifying by sex, all types of reported AEs, except vomiting, cough, and itching and skin rash, were significantly higher in female participants than male participants (Fig. 5). Age group was significantly associated with the occurrence of AEs (p < 0.001); participants between 10 and 15 years of age reported more AEs (24.8%) than those between 5 and 9 years of age (13.4%). The type of food taken before MDA was significantly associated with the occurrence of AEs (p < 0.001); study participants who had a fatty meal before MDA reported more AEs (30.8%), followed by those who had a high protein meal (29.5%) and those who had a carbohydrate meal (20.4%). The number of praziquantel tablets administered was significantly associated with the occurrence of AEs (p < 0.001). Study participants who had taken more than three tablets reported more AEs (29.6%), followed by study participants who had taken two to three tablets (21.8%) and those who had taken one tablet (13.5%). Other variables such as stunting, concomitant medication, taking any traditional medicine, and having any chronic medical condition were not significantly associated with the occurrence of AEs.
3.6 Risk Factors Associated with Post-MDA AEs
We analyzed risk factors associated with developing any AE following MDA using univariate and multivariate analysis and the results are presented in Table 4. Using log binomial regression in univariate analysis, sex, age category, wasting, type of food taken before MDA, and number of praziquantel tablets were significant predictors of developing at least one post-MDA AE. In multivariate analysis using log binomial regression for all variables from univariate analysis with a p value < 0.2, sex, age category, type of food taken, and number of praziquantel tablets administered were significant predictors of developing any post-MDA AEs.
4 Discussion
This observational active safety surveillance study investigated the incidence and associated factors of AEs following mass praziquantel and albendazole administration among children in Rwanda. Our results indicate that about one-fifth of the children who received praziquantel and albendazole as MDA experienced mild-to-moderate AEs, mostly within the first 2 days after MDA. Most AEs were transient and resolved within 1 week after MDA. The overall cumulative incidence of experiencing at least one type of AE was 20.6%. Different studies from SSA reported varying incidence rates of AEs associated with mass praziquantel and albendazole administration. Studies conducted in Kenya (25.3%), Tanzania (28.5%), Angola (55.9%), and Ethiopia (83%) reported higher incidences than our study finding [19, 24, 25, 34]. Differences in the reported incidence of AEs can be due to variation in the studies, such as data collection methods, AE definition, and/or infection status of the study population (treatment in infected children versus preventive chemotherapy in the target population with unknown infection status). The lower incidence of AEs in our study could be due to our stringent definition set for MDA-associated AEs that excludes pre-existing clinical symptoms.
The incidence rates of MDA-associated AEs were significantly different among schools and the four study districts (Table 3). Children attending schools in the Rubavu district had the highest AE incidence, possibly due to a higher prevalence of STH (92%), compared with the other districts Rutsiro (54%), Nyamasheke (60%), and Rusizi (87%) [4]. Study participants in the Rubavu district may have also had higher infection intensity compared with participants in the other districts. Children with moderate to heavy infection intensity are more likely to experience AEs compared with those with light infection intensity [18,19,20]. AEs may be induced by drugs or immunologic reaction due to the killing of the parasites mostly in heavy infection intensity [25, 35]. The occurrence of AEs could be influenced by other factors such as age, co-infections or comorbidities, concomitant medication, nutritional status, socioeconomic conditions, and environmental exposure [25, 36, 37]. A recent study from Kenya reported concomitant medications and chronic illness as significant risk factors for developing AEs following diethylcarbamazine citrate and albendazole MDA [38]. In our study, we did not find any significant association between chronic illness/comorbidity or concomitant traditional medicine use with AEs. This could be because of the small number of children who had chronic illness/comorbidity (2.7%) in our study population, and hence a lack of adequate power to detect any such association. On the other hand, we found that children who reported pre-MDA events experienced more post-MDA AEs (28%) compared with children who did not report pre-MDA events (19%). The association of having pre-MDA clinical symptoms with higher incidence of MDA-associated AEs has also been reported recently [38, 39]. Therefore, children with underlying clinical symptoms should be closely monitored after MDA.
The incidence of AEs varies depending on the type of AEs. A meta-analysis that evaluated the clinical efficacy and tolerability of praziquantel in the treatment of intestinal and urinary schistosomiasis reported that the incidence of AEs ranged from 2.3% for urticaria to 31.1% for abdominal pain [40]. The most observed AEs in our study were headache (21%), dizziness or fainting (15.2%), nausea (12.8%), and stomach pain (12.2%), as similarly reported by other studies from Angola, Ethiopia, Kenya, and Tanzania [19, 24, 25, 34]. Wide interindividual variations in plasma praziquantel concentrations partly due to pharmacogenetic variations has recently been reported [41, 42]. Any association of praziquantel pharmacokinetics and pharmacogenetic variation with susceptibility to treatment-induced AEs remains to be investigated. Generally, the AEs occurred within days 1 and 2 of post drug administration and were transient; most resolved by day 3 (Fig. 4). These findings are in line with previous studies where the majority of the reported AEs (80.8%) resolved within a day or two [24, 43].
Among the reported AEs, 91.3% were mild, 8.4% were moderate, and 0.3 % were graded as severe, which is in line with reports from other studies, including a randomized controlled trial and meta-analysis [19, 24, 44, 45]. However, a recent study conducted in India reported severe and serious AEs among children who received albendazole alone [46]. The severity of AEs could potentially be due to several factors, including, but not limited to, infection intensity and nutritional and health status. Thus, safety surveillance during praziquantel and albendazole mass administration in various settings is imperative.
The current study revealed that older age, having pre-MDA events, type of food taken before MDA, and increased number of praziquantel tablets taken were significant risk factors for developing at least one AE post MDA (Table 4). All study participants received one tablet of albendazole (400 mg) regardless of age, but the dose of praziquantel was based on the height of the child, corresponding to 40 mg/kg as recommended by the WHO MDA guideline [31]. Thus, the observed higher incidence of AEs among older children could be due to the increased number of praziquantel tablets administered. Indeed, taking two or more praziquantel tablets was a significant predictor of AEs; however, after adjusting for the number of praziquantel tablets, the older age group still had an increased risk for AEs. This could be due to the higher STH prevalence in the older age group, which might be associated with a higher risk of AEs, as previously described [4, 36]. Interestingly, children who had a fatty or high protein diet before MDA had a higher risk of experiencing more AEs than those who had a carbohydrate meal. In line with our findings, a recent study reported the association of having a fatty or high protein meal before MDA with a higher incidence of AEs following mass diethylcarbamazine and albendazole administration for the elimination of lymphatic filariasis in Kenya [38]. Altered drug absorption and bioavailability with a fatty or high protein diet has been previously reported [47]. Accordingly, food–drug interactions influencing drug absorption could possibly alter the susceptibility to AEs following praziquantel and albendazole MDA.
Our findings indicate that females are at a higher risk of experiencing more AEs than males. Similar to our findings, another study assessing the safety of praziquantel and albendazole in Kenya reported that females and older children (≥ 10 years) experienced more AEs [24]. A higher incidence of MDA-associated AEs in females than males who received preventive chemotherapy for the prevention of lymphatic filariasis has also been recently reported [38, 39]. Generally, women have a higher risk of developing AEs compared with men, however the reason for this is unclear but is possibly due to physiological and sex-related hormonal differences, which can affect drug metabolism [38, 48, 49].
To our knowledge, this is the first and largest active CEM study to investigate the incidence, type, severity, and predictors for AEs following mass praziquantel and albendazole administration for the control and elimination of schistosomiasis and STHs in Rwanda and SSA. The strength of our study is the large sample size, thereby enhancing the ability to detect rare AEs as recommended by the WHO. Secondly, assessment of the AEs was conducted using active surveillance for up to 7 days post MDA. This provided a chance to explore the immediate AEs following drug administration and those that can occur at a later stage. However, as mass albendazole and praziquantel administration is given to all eligible school children without a prior diagnosis, we could not compare the incidence of AEs between schistosomiasis and/or STH-infected versus healthy children, and this may subsequently be considered a limitation of our study.
5 Conclusions
Single-dose praziquantel and albendazole administered during MDA for the control of schistosomiasis and soil-transmitted helminthiasis among school children is safe and tolerable. However, the finding that one in five children experience transient mild-to-moderate AEs and few cases of severe AEs underlines the need to integrate pharmacovigilance in MDA campaigns. Our study suggests being female, older age, taking two or more praziquantel tablets and having pre-existing clinical conditions or symptoms are independent risk factors for AEs following praziquantel and albendazole MDA. Our study highlights the importance of integrating pharmacovigilance into the national NTD public health program and safety monitoring during MDA for the timely detection and management of AEs.
References
World Health Organization. Schistosomiasis Fact Sheet. Geneva; World Health Organization; 2020. https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. Accessed 31 Jan 2020.
World Health Organization. Soil-transmitted helminth infections. Geneva: World Health Organization; 2021. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. Accessed 15 Jan 2022.
Gebreyesus TD, Tadele T, Mekete K, Barry A, Gashaw H, Degefe W, et al. Prevalence, intensity, and correlates of schistosomiasis and soil-transmitted helminth infections after five rounds of preventive chemotherapy among school children in Southern Ethiopia. Pathogens. 2020;9:1–14.
Kabatende J, Mugisha M, Ntirenganya L, Barry A, Ruberanziza E, Mbonigaba JB, et al. Prevalence, intensity, and correlates of soil-transmitted helminth infections among school children after a decade of preventive chemotherapy in Western Rwanda. Pathogens. 2020;9:1–20.
Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8: e2865.
Global Atlas of Helminth Infections. Global burden; 2018. http://www.thiswormyworld.org/worms/global-burden. Accessed 21 May 2022.
Hall A, Hewitt G, Tuffrey V, De Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236.
Pabalan N, Singian E, Tabangay L, Jarjanazi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1): e0005523.
Rujeni N, Morona D, Ruberanziza E, Mazigo HD. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: an update on their epidemiology and control. Infect Dis Poverty. 2017;6:1–11.
World Health Organization. Eliminating soil-transmitted helminthiases as a public health problem in children. Progress report 2001−2010 and strategic plan 2011−2020; Geneva; World Health Organization; 2012. https://www.who.int/publications/i/item/9789241503129. Accessed 21 Jan 2021.
World Health Organization. Update on the global status of implementation of preventive chemotherapy (PC). World Health Organization; 2020. https://www.who.int/neglected_diseases/preventive_chemotherapy/PC_Update.pdf?ua=. Accessed 26 Feb 2021.
World Health Organization. Guideline: preventive chemotherapy to control soil-transmitted helminths infections in at risk population groups. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2017.
Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Rwanda: 2019. https://espen.afro.who.int/countries/rwanda. Accessed 6 Sept 2021.
Barry A, Olsson S, Khaemba C, Kabatende J, Dires T, Fimbo A, et al. Comparative assessment of the pharmacovigilance systems within the neglected tropical diseases programs in East Africa—Ethiopia, Kenya, Rwanda, and Tanzania. Int J Environ Res Public Health. 2021;18:1–13.
Minzi OM, Mnkugwe RH, Ngaimisi E, Kinung’hi S, Hansson A, Pohanka A, et al. Effect of dihydroartemisinin-piperaquine on the pharmacokinetics of praziquantel for treatment of schistosoma mansoni infection. Pharmaceuticals. 2021;14:1–12.
Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf. 2013;36:75–81.
World Health Organization. Assuring Safety of Preventive Chemotherapy Interventions for the Control of Neglected Tropical Diseases. Geneva: World Health Organization; 2011. Available at: https://apps.who.int/iris/handle/10665/44683. Accessed 10 Jan 2021.
Zwang J, Olliaro P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: a meta-analysis. Parasites Vectors. 2017;10:1–16.
Mnkugwe RH, Minzi OS, Kinungʹhi SM, Kamuhabwa AA, Aklillu E. Efficacy and safety of praziquantel for treatment of schistosoma mansoni infection among school children in Tanzania. Pathogens. 2020;9:1–12.
Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, et al. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003.
Barry A, Olsson S, Minzi O, Bienvenu E, Makonnen E, Kamuhabwa A, et al. Comparative assessment of the national pharmacovigilance systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020;43:339–50.
Kiguba R, Olsson S, Waitt C. Pharmacovigilance in low- and middle-income countries: a review with particular focus on Africa. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15193.
World Health Organization. The Safety of Medicines in Public health programmes: pharmacovigilance an essential tool. WHO Library Catalog Data; 2006:61. https://apps.who.int/iris/handle/10665/43384. Accessed 20 May 2022.
Njenga SM, Ng’Ang’a PM, Mwanje MT, Bendera FS, Bockarie MJ. A school-based cross-sectional survey of adverse events following co-administration of albendazole and praziquantel for preventive chemotherapy against urogenital schistosomiasis and soil-transmitted helminthiasis in Kwale County, Kenya. PLoS One. 2014;9:6–10.
Lemos M, Pedro JM, Fançony C, Moura S, Brito M, Nery SV, et al. Schistosomiasis and soil-transmitted helminthiasis preventive chemotherapy: adverse events in children from 2 to 15 years in Bengo province, Angola. PLoS One. 2020;15:1–13.
Raso G, N’Goran EK, Toty A, Luginbühl A, Adjoua CA, Tian-Bi NT, et al. Efficacy and side effects of praziquantel against Schistosoma mansoni in a community of western Côte d’Ivoire. Trans R Soc Trop Med Hyg. 2004;98:18–27.
Yimer G, Amogne W, Habtewold A, Makonnen E, Ueda N, Suda A, et al. High plasma efavirenz level and CYP2B66 are associated with efavirenz-based HAART-induced liver injury in the treatment of naïve HIV patients from Ethiopia: a prospective cohort study. Pharmacogenomics J. 2012;12:499–506.
Mugusi S, Ngaimisi E, Janabi M, Minzi O, Bakari M, Riedel KD, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS One. 2012;7:1–9.
World Health Organization. Cohort event monitoring (CEM) for safety signal detection after vaccination with COVID-19 vaccines. Geneva: World Health Organization; 2021. https://www.who.int/publications/i/item/10665338400. Accessed 10 Feb 2022.
World Health Organization. WHO Anthroplus Software; Software for assessing Growth and Development of the World’s Children and Adolescents. Department of Nutrition for Health and Development. Geneva: World Health Organization; 2009.
World Health Organization. Preventive chemotherapy in human helminthiasis. Geneva: World Health Organization; 2006. http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf. Accessed 30 Jan 2021.
Montresor A, Odermatt P, Muth S, Iwata F, Raja’a YA, Assis AM, et al. The WHO dose pole for the administration of praziquantel is also accurate in non-African populations. Trans R Soc Trop Med Hyg. 2005;99:78–81.
Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events (CTCAE).v.5.0 [5x7]. Cancer Therapy Evaluation Program; 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 11 Oct 2021.
Erko B, Degarege A, Tadesse K, Mathiwos A, Legesse M. Efficacy and side effects of praziquantel in the treatment of Schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed. 2012;2:235–9.
Hong S. Albendazole and praziquantel: review and safety monitoring in Korea. Infect Chemother. 2018;50:1–10.
Samuel F, Degarege A, Erko B. Efficacy and side effects of albendazole currently in use against Ascaris, Trichuris and hookworm among school children in Wondo Genet, southern Ethiopia. Parasitol Int. 2014;63:450–5.
Sousa-Figueiredo JC, Betson M, Atuhaire A, Arinaitwe M, Navaratnam AMD, Kabatereine NB, et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis. 2012;6(10): e1864. https://doi.org/10.1371/journal.pntd.0001864.
Khaemba C, Barry A, Omondi WP, Bota K, Matendechero S, Wandera C, et al. Safety and tolerability of mass diethylcarbamazine and albendazole administration for the elimination of lymphatic filariasis in Kenya: an active surveillance study. Pharmaceuticals (Basel). 2021;14(3):264.
Fimbo AM, Minzi OM, Mmbando BP, Gurumurthy P, Kamuhabwa AAR, Aklillu E. Safety and tolerability of ivermectin and albendazole mass drug administration in lymphatic filariasis endemic communities of Tanzania: a cohort event monitoring study. Pharmaceuticals (Basel). 2022;15(5):594.
Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis: a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8(11): e3286.
Mnkugwe RH, Minzi O, Kinung’hi S, Kamuhabwa A, Aklillu E. Effect of pharmacogenetics variations on praziquantel plasma concentrations and schistosomiasis treatment outcomes among infected school-aged children in Tanzania. Front Pharmacol. 2021;12:712084.
Mnkugwe RH, Ngaimisi KE, Kinung’hi S, Kamuhabwa AAR, Minzi OM, Aklillu E. Optimal single sampling time-point for monitoring of praziquantel exposure in children. Sci Rep. 2021;11(1):17955.
Njomo DW, Tomono N, Muhoho N, Mitsui Y, Josyline KC, Mwandawiro CS. The adverse effects of albendazole and praziquantel in mass drug administration by trained schoolteachers. Afr J Health Sci. 2010;17:10–4.
Coulibaly JT, Panic G, Silué KD, Kovač J, Hattendorf J, Keiser J. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob Health. 2017;5:e688–98.
Olliaro PL, Coulibaly JT, Garba A, Halleux C, Keiser J, King CH, et al. Efficacy and safety of single 40 mg/kg oral praziquantel in the treatment of schistosomiasis in preschool-age versus school-age children: an individual participant data meta-analysis. PLoS Negl Trop Dis. 2020;14(6): e0008277.
Agrawal P, Srivastava B, Bhardwaj R, Gaur S. Adverse events of albendazole due to mass drug administration. Int J Basic Clin Pharmacol. 2017;6:1674–7.
Ayo JA, Agu H, Madaki I. Food and drug interactions: its side effects. Nutr Food Sci. 2005;35:243–52.
de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85:1507–15.
Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17: 100188.
Acknowledgements
The authors would like to extend their sincere thanks to the school directors and teachers from the schools that participated in the study for their support during the data collection. They also thank the parents/guardians and school children from all eight schools that participated in the study. The authors also appreciate the technical support from the National Reference Laboratory, and data managers from hospitals and health centers who contributed to make this study feasible.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was conducted as part of the Pharmacovigilance Infrastructure and Postmarketing Surveillance System Capacity Building for Regional Medicine Regulatory Harmonization in East Africa (PROFORMA) project funded by the European and Developing Countries Clinical Trials Partnership (EDCTP) 2 program supported by the European Union (Grant number CSA2016S-1618) and the Swedish International Development Cooperation Agency (SIDA).
Author contributions
Conceptualization: AB, JK, MM, LN, EB and EA. Data curation: JK, AB, MM, LN, and EA. Formal analysis: AB, JK, LN, and MM. Investigation: JK, AB, EB, MM, and EA. Methodology: AB, JK, EB, and EA. Supervision: EB, UB, and EA. Writing—original draft: JK and AB. Writing—review and editing: AB, JK, MM, LN, EB, UB, and EA. All authors read and approved the final version.
Conflict of interest
Joseph Kabatende, Abbie Barry, Michael Mugisha, Lazare Ntirenganya, Ulf Bergman, Emile Bienvenu, and Eleni Aklillu declare no conflicts of interest.
Ethics approval
This study was approved by the Rwandan National Ethics Committee (Review Approval Notice No. 0064/RNEC/2019) and the National Health Research Committee of the Ministry of Health, Rwanda (NHRC/2018/PROT/042).
Consent to participate
Participant consent was documented on a written informed consent form, which was accompanied by written information for parents/guardians, and the informed consent forms were approved by the same Ethics Committees that approved this protocol.
Consent for publication
Not applicable.
Availability of data and material
Data are available upon reasonable request owing to privacy and ethical restrictions from the authors.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kabatende, J., Barry, A., Mugisha, M. et al. Safety of Praziquantel and Albendazole Coadministration for the Control and Elimination of Schistosomiasis and Soil-Transmitted Helminths Among Children in Rwanda: An Active Surveillance Study. Drug Saf 45, 909–922 (2022). https://doi.org/10.1007/s40264-022-01201-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01201-3