Abstract
Background
Children carry most of the schistosomiasis burden. While school-aged children are the principal target group of preventive chemotherapy with praziquantel, limited information on efficacy and safety exists for preschool-aged children.
Methods
Here, we conducted a meta-analysis of clinical trials of praziquantel for treating children with any form of schistosomiasis. Efficacy was reported as cure rate (CR) and egg reduction rates (ERR); statistical corrections were applied based on methodological disparities across trials to derive the predicted geometrical mean ERR (pERRgm). Safety was reported as frequencies of adverse events.
Results
Forty-seven comparative and non-comparative studies were identified, enrolling 15,549 children of whom 14,340 (92%) were assessed between 3 and 8 weeks post-treatment with praziquantel 40 mg/kg (the WHO-recommended treatment, n = 8,380, 56%) or comparators (n = 5,960, 44%). The median age was 10 years (range 1–19), 11% (n = 1,694) were preschool-aged. The CR and pERRgm with praziquantel 40 mg/kg were respectively: S. haematobium, 73.6% (95% CI: 63.5–81.40, 25 study arms) and 94.7% (95% CI: 92.7–96.4); S. mansoni, 76.4% (95% CI: 71.5–81.0, 34 arms) and 95.3% (95% CI: 94.2–96.2); S. mansoni/S. haematobium, 67.6% (95% CI: 54.1–80.7, 5 arms) and 93.4% (95% CI: 89.9–96.2); S. japonicum, 94.7% (95% CI: 92.2–98.0) and 98.7% (95% CI: 98.3–99.2).
Mixed-effect multivariate analysis found no significant difference between preschool- and school-aged children for CR or pERRgm in S. haematobium (P = 0.309 and P = 0.490, respectively) or S. mansoni (P = 0.982 and P = 0.895) after controlling for time of assessment, formulation, intensity of infection and detection method. Praziquantel was reportedly safe at all ages, with only mild reported adverse events which cleared rapidly after treatment.
Conclusions
Praziquantel 40 mg/kg was effective at reducing infection intensity in all Schistosoma species without differences between preschool- and school-aged children. However, conclusions should be tempered because of the limited number of preschool-aged children enrolled, disparities in study procedures and limited information made available in publications, as well as the current imperfect test-of-cure. Also, although reportedly well-tolerated, safety was inconsistently assessed. Studies in target groups, individual-data meta-analysis and standardised methodologies are needed for more robust evidence-base.
Similar content being viewed by others
Background
Children carry most of the schistosomiasis burden [1]. Schistosomiasis treatment and control relies largely upon preventive chemotherapy (PC) with praziquantel (PZQ) directed primarily at school-aged children living in schistosomiasis-endemic areas [2]. Preschool-aged children have traditionally been excluded from PC and treated on confirmation of infection [3], but are recognized as a vulnerable group [4–6]. Emphasis is now shifting from control to elimination of schistosomiasis [7], meaning that all the infected groups should be treated, including preschool-aged children (1–4 years-old), provided a suitable formulation is available. PZQ is registered for use in children from four years of age, and can be given routinely in PC programmes to children measuring 94 cm or more (recently extended to 60 cm [8]) but the absence of paediatric formulations is a practical limitation to having younger children treated routinely.
Praziquantel has been in use now for over three decades, and has been given to millions of people, mostly children - close to 40 million school-aged children in 2013 [9]. However, several unresolved questions still exist about this drug (reviewed in Stothard et al. [10]), including its pharmacokinetic and pharmacodynamic properties [11].
The World Health Organization (WHO) recommends using PZQ at 40 mg/kg, a recommendation generally supported by systematic reviews [12–14] and to express treatment outcome in terms of egg reduction rate (ERR) calculated as the difference of the arithmetic mean egg counts (preferred over geometric means) between pre- and post-treatment samples [3]. It is worth adding, as a note of caution, that we only have imprecise methods to detect Schistosoma infection that are based on the detection of eggs in excreta (faeces or urine) as opposed to adult worms, and that these methods lack sensitivity (for instance, the widely-used Kato-Katz method cannot detect less than 24 eggs per gram of stools). Therefore, the (temporary) reduction or cessation of egg-excretion should not be interpreted as being equivalent to killing of adult worms. This has profound implications both for the estimation of schistosomiasis cases and for the assessment of drug efficacy [15].
This meta-analysis was conducted to assess the efficacy and safety of PZQ in preschool- and school-aged children and whether these outcomes differ with subject’s age. This question is particularly relevant in view of the inclusion of preschool-aged children as a target of schistosomiasis control through PC, and whether any dose adjustment should be required.
Methods
This is a meta-analysis of aggregated data; eligible studies were comparative and non-comparative clinical trials where PZQ had been given at any dose to preschool- and school-aged children and adolescents) for treating intestinal or urinary schistosomiasis, and where outcome was assessed within 2 months (3 to 8 weeks) post-treatment. The study was conducted according to the PRISMA guideline (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [16]. The PRISMA checklist was used to ensure inclusion of relevant information in the analysis (Additional file 1: Table S1).
Published studies were identified by the Cochrane collaboration through electronic searches from January 1, 1990, up to November 2015 of MEDLINE, EMBASE, LILACS, the Cochrane Infectious Diseases Group’s trials register and the Cochrane Central Register of Controlled Trials (CENTRAL) using the search term “praziquantel”, regardless of language (English, French, and Portuguese) and might have failed to identify articles published in other languages.
To qualify for inclusion, trials were to be on PZQ mono-therapy at any dosage and dosing regimen, using any formulation and brand, and could be either non-comparative or comparative (randomized controlled trial, quasi-randomised trials). The search identified 828 studies of PZQ in schistosomiasis in children, of which 47 had an assessment within 8 weeks (subjects treated with praziquantel, control treatment or placebo). A previous paper reported the results of a broader meta-analysis conducted up to 2012 including subjects of all ages [14].
Efficacy outcomes are reported as cure rate (CR) and egg reduction rate (ERR). CR, defined as the conversion from a positive test pre-treatment to a negative test up to 8 weeks post-treatment, is reported as provided in the articles on a per-protocol population. ERR, defined as the proportional reduction in the mean eggs per gram of faeces (intestinal schistosomiasis) or per ml of urine (urinary schistosomiasis) post-treatment vs pre-treatment is presented based on the arithmetic (ERRam) or geometric (ERRgm) mean ERR provided in the articles, respectively, 5 and 31 of the studies included in this review (11 studies did not report ERR). Since ERR was inconsistently assessed on all patients in the per-protocol population or only those who had countable eggs in their excreta post-treatment (‘uncured’), a logarithmic correlation between the CR and the ERRgm (log-transformed) was applied to correct the ERRgm results based on uncured patients and to help predict the ERRgm in studies that reported only the CR.
Tolerability is assessed by calculating the incidence of adverse events (AE) defined as any sign or symptom occurring after treatment, irrespective of whether that sign or symptom was present at baseline or not, of its severity and drug-event relationship. The mean incidence is presented for the PZQ 40 mg/kg treatment groups (all brands) excluding PZQ 40 mg/kg syrup, and Levo-PZQ 20 mg/kg.
The 95% confidence intervals (CI) for the mean CR, ERR, and AE are calculated using a bootstrap resampling method based on statistical bias between studies with a maximum of 1,000 replicates [17]. For randomized controlled trials assessing the efficacy (CR) and tolerability (AE) of PZQ vs other drugs, placebo, or comparing different PZQ dosing regimens, risk ratios (RR) with 95% CI, meta-analysis regression with random effect on the study/site is used and the pooled RR is presented using the DerSimonian & Laird procedure for random effects models [18]. Heterogeneity was expressed as I2 [19].
Children’s age groups were differently categorized whether the study was school-based (between 5 and 17 years of age) or community based including (i) preschool-aged (between 1 and 5 or 7 years) and (ii) school-aged (between 4 and 19 years) according to study definition. It should be noted that studies used categories which only partly conform with the WHO age classification for PC: preschool-aged 1–4-year-old; school-aged children 5–14-year-old.
Age-groups were inconsistently defined and generally broader in community-based studies; for example, children in nursery were enrolled as school-aged in a community based study [20]. As the studies had disparate age ranges, a median age (in year) was calculated from the reported range. A multivariate mixed-effect model was used to assess the age-dependency of CR and ERR with random effect on the site in an attempt to account for any potential statistical heterogeneity across studies while controlling for potential confounders such as: (i) endpoint of assessment (analysed as a continuous variable, in week); (ii) differences in diagnostic approaches (binary, single vs multiple smears); (iii) infection intensities before treatment, categorized as light (<100 egg per gram of faeces), moderate (100–399), and heavy (≥400) for S. mansoni, and categorized as light (<50 eggs/10 ml urine) and heavy (≥50) for S. haematobium; (iv) year of study.
Graphical displays for CR and ERR by age group were illustrated using forest plots [21]. Data were analysed using Stata v13 (Stata Corp.).
We also provide more detailed narrative summaries of the studies treating specifically preschool-aged children and those reporting results for narrower age-groups, where more direct comparisons are possible between more homogenous populations.
Results
This analysis is based on 47 studies [20, 22–67] enrolling 15,549 children aged 6 months to 19 years (of whom 1,694 (11%) were preschool-aged children). Of these 14,340 were assessed for efficacy between 3 and 8 weeks after treatment (8% attrition, acceptable risk of attrition bias) (Table 1, Additional file 2: Table S2) of whom 11% (n = 1,506) were preschool-aged (5 studies) and 89% (n = 12,834) school-aged (42 studies), 48% (n = 6,897) infected with S. mansoni, 39% (n = 5,552) with S. haematobium, 8% (n = 1,196) with S. japonicum, and 5% (n = 695) co-infected with S. mansoni/S. haematobium. Fifty-eight percent (58%, n = 8,380) of the subjects were treated with PZQ 40 mg/kg (tablets, n = 8,037 or syrup, n = 343), 14% (n = 2,013) with other doses of PZQ, and 30% (n = 4,302) with other drugs or placebo. Outcomes were measured within 4 weeks post-treatment in 56% (n = 8,042) and within 4 to 8 weeks in 44% (n = 6,298) of the children. No study enrolled both preschool- and school-aged children.
The 1,694 pre-school-aged children were treated at five sites in Uganda, Niger and Ivory Coast with PZQ 40 mg/kg (tablet or syrup) or PZQ 2*40 mg/kg for S. mansoni and S. haematobium. The attrition at the one-month post-treatment assessment was 11%. Of the 1,506 children assessed, 12% (n = 179) were infected with S. haematobium and were either treated with PZQ 40 mg/kg tablet in a single-arm study (n = 18) [22] or with syrup only also in a single-arm study (n = 161) [23] and 88% (n = 1,327) were infected with S. mansoni: a two-arm study comparing crushed tablets and syrup, both given at 40 mg/kg (n = 203) [24]; a two-arm study [25] comparing PZQ tablets given as a single dose of 40 mg/kg (n = 357) to a double-dose of 2*40 mg/kg (n = 339); two single-arm studies [22, 26] with crushed tablets only (n = 369 and 35, respectively); and a single-arm study (with syrup only; n = 88) [23].
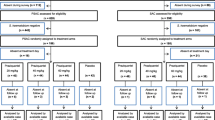
Treatment outcome was reported as CR in 14,157 children and as ERR (all calculations methods) in 10,033 patients overall, of whom 58% (n = 8,199) and 62% (n = 5,958), respectively, were treated with PZQ 40 mg/kg (Fig. 1). The breakdown by species and reported outcome was: both CR and ERR, 62% of the subjects; CR only, 33%; and ERR only, 5%. For S. haematobium, the outcome was reported as CR for 4,612 patients and 3,963 for ERR; for S. mansoni this was 6,897 and 5,138, respectively; for S. mansoni/S. haematobium, 695 and 111; and for S. japonicum, 1,196 and 403.
Of the 36 studies reporting outcomes as ERR, 31 used the ERRgm and 5 the ERRam (2 studies reported both) (Table 2). The ERRgm was calculated using a log-transformation applied to the entire per-protocol population for 24% of subjects, on uncured patients only in 16%, and not specified in 26%. No study reported ERRam on uncured subjects.
Egg counts were reported using different diagnostic approaches (number of specimens and tests): from one to three days and one to three stools per day tested in simple or duplicate or triplicate slides. The most frequent approach for S. mansoni was one test on one stool sample collected on the same day and for S. haematobium was one test on two separate urine samples, collected on the same day. Subjects receiving PZQ 40 mg/kg for S. mansoni had a mean egg count before treatment of 376 eggs per gram of faeces (epg, range 1.2–2,662); the mean was higher in studies using arithmetic means (854, range 144–2,662 epg) than those using geometric means (276, range 1.2–1,065 epg); for S. haematobium 105 eggs per ml of urine (range 1–630), also higher with arithmetic than geometric means (120 and 108, respectively); and for S. japonicum, mean epg 297 (range 60–255; studies reporting only geometric means).
Efficacy of PZQ 40 mg/kg
The calculated median age of the total 8,380 subjects treated with the PZQ 40 mg/kg was 9 years (range 1 month to 19 years) and clustered around 6 to 15 years of age. The median age of school-children varied widely across sites from 4 to 19 years, mostly depending on whether the study was school- or community-based.
The overall CR in children treated with PZQ 40 mg/kg (tablet and syrup) was 73.6% (95% CI: 64.1–82.3, 25 study groups, 3,294 children) for S. haematobium and 76.4% (95% CI: 71.5–81.0, 34 groups, 4,157 children) for S. mansoni (Fig. 2). The overall reported ERRgm for S. haematobium was 90.9% (95% CI: 86.0–95.6, 21 groups, 2,586 children) but was 81.0% for ERR based on uncured patients and 99.5% for ERRgm log-transformed; for S. mansoni it was 84.3% (95% CI: 79.0–89.3, 22 groups, 3,233 children), from 79.7% for ERR based on uncured patients to 92.0% for ERRgm log-transformed. For S. japonicum the CR was 94.7% (95% CI: 92.1–98.0, three groups, 406 children) and the ERRgm was 95.0% (95% CI: 90.1–99.9, one study, 203 children); in mixed S. haematobium/S. mansoni infections, the CR was 67.6% (95% CI: 53.6–82.4, three studies, 342 children) and the ERRgm 98.0% (95% CI: 90.9–99.9, one study, 54 children).
Using the reported CR and ERRgm with log-transformation calculation from 34 study arms (Fig. 3), a logarithmic correlation was found (y = 0.158 ln(x) + 0.995, R 2 = 0.34) with which we extrapolated the corresponding predicted ERRgm (pERRgm) for all PZQ groups, whatever the original calculation method. As a result, in PZQ 40 m/kg groups, the overall pERRgm was 94.7% (95% CI: 92.7–96.4) for S. haematobium, 95.3% (95% CI: 94.2–96.2) for S. mansoni, 93.4% (95% CI: 89.9–96.2) for S. mansoni/S. haematobium, and 98.7% (95% CI: 98.3–99.2) for S. japonicum (Table 3).
The ratio between predicted and reported log-transformed ERRs averaged 1.1 (0.93–1.4). The sensitivity analysis restricted to the studies reporting log-transformed ERRgm showed that the pERRgm was on average 0.8% higher than the reported ERRgm (using logarithmic transformation). Similarly, the pERRgm was on average 24% higher than the reported ERRgm based on uncured patients, 12% higher than the reported ERRgm with unspecified method and 3% higher than the reported ERRam.
Based on reported ERRs (all methods), 45% (26/47) of the study arms treated with PZQ 40 mg/kg for any species would not meet the 90% ERR threshold, compared to 15% (7/47) after statistical correction (Additional file 3: Table S3).
Efficacy of PZQ 40 mg/kg by species and age groups
Schistosoma haematobium
The CR was 87.3% (95% CI: 85.7–88.9, 2 groups) in preschool-aged children, and 71.4% (95% CI: 62.3–80.5, 22 groups) in school-aged children; the pERRgm was 97.1% (95% CI: 97.1–97.7) and 94.4% (95% CI: 92.4–96.3), respectively. The mixed-effect model did not detect any difference in CR or pERRgm between preschool- and school-aged children (P = 0.309, P = 0.490, respectively), syrup and tablet formulation (P = 0.925, P = 0.949), week of assessment (P = 0.287, P = 0.337), light vs more intense infection (P = 0.199, P = 0.086), one vs multiple samples (P = 0.723, P = 0.660).
Schistosoma mansoni
The CR was 82.0% (95% CI: 72.6–90.0, 9 groups) in preschool-aged children, and 74.4% (95% CI: 68.8–79.5, 25 groups) in school-aged children; the pERRgm was 96.4% (95% CI: 72.6–90.0) and 94.9% (95% CI: 93.6–95.9), respectively. The mixed-effect model did not detect any difference in CR or pERRgm between preschool- and school-aged children (P = 0.982, P = 0.895, respectively), syrup and tablet formulation (P = 0.956, P = 0.959), week of assessment (P = 0.791, P = 0.784), light vs more intense infection (P = 0.932, P = 0.837), one vs multiple samples (P = 0.741, P = 0.631).
Schistosoma mansoni/S. haematobium
The CR was 71.9% (95% CI: 61.8–87.7) in school-aged children under 10 years old in three studies, and 61.0% (95% CI: 45.5–76.6) in older school-aged children in two studies. The ERR was only recorded in one study for under 10’s and was 98.0% (95% CI: 94.6–100). The overall CR was 67.6% (95% CI: 54.1–80.7, 5 groups) and the corresponding pERRgm was 93.4% (95% CI: 89.9–96.2). Using multivariate analysis, no correlation was detected between age and CR (P = 0.625).
Schistosoma japonicum
The CR was 92.1% (95% CI: 86.8–97.4) in school-aged children under 10 years old in one study, and 96.0% (95% CI: 94.0–98.0) in older schoolchildren in two studies. The overall CR was 94.7% (95% CI: 92.2–98.0), the reported ERR was 95.0% (95% CI: 90.1–99.9) and the pERRgm was 98.7% (95% CI: 98.3–99.2).
PZQ 40 mg/kg vs comparators
In studies comparing PZQ 40 mg/kg to other PZQ regimens for treating S. haematobium, and using meta-analysis regression (Additional file 4: Figure S1), no difference was detected vs PZQ 30 mg/kg (RR = 0.94, 95% CI: 0.87–1.01, P = 0.106) and split-dose PZQ 2*20 mg/kg (RR = 0.98, 95% CI: 0.94–1.04, P = 0.590); subjects treated with PZQ 40 mg/kg were at a significantly lower risk of failure than artesunate alone (RR = 0.54, 95% CI: 0.36–0.83, P = 0.005) or in combination (AS + SP, RR = 0.83, 95% CI: 0.79–0.88, P = 0.001; AS + MQ, RR = 0.69, 95% CI: 0.47–1.02, P = 0.066), mefloquine alone (RR = 0.24, 95% CI: 0.10–0.58, P = 0.001); metrifonate 10 mg/kg (RR = 0.15, 95% CI: 0.04–0.58, P = 0.005), albendazole (RR = 0.43, 95% CI: 0.30–0.61, P = 0.001). There was no difference between PZQ 40 mg/kg and PZQ 60 mg/kg, P = 0.750; ASMQ + PZQ, P = 0.906; PZQ + MQ, P = 0.499; albendazole + PZQ 40 mg/kg, P = 0.561. Only PZQ 40 mg/kg in combination with artesunate performed better than PZQ 40 mg/kg (RR = 1.17, 95% CI: 1.03–1.33, P = 0.019).
Similarly, patients treated with PZQ 40 mg/kg for S. mansoni (Additional file 5: Figure S2), were at a significantly lower risk of failure than lower doses of PZQ (10 mg/kg, RR = 0.35, 95% CI: 0.22–0.55; 20 mg/kg, RR = 0.56, 95% CI: 0.41–0.75; 30 mg/kg, RR = 0.81, 95% CI: 0.70–0.92); artesunate combined with SP (RR = 0.59, 95% CI: 0.46–0.75, P = 0.001) or artesunate with sulfalene (RR = 0.21, 95% CI: 0.12–0.34, P = 0.001), mirazid 300 mg/kg (RR = 0.15, 95% CI: 0.06–0.35, P = 0.001), albendazole (RR = 0.60, 95% CI: 0.43–0.84, P = 0.003), arachidonic acid (RR = 0.72, 95% CI: 0.55–0.96, P = 0.023). No difference was detected between PZQ 40 mg/kg formulated as tablet or syrup (RR = 1.01, 95% CI: 0.88–1.15, P = 0.884), PZQ 60 mg/kg (RR = 1.05, 95% CI: 0.98–1.12, P = 0.141), PZQ 2*40 mg/kg (RR = 1.03, 95% CI: 0.96–1.10, P = 0.401), PZQ 40 mg/kg + albendazole (RR = 1.01, 95% CI: 0.78–1.31, P = 0.921), PZQ + arachidonic acid (RR = 1.29, 95% CI: 0.88–1.88, P = 0.193) and oxamniquine at 15, 30, 40, 50 mg/kg (P = 0.254, P = 0.744, P = 0.080, P = 0.056, respectively).
No difference was detected in mixed S. mansoni/S. haematobium infection between PZQ 40 mg/kg and PZQ 10 mg/kg (RR = 0.15, 95% CI: 0.02–1.08, P = 0.060), 20 mg/kg (RR = 0.63, 95% CI: 0.35–1.15, P = 0.135), 30 mg/kg (RR = 0.87, 95% CI: 0.67–1.12, P = 0.278), split dose 2*20 mg/kg (RR = 1.07, 95% CI: 0.88–1.31, P = 0.494), and oltipraz 30 mg/kg (RR = 1.05, 95% CI: 0.92–1.19, P = 0.481).
No difference was detected in patients treated for S. japonicum between PZQ 40 mg/kg and PZQ 60 mg/kg (RR = 1.02, 95% CI: 0.97–1.07, P = 0.461) or PZQ 40 mg/kg + albendazole (RR = 0.99, 95% CI: 0.94–1.04, P = 0.767).
Safety: adverse events (AEs)
Overall, 22 studies recorded AEs in 6,713 children. One additional tolerability study was included [68] to the 21 studies considered for the efficacy analysis. The proportion of children treated with PZQ 40 mg/kg was 67% (n = 4,480), 22% of whom were preschool-aged (n = 980). The syrup formulation was tested on half of the pre-school aged children. Out of the three studies reporting AEs, one compared PZQ 40 mg/kg tablet and syrup formulation [24]. The two other studies were not comparative: one tested PZQ 40 mg/kg tablet [22] and the other one the PZQ 40 mg/kg liquid syrup formulation [23]. Five thousand, nine hundred and twenty-six AEs were reported within 48 h of treatment in PZQ 40 mg/kg recipients. Most of the signs and symptoms recorded were gastro-intestinal, neurological and dermatological.
On average 55.5% (95% CI: 39.4–78.2) of the 1,606 patients treated in 10 studies with PZQ 40 mg/kg experienced at least one of the AEs listed (Fig. 4). Signs and symptoms were not sought systematically in all studies. The incidence of AEs ranged from 6.1% for itching/rash (studied in 10 studies) to 35.5% for drowsiness (studied in 3 studies). In preschool-aged children in Uganda [18], no significant difference was detected between PZQ 40 mg/kg tablet (n = 254) and syrup (n = 249) in 15 different signs or symptoms screened.
One study analyzed age trends in schoolchildren aged between 6 and 19 years old [46]. Authors found more AEs in older subjects (P = 0.008) and differences between sites.
Summary of efficacy and safety finding in trials in preschool-aged children: a narrative review
Five studies [16–20] enrolled the totality of the 1,694 preschool-aged children, of whom 89% (n = 1,506) were assessed for efficacy, 988 for S. mansoni and 179 for S. haematobium treated with PZQ 40 mg/kg (90% with tablet formulation).
In Navaratnam et al. [24], 203 preschool-aged children aged 1.5 to 5 years were randomly assigned to PZQ 40 mg/kg (crushed) tablet or syrup formulation. The pre-treatment arithmetic mean egg count was 317.6 in the tablet group and 292.1 in the syrup group. No difference was detected between the syrup and tablet formulations for CR (80.9 vs 81.7%) and ERRam (86.1 vs 89.0%). There was a significant correlation between pre-treatment infection intensity and efficacy when assessed as CR: 88.6% in light, 74.5% in moderate and 67.4% in heavy infection. Non-compliance to PZQ syrup was 11.1% (95% CI: 8.6–14.0) and that of PZQ tablet was 14.7% (95% CI: 12.0–18.0) with no significant difference detected (OR = 1.33, 95% CI: 0.93–1.92, P =0.110). Infants 12 months of age were 20.3 times more likely (P = 0.001) to react negatively to the syrup formulation than 5 year olds. Amelioration of vomiting (OR = 1.65, P = 0.006) and peri-rectal bleeding (OR = 1.40, P = 0.007) was significantly higher with crushed PZQ tablet. The most common AEs in both treatment arms were dizziness, drowsiness and fatigue. This was the only study to report dizziness in preschool-aged children, and the incidence seemed to be higher than what reported in 13 studies in school-aged children (Table 4).
In Sousa-Figueiredo et al. [26], 305 patients aged from 5 months to 7 years were treated for S. mansoni. The overall pre-treatment egg count was 144.1 or 5.99 eggs using the arithmetic and geometric mean respectively. Children under 4 years-old had a lower CR (40.8%) and ERRam (75.4%), than 4 to 7 years-old (60.8 and 82.9%, respectively). Children with history of previous treatment had a lower CR (41.7%) and ERRam (72.8%) than treatment-naive children (77.6 and 92.1%, respectively). PZQ tablets proved to be safe, with only mild, transient reported adverse events. At the6-month and 12-month follow-up times, the number of cases of dizziness, sleepiness, fatigue, cramps, nausea, sweating and night fevers post-treatment decreased significantly compared to baseline. No symptom became more prevalent with time.
In Nalugwa et al. [25], Ugandan preschool-aged children aged from 12 to 60 months were randomized to receive PZQ 40 mg/kg or 2*40 mg/kg tablets (2 weeks apart). By week 4, no difference was detected between treatment for CR (83.2 and 85.5%, P = 0.390, respectively) or ERRgm (98.9 and 99.3%, respectively). Efficacy was lower in children with higher infection intensity and higher in younger children.
Two studies treated preschool-aged children for S. mansoni and S. haematobium with PZQ 40 mg/kg tablet (Côte d’Ivoire, n = 53 [16]) and syrup (Niger, up to 67 months, n = 249 [17]). In Côte d’Ivoire the reported efficacy was high against both S. mansoni (CR, 88.6%; ERR, 96.7%) and S. haematobium (CR, 88.9%; ERR, 98.0%); in Niger, the reported efficacy was comparatively lower for both S. haematobium (CR, 85.7%; ERR, 69.4%) and S. mansoni (CR, 75.0%; ERR, 66.7%). Both trials reported only mild AEs (only one case of face and body inflammation that was graded as a moderately severe, [16]) and no serious AE.
Discussion
This analysis is based on a systematic review of comparative and non-comparative studies of PZQ for treating intestinal and urinary schistosomiasis, and concentrates on the efficacy and safety of the WHO-recommended standard dose of 40 mg/kg in preschool- and school-aged children. The main question that we attempted to answer was whether efficacy varied with the subject’s age, and more specifically if PZQ at 40 mg/kg is as effective in preschool-aged children as in older children, so that it can be used more broadly in this younger age category. These analyses do not indicate any age-effect on efficacy, whether expressed as CR or ERRgm, or a dose-effect between 40 and 60 mg/kg, for any Schistosoma species. However, one should bear in mind the limitations of this aggregate-data meta-analysis.
First, only five studies compared efficacy explicitly between age-groups [25, 26, 38, 52, 54]. Secondly, age trends could not be calculated reliably because age categories as reported in the papers are often broad and inconsistent across studies, so that the calculated median age may not reflect the range of responses within each individual group. Following the WHO age classification for PC, the preschool-aged children age-group should include 1–4-year-olds and the school-aged group 5–14-year-olds. Thirdly, preschool-aged children are under-represented: 11% of all subjects treated with PZQ 40 mg/kg. Lastly, and importantly, methodology was insufficiently standardized: studies differed in terms of diagnostic approach used (number of samples and smears), outcome measures (CR, ERR using geometric or arithmetic mean), duration of follow-up, and safety reporting. ERR was predominantly calculated using geometric means, which are less apt to identify outliers (patients responding less well) and tend to show higher efficacy, compared to arithmetic means or individual-patient response distributions [3, 69]. It is therefore difficult to apply the WHO-recommended threshold of 90% ERRam [3] to the results of these studies and meta-analysis. Moreover, when the geometric mean egg count was used, some studies calculated the ERR using the geometric mean as the difference between egg counts on all the patients, pre-treatment, and only the positive subjects at the time of the endpoint assessment. This method is inappropriate and biases the result because the denominator changes from the baseline to the endpoint time, overestimates the egg counts post-treatment and underestimates efficacy (in our calculations here by ~ 24%).
To mitigate these shortcomings, we recalculated the ERRgm and applied multivariate analyses. The predicted ERRgm (pERRgm) provided a good fit with the reported ERRgm using log-transformation; only three data points (the lowest reported ERRgm) out of 34 fell well below the trend line, which demonstrates a good regression fit. Of these, two are from the same study (S. mansoni infection, moderate intensity, Kenya [62]). There might be multiple reasons for this. One is mathematics (the geometric mean wipes out extreme values, so the contribution of individual outliers in the response distribution to the overall response is minimised). Another one is genetic heterogeneity between schistosome populations from different geographical regions, which means that otherwise generalizable trends might not apply to some specific local situations. A third one is the background prevalence of infection (which could not be estimated here, but appears to play an important role [10]). Predicting ERRgm from CR has therefore its limitations, but it allowed including a broader range of studies and a larger number of subjects that would have otherwise been possible, making it possible to expand the utility and comparability of older studies; it is expected that this will not be required with newer studies adopting the WHO recommendation to express results as ERRam [3].
With respect to the WHO-recommended threshold of 90% efficacy by ERRam, and with all the above-mentioned provisos, using our statistical correction, we found that 85% of the study arms treated with PZQ 40 mg/kg for any species would meet this criterion.
The multivariate analyses with random effect on the study site to account for differences between studies using PZQ 40 mg/kg in S. mansoni or S. haematobium found no relationship between efficacy (whether measured as CR or ERR), and age (preschool vs school children aged), duration of follow-up (one vs two months), formulation (syrup vs tablet), diagnostic approach (single vs multiple smears), and infection intensity (light vs others). It is however worth noting that the effects of risk factors on efficacy varied across individual studies. Sousa-Figueiredo et al. (Uganda, S. mansoni) [26] found that the risk of not clearing the infection was significantly higher in children less than four years-old than four to seven year-old by a factor of 3.5, and in children with heavy infections by a factor of circa two. Thiong’o et al. [62] (Kenya, 5–9, 10–14, 15+ years) found a significant trend in four study sites for efficacy measured as CR to decrease with age: ranging 59–92, 72–79, 80–93, 52–78%, and ERR ranging 99–100, 99–100, 93–98, 75–91); of note, older children were more heavily infected and had higher ERRgm than younger children with lower infection intensity before treatment. Gryseels et al. [54] in Burundi found opposite trends in CRs after treatment with doses of 20, 30 and 40 mg/kg in subjects aged < 20 (from ~ 52 to 78%) and 20+ years (from 80 to ~ 94%). A recent study conducted on 508 Zimbabwean children treated with PZQ 40 mg/kg for S. haematobium not included in this review [70] found no difference in CR and ERR between children aged one to five (both 100%) and six to 10 years (94% and 97.9%, respectively). A previous systematic review and meta-analysis [10] which compared CR in similar numbers of participants (1,026 preschool-aged and 12,906 school-aged children), also did not detect significant differences in the combined CRs for S. mansoni and S. haematobium (73.6 vs 71.1%, respectively), but rather found a correlation between lower CR and higher background infection prevalence, especially for younger children. Here, we confirmed and extended these findings by including also ERRgm (see above), though both CR and ERRgm are probably inaccurate for the reasons listed above. These disparate findings likely reflect a range of situations in terms of infection prevalence and intensity in younger and older children which also depends on the deployment and coverage of preventive chemotherapy cycles in the population.
These findings must be considered in the light of the current imperfect test-of-cure; counting eggs in excreta (using the available low-sensitivity methods) cannot distinguish between definitive (adult worm killing) and temporary effects (suspended egg excretion). These methods, while useful in quantifying effects of PC programmes at the population level, are inaccurate when it comes to assess the real drug efficacy and detect early signals of reduced efficacy and potential resistance.
The other question was whether higher levels of efficacy could be achieved using 60 mg/kg instead of 40 mg/kg (and in which age-group). No evidence of difference emerged from this meta-analysis in children or previous meta-analyses in overall population [12–14]. However, a definitive conclusion cannot be reached on this question, both because the numbers are relatively small (S. mansoni: PZQ 40 mg/kg, n = 492 vs PZQ 60 mg/kg, n = 473; S. haematobium: PZQ 40 mg/kg, n = 74 vs PZQ 60 mg/kg, n = 77), and because ERRs do not capture the distribution of individual responses, and particularly outliers [69]. We also know that better efficacy can be achieved with longer treatment and higher doses - conditions that are however not feasible in routine PC.
Compared to our previous systematic review and meta-analysis, this was focused on the efficacy and safety of PZQ in children; here, the calculated median age was 10 years old, and the range across all studies was one to 19 years, for a total of 14,340 subjects with an efficacy outcome. The previous analysis (n = 19,499) had all treatments and age-groups (age range 1–75 years old). In addition, here we explored different approaches for ERR calculations methods and applied a statistical correction to standardize ERR outcomes.
Praziquantel was well tolerated in all age groups, though approximately half of the subjects experienced an adverse event. It must be however noted that safety was variably and inconsistently assessed; few AEs were assessed systematically across most studies. The frequency of certain AEs in each age-group may simply reflect the fact that these were recorded in certain studies, and not others. Also, it is very difficult to detect true signal from background noise: the absence of pre-exposure information on presence and severity of signs/symptom, prevents assessing whether the events reported were related or not to treatment. It is all-important that clinical researchers be reminded of the critical relevance of collecting safety information in the controlled environment of clinical trials, even for drug which are widely regarded as well-tolerated: this is especially true for drugs, like praziquantel, that are used predominantly in children, who might not complain and cannot verbalize discomfort, with the risk of risks being underrated events being underreported [71]. A simple way to doing that is to collect and grade signs and symptoms at enrolment to allow comparison between pre- and post-drug exposure, to identify treatment-emergent signs and symptoms. Child-friendly questionnaires including drawings should be considered, especially in young children.
Conclusions
In summary, PZQ at the standard dose of 40 mg/kg was generally effective at reducing intensity infection of all Schistosoma species at all ages of those studied, but efficacy varied across studies and age-ranges. This conclusion should be tempered to reflect the above-mentioned limitations and will require further studies and pooling of data for an individual patient data meta-analysis. It is indeed possible that exposure to infection and infection intensity increase with age in the absence of effective PC. This confounds analysis and makes it difficult to conclude reliably as to whether the same PZQ dose should be applied in preschool-aged children. It is also worth mentioning that these conclusions have practical implications for the solid oral form of praziquantel, as other formulations are not recommended for use by the WHO. This report also highlights the need for consistent methodologies to be used in designing clinical trials for schistosomiasis to assess and report on efficacy and safety.
Abbreviations
- AE:
-
Adverse event
- AQ:
-
Amodiaquine
- ARA:
-
Arachidonic acid
- AS:
-
Artesunate
- CI:
-
Confidence interval
- CR:
-
Cure rate
- epg:
-
Eggs per gram
- ERR:
-
Egg reduction rate
- ERRam:
-
Egg reduction rate using arithmetic mean
- ERRgm:
-
Egg reduction rate using geometric mean
- OX:
-
Oxamniquine
- PC:
-
Preventive chemotherapy
- pERRgm:
-
Predicted egg reduction rate using geometrical mean
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PZQ:
-
Praziquantel
- RR:
-
Risk ratio
- sh:
-
S. haematobium
- sj:
-
S. japonicum
- sm:
-
S. mansoni
- SP:
-
Sulfadoxine-pyrimethamine
- WHO:
-
World Health Organization
References
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25.
World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. 2006. Available: http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf (Accessed 2 May 2014).
World Health Organization. Assessing the Efficacy of Anthelminthic Drugs Against Schistosomiasis and Soil-Transmitted Helminthiases. 2013. Available: http://apps.who.int/iris/bitstream/10665/79019/1/9789241564557_eng.pdf (Accessed June 2016).
World Health Organization. Report of a meeting to review the results of studies on the treatment of schistosomiasis in pre-school-age children. 2010. Available: http://whqlibdoc.who.int/publications/2011/9789241501880_eng.pdf (Accessed 2 May 2014).
Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197–205.
Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23:83–6.
World Health Assembly resolution. 2012. WHA65.21 http://www.who.int/neglected_diseases/Schistosomiasis_wha65/en/. Accessed 2 June 2016.
Sousa-Figueiredo JC, Betson M, Stothard JR. Treatment of schistosomiasis in African infants and preschool-aged children: downward extension and biometric optimization of the current praziquantel dose pole. Int Health. 2012;4:95–102.
World Health Organization. Schistosomiasis: number of people treated worldwide in 2013. Wkly Epidemiol Rec. 2015;5:25–32.
Stothard JR, Sousa-Figueiredo JC, Navaratnam A. Advocacy, policies and practicalities of preventive chemotherapy campaigns for African children with schistosomiasis. Expert Rev Anti Infect Ther. 2013;11:733–52.
Olliaro P, Delgado-Romero P, Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemother. 2014;69:863–70.
Danso-Appiah A, Olliaro P, Utzinger J. Drugs for treating Schistosoma mansoni infection. Cochrane Database Syst Rev. 2013;2:CD000528.
Kramer CV, Zhang F, Sinclair D, Olliaro PL. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev. 2014;8:CD000053.
Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8:e3286.
de Vlas SJ, Gryseels B, van Oortmarssen GJ, Polderman AM, Habbema JD. A pocket chart to estimate true Schistosoma mansoni prevalences. Parasitol Today. 1993;9:305–7.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Knopp S, Hattentorf J, Speich B, Rinaldi L, Mohammed KA, Khamis IS, et al. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. Clin Infect Dis. 2010;51:1420–8.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Juni B, Altman DG. Egger M Systematic reviews of healthcare: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6.
Inyang-Etoh PC, Ejezie GC, Useh MF, Inyang-Etoh EC. Efficacy of a combination of praziquantel and artesunate in the treatment of urinary schistosomiasis in Nigeria. Trans R Soc Trop Med Hyg. 2009;103:38–44.
Pocock SJ, Travison TG, Wruck LM. How to interpret figures in reports of clinical trials. BMJ. 2008;336:1166–9.
Coulibaly JT, N’gbesso YK, Knopp S, Keiser J, N’Goran EK, Utzinger J. Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6:e1917.
Garba A, Lamine MS, Djibo A, Tahirou A, Aouami MA, Alfari A, et al. Safety and efficacy of praziquantel syrup (Epiquantel®) against Schistosoma haematobium and Schistosoma mansoni in preschool-aged children in Niger. Acta Trop. 2013;128:318–25.
Navaratnam AM, Sousa-Figueiredo JC, Stothard JR, Kabatereine NB, Fenwick A, Mutumba-Nakalembe MJ. Efficacy of praziquantel syrup versus crushed praziquantel tablets in the treatment of intestinal schistosomiasis in Ugandan preschool children, with observation on compliance and safety. Trans R Soc Trop Med Hyg. 2012;106:400–7.
Nalugwa A, Nuwaha F, Tukahebwa EM, Olsen A. Single versus double dose praziquantel comparison on efficacy and schistosoma mansoni re-infection in preschool-age children in Uganda: a randomized controlled trial. PLoS Negl Trop Dis. 2015;9:e0003796.
Sousa-Figueiredo JC, Betson M, Atuhaire A, Arinaitwe M, Navaratnam AM, Kabatereine NB, et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis. 2012;6:e1864.
Borrmann S, Szlezak N, Faucher JF, Matsiegui PB, Neubauer R, Binder RK, et al. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double-blind, randomized, placebo-controlled study. J Infect Dis. 2001;184:1363–6.
Davis A, Biles JE, Ulrich AM. Initial experiences with praziquantel in the treatment of human infections due to Schistosoma haematobium. Bull World Health Organ. 1979;57:773–9.
De Clercq D, Vercruysse J, Kongs A, Verlé P, Dompnier JP, Faye PC. Efficacy of artesunate and praziquantel in Schistosoma haematobium infected schoolchildren. Acta Trop. 2002;82:61–6.
Keiser J, N’Guessan NA, Adoubryn KD, Silué KD, Vounatsou P, Hatz C. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50:1205–13.
Keiser J, Silué KD, Adiossan LK, N’Guessan NA, Monsan N, Utzinger J, et al. Praziquantel, mefloquine-praziquantel, and mefloquine-artesunate-praziquantel against Schistosoma haematobium: a randomized, exploratory, open-label trial. PLoS Negl Trop Dis. 2014;8:e2975.
Latham MC, Stephenson LS, Kurz KM, Kinoti SN. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. Am J Trop Med Hyg. 1990;43:170–9.
McMahon JE, Kolstrup N. Praziquantel: a new schistosomicide against Schistosoma haematobium. BMJ. 1979;2:1396–9.
Midzi N, Sangweme D, Zinyowera S, Mapingure MP, Brouwer KC, Munatsi A, et al. Efficacy and side effects of praziquantel treatment against Schistosoma haematobium infection among primary school children in Zimbabwe. Trans R Soc Trop Med Hyg. 2008;102:759–66.
N’Goran EK, Utzinger J, Gnaka HN, Yapi A, N’Guessan NA, Kigbafori SD, et al. Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am J Trop Med Hyg. 2003;68:24–32.
Oyediran AB, Kofie BA, Bammeke AO, Bamgboye EA. Clinical experience with praziquantel in the treatment of Nigerian patients infected with S. haematobium. Arzneimittelforschung. 1981;31:581–4.
Ojurongbe O, Sina-Agbaje OR, Busari A, Okorie PN, Ojurongbe TA, Akindele AA. Efficacy of praziquantel in the treatment of Schistosoma haematobium infection among school-age children in rural communities of Abeokuta. Nigeria Infect Dis Poverty. 2014;3:30.
Ouldabdallahi M, Ousmane B, Ouldbezeid M, Mamadou D, Konaté L, Chitsulo L. Comparison of the efficacy and safety of praziquantel administered in single dose of 40 versus 60 mg/kg for treating urinary schistosomiasis in Mauritania. Bull Soc Pathol Exot. 2013;106:167–9.
Senghor B, Diaw OT, Doucoure S, Sylla SN, Seye M, Talla I, et al. Efficacy of praziquantel against urinary schistosomiasis and reinfection in Senegalese school children where there is a single well-defined transmission period. Parasit Vectors. 2015;8:362.
Sissoko MS, Dabo A, Traore H, Diallo M, Traoré B, Konaté D, et al. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One. 2009;4:e6732.
Stete K, Krauth SJ, Coulibaly JT, Knopp S, Hattendorf J, Müller I, et al. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with praziquantel in school-aged children. Parasit Vectors. 2012;5:298.
Tchuente LA, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J Trop Med Hyg. 2004;71:778–82.
Wilkins HA, Moore PJ. Comparative trials of regimes for the treatment of urinary schistosomiasis in The Gambia. J Trop Med Hyg. 1987;90:83–92.
Belizario Jr VY, Amarillo MLE, Martinez RM, Mallari AO, Tai CMC. Efficacy and safety of 40 mg/kg and 60 mg/kg single doses of praziquantel in the treatment of schistosomiasis. Pediatr Infect Dis J. 2008;3:27–34.
Olliaro PL, Vaillant MT, Belizario VJ, Lwambo NJ, Ouldabdallahi M, Pieri OS, et al. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis. 2011;5:e1165.
Olds G, King C, Hewlett J, Olveda R, Wu G, Ouma J, et al. Double-blind placebo controlled study of the concurrent administration of albendazole and praziquantel in school-aged children infected with schistosomiasis. Am J Trop Med Hyg. 1998;179:309–10.
Barakat R, El Morshedy H. Efficacy of two praziquantel treatments among primary school children in an area of high Schistosoma mansoni endemicity, Nile Delta. Egypt Parasitology. 2011;138:440–6.
Barakat R, Abou El-Ela NE, Sharaf S, El Sagheer O, Selim S, Tallima H, et al. Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. Am J Trop Med Hyg. 2015;92:797–804.
Berhe N, Gundersen SG, Abebe F, Birrie H, Medhin G, Gemetchu T. Praziquantel side effects and efficacy related to Schistosoma mansoni egg loads and morbidity in primary school children in north-east Ethiopia. Acta Trop. 1999;72:53–63.
Botros S, Sayed H, El-Dusoki H, Sabry H, Rabie I, El-Ghannam M, et al. Efficacy of mirazid in comparison with praziquantel in Egyptian Schistosoma mansoni-infected school children and households. Am J Trop Med Hyg. 2005;72:119–23.
Degu G, Mengistu G, Jones J. Praziquantel efficacy against schistosomiasis mansoni in schoolchildren in north-west Ethiopia. Trans R Soc Trop Med Hyg. 2002;96:444–5.
Erko B, Degarege A, Tadesse K, Mathiwos A, Legesse M. Efficacy and side effects of praziquantel in the treatment of schistosomiasis mansoni in schoolchildren in Shesha Kekele Elementary School, Wondo Genet, Southern Ethiopia. Asian Pac J Trop Biomed. 2012;2:235–9.
Friis H, Byskov J. The effect of praziquantel against Schistosoma mansoni-infections in Botswana. Trop Geogr Med. 1989;41:49–51.
Gryseels B, Nkulikyinka L, Coosemans MH. Field trials of praziquantel and oxamniquine for the treatment of schistosomiasis mansoni in Burundi. Trans R Soc Trop Med Hyg. 1987;81:641–4.
Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, et al. Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. Am J Trop Med Hyg. 1997;56:511–4.
Massoud AA, el Kholy AM, Anwar WA. Assessment of efficacy of praziquantel against Schistosoma mansoni infection. J Trop Med Hyg. 1984;87:119–21.
Metwally A, Bennett J, Botros S, Ebeid F, el attar Gel D. Impact of drug dosage and brand on bioavailability and efficacy of praziquantel. Pharmacol Res. 1995;31:53–9.
Mohamed AA, Mahgoub HM, Magzoub M, Gasim GI, Eldein WN, Ahmed Ael A, et al. Artesunate plus sulfadoxine/pyrimethamine versus praziquantel in the treatment of Schistosoma mansoni in eastern Sudan. Trans R Soc Trop Med Hyg. 2009;103:1062–4.
Obonyo CO, Muok EM, Mwinzi PN. Efficacy of artesunate with sulfalene plus pyrimethamine versus praziquantel for treatment of Schistosoma mansoni in Kenyan children: an open-label randomised controlled trial. Lancet Infect Dis. 2010;10:603–11.
Selim S, El Sagheer O, El Amir A, Barakat R, Hadley K, Bruins MJ, et al. Efficacy and safety of arachidonic acid for treatment of Schistosoma mansoni-infected children in Menoufiya, Egypt. Am J Trop Med Hyg. 2014;91:973–81.
Simonsen PE, Nega A, Furu P. Intestinal schistosomiasis among children in a labour village of Wonji Sugar Estate, Ethiopia. East Afr Med J. 1990;67:532–8.
Thiong’o FW, Mbugua GG, Ouma JH, Sturrock RK. Efficacy of oxamniquine and praziquantel in school children from two Schistosoma mansoni endemic areas. East Afr Med J. 2002;79:29–33.
Teesdale CH, Chitsulo L, Pugh RN. Oxamniquine dosage in Malawi. East Afr Med J. 1984;61:40–4.
Utzinger J, N’Goran EK, N’Dri A, Lengeler C, Tanner M. Efficacy of praziquantel against Schistosoma mansoni with particular consideration for intensity of infection. Trop Med Int Health. 2000;5:771–8.
El Tayeb M, Daffalla AA, Kardaman MW, See R, Fenwick A. Praziquantel and oltipraz: the treatment of schoolchildren infected with Schistosoma mansoni and/or Schistosoma haematobium in Gezira, Sudan. Ann Trop Med Parasitol. 1988;82:53–7.
Kardaman MW, Fenwick A, El Igail AB, et al. Treatment with praziquantel of schoolchildren with concurrent Schistosoma mansoni and S. haematobium infections in Gezira, Sudan. J Trop Med Hyg. 1985;88:105–9.
Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. J Trop Med Hyg. 1988;91:13–7.
Jaoko WG, Muchemi G, Oguya FO. Praziquantel side effects during treatment of Schistosoma mansoni infected pupils in Kibwezi. Kenya East Afr Med. 1996;73:499–501.
Olliaro PL, Vaillant M, Diawara A, Coulibaly JT, Garba A, Keiser J, et al. Toward measuring schistosoma response to praziquantel treatment with appropriate descriptors of egg excretion. PLoS Negl Trop Dis. 2015;9:e0003821.
Wami WM, Nausch N, Midzi N, Gwisai R, Mduluza T, Woolhouse ME, Mutapi F. Comparative assessment of health benefits of praziquantel treatment of urogenital schistosomiasis in preschool and primary school-aged children. Biomed Res Int. 2016;2016:9162631.
World Health Organization (WHO). Promoting safety of medicines for children. 2007. http://www.who.int/medicines/publications/essentialmedicines/Promotion_safe_med_childrens.pdf Accessed 21 Sept 2016.
Acknowledgements
We thank A. Garba and A. Montresor for critically reviewing the manuscript. PO is a staff member of the WHO; the authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Funding
The work of JZ was supported financially by the schistosomiasis paediatric consortium (http://www.pediatricpraziquantelconsortium.org) which however was not involved in the analyses and conclusions of this paper.
Availability of data and materials
The authors confirm that all data underlying the findings are fully available without restriction. The data are available as can be found in the publications extracted from eligible studies identified by the Cochrane collaboration through electronic searches from January 1, 1990, up to November 2015 of MEDLINE, EMBASE, LILACS, the Cochrane Infectious Diseases Group’s trials register and the Cochrane Central Register of Controlled Trials (CENTRAL) using the search term ‘praziquantel’ published in English, French or Portuguese.
Authors’ contributions
The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication. JZ, PO designed the analysis, interpreted the data and prepared the manuscript; JZ pooled and analysed the data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Patients’ consent for publication was obtained in every study and can be found in the corresponding publications included in this meta-analysis.
Ethics approval and consent to participate
Ethical clearance was obtained in every study and can be found in the corresponding publications included in this meta-analysis. Patients’ consent to participate was obtained in every study and can be found in the corresponding publications included in this meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
PRISMA checklist. (DOC 70 kb)
Additional file 2: Table S2.
Study design characteristics. (DOCX 20 kb)
Additional file 3: Table S3.
Reported efficacy and predicted egg reduction rate by age group and site, PZQ 40 mg/kg. (DOCX 32 kb)
Additional file 4: Figure S1.
Forest plot for PZQ 40 mg/kg and comparator treatments for S. haematobium, cure rate (CR) and risk ratio (RR). Abbreviations: pzq40, praziquantel 40 mg/kg; pzq20, praziquantel 20 mg/kg; pzq30, praziquantel 30 mg/kg; pzq60, praziquantel 60 mg/kg; RR, risk ratio; comp, comparator group; AS, artesunate; MQ, mefloquine; ASMQ, artesunate + mefloquine; ASSP, artesunate + sulfadoxine-pyrimethamine; met10, metrifonate 10 mg/kg. (TIF 7292 kb)
Additional file 5: Figure S2.
Forest plot for PZQ 40 mg/kg and comparator treatments for S. mansoni, cure rate (CR) and risk ratio (RR). I2 is calculated for pooled subgroups as = 100%*(Q - df)/Q, where Q is Cochran’s heterogeneity statistic and df the degrees of freedom. Abbreviations: pzq40, praziquantel 40 mg/kg; pzq10, praziquantel 20 mg/kg; pzq20, praziquantel 20 mg/kg; pzq30, praziquantel 30 mg/kg; pzq60, praziquantel 60 mg/kg; pzq syrup, praziquantel syrup 40 mg/kg; ARA arachidonic acid; ox, oxamniquine; RR, risk ratio; comp, comparator group; I2, Higgins’ I squared. (TIF 8364 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zwang, J., Olliaro, P. Efficacy and safety of praziquantel 40 mg/kg in preschool-aged and school-aged children: a meta-analysis. Parasites Vectors 10, 47 (2017). https://doi.org/10.1186/s13071-016-1958-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1958-7