Abstract
Background and Objective
Viloxazine extended-release (ER) [Qelbree®] is a nonstimulant attention-deficit/hyperactivity disorder (ADHD) treatment. In vitro studies suggested potential for viloxazine to inhibit cytochrome 450 (CYP) enzymes 1A2, 2B6, 2D6 and 3A4. This clinical study therefore evaluated viloxazine ER effects on index substrates for CYP1A2, 2D6, and 3A4, and secondarily evaluated the impact of CYP2D6 polymorphisms on viloxazine pharmacokinetics.
Methods
Thirty-seven healthy subjects received a modified Cooperstown cocktail (MCC; caffeine 200 mg, dextromethorphan 30 mg, midazolam 0.025 mg/kg) on Day 1, viloxazine ER 900 mg/day on Days 3–5, and a combination of viloxazine ER 900 mg and MCC on Day 6. Viloxazine ER effects on MCC substrates were evaluated using analysis of variance. The impact of CYP2D6 genetic polymorphisms on steady-state viloxazine plasma concentrations was evaluated using Student’s t test assessing pharmacokinetic parameter differences between poor versus extensive metabolizers.
Results
The least squares geometric mean ratio [GMR%] (90% CI) of MCC substrate + viloxazine ER/MCC substrate alone for caffeine maximum concentration (Cmax), area under the plasma concentration-time curve from time 0 to the last quantifiable concentration (AUCt), and area under the plasma concentration-time curve from time 0 extrapolated to infinity (AUC∞) was 99.11 (95.84–102.49), 436.15 (398.87–476.92), and 583.35 (262.41–1296.80), respectively; 150.76 (126.03–180.35), 185.76 (155.01–222.61), and 189.71 (160.37–224.42) for dextromethorphan Cmax, AUCt, and AUC∞, respectively; and 112.81 (104.71–121.54), 167.56 (153.05–183.45), and 168.91 (154.38–184.80) for midazolam Cmax, AUCt, and AUC∞, respectively. At steady state, viloxazine least squares GMR (90% CI) for poor/extensive CYP2D6 metabolizers were Cmax 120.70 (102.33–142.37) and area under the plasme concentration-time curve from time 0 to 24 hours (AUC0–24 125.66 (105.36–149.87)).
Conclusion
Viloxazine ER is a strong CYP1A2 inhibitor and a weak CYP2D6 and CYP3A4 inhibitor. CYP2D6 polymorphisms did not meaningfully alter the viloxazine ER pharmacokinetic profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Viloxazine extended-release is a strong inhibitor of cytochrome P450 (CYP) 1A2 and a weak inhibitor of CYP2D6 and CYP3A4. |
The pharmacokinetic profile of viloxazine is not impacted by CYP2D6 polymorphisms to a clinically relevant extent, suggesting that despite being a CYP2D6 substrate, CYP2D6 does not represent the only metabolic pathway for viloxazine, and metabolism pathway switching may take place. |
1 Introduction
The extended-release (ER) capsule formulation of viloxazine (viloxazine ER; Qelbree®) is the first new molecule to be approved for the treatment of attention-deficit/hyperactivity disorder (ADHD) in over a decade in the United States (US). Viloxazine ER is a nonstimulant ADHD treatment approved for use in both children (≥ 6 years of age) and adults. Originally approved in the 1970s in Europe as an immediate-release formulation for the treatment of depression, the current ER formulation prolongs drug release and absorption, allowing for once-daily dosing and providing steadier plasma concentrations [1]. Although classified as a selective norepinephrine reuptake inhibitor, viloxazine also displays serotonergic activity in preclinical experiments [2]. Its mechanistic action to treat ADHD is unclear; however, it is thought to be through inhibiting the reuptake of norepinephrine [3]. In addition to norepinephrine and dopamine, viloxazine increases serotonin in the prefrontal cortex at clinically used doses but does not show relevant activity as a serotonin reuptake inhibitor [1]. Additionally, it shows functional activity as a 5HT2C partial agonist and 5HT7 antagonist in preclinical research [4]. Its more modest affinity at norepinephrine transporters (less potent norepinephrine transporter binding affinity than atomoxetine and reboxetine) is consistent with a low incidence of cardiovascular effects observed in ADHD clinical studies [5]. Pharmacokinetically, viloxazine is primarily metabolized in humans through 5-hydroxylation followed by glucuronidation [6]. Hepatic cytochrome P450 (CYP) 2D6 is the major isoenzyme responsible for hydroxylation to 5-hydroxyviloxazine with minor involvement of CYP1A2, 2B6, 2C9, 2C19, and 3A4; subsequent glucuronidation to 5-hydroxyviloxazine glucuronide (5-HVLX-gluc) is mediated by uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A9 and UGT2B15 [6].
The potential for a drug to produce relevant inhibitory interactions can be evaluated by comparing its maximum therapeutic concentrations (Cmax) to the inhibitory constants (ki) for CYP enzymes seen in in vitro studies. A Cmax/ki ratio > 0.1 indicates potential for the drug to produce an inhibitory effect and warrants further in vivo studies, per US FDA Draft Drug Interaction Studies Guidance for Industry [7]. Viloxazine showed inhibitory ratios > 0.1 for CYP1A2, 2D6, and 3A4 (60.1, 0.587, and 0.278, respectively, based on ratios calculated using ki values of 0.124 μg/mL [caffeine], 12.7 μg/mL [dextromethorphan], and 26.8 μg/mL [midazolam], respectively, and Cmax values for children 6–8 years of age). Therefore, the following clinical study was conducted to better evaluate viloxazine effects on these enzymes.
In general, many drugs interact with the hepatic CYP system (either as substrates, inducers, or inhibitors) [8, 9]. CYP enzymes are especially critical in the metabolism of antipsychotics and antidepressants [10], and the potential for drug interactions with viloxazine ER warrants consideration. Notably, CYP metabolic activity has been shown to vary due to underlying genetic polymorphisms. In particular, CYP2D6, which functions in the metabolism of ~ 20% of commonly used drugs, is highly polymorphic; poor metabolizer (PM), intermediate metabolizer (IM), and extensive metabolizer (EM) phenotypes have been identified based on enzyme activity scores [11, 12]. It is therefore important to consider the metabolic status of CYP2D6 and the potential of drug interactions in patients who are PMs of CYP2D6 when using treatments such as viloxazine ER that are metabolized by or affect this enzyme.
The primary objective of the current study is to assess the effects of viloxazine ER on the pharmacokinetic profiles of caffeine, dextromethorphan, and midazolam, known index substrates for CYP1A2, 2D6, and 3A, respectively (CYP enzymes known to be affected by viloxazine), in healthy adults to evaluate the extent of potential drug interactions. A secondary objective was to evaluate the impact of CYP2D6 genetic polymorphism phenotype (i.e., PMs vs. EMs) on the steady-state pharmacokinetics of viloxazine ER. Safety and tolerability were also evaluated.
2 Methods
The trial conduct was reviewed and approved by IntegReview Institutional Review Board (IRB; Austin, TX, USA) and conducted in accordance with the Helsinki Declaration and the International Council for Harmonisation Note for Guidance on Good Clinical Practice. All subjects provided written informed consent. The trial was conducted by Worldwide Clinical Trials Early Phase Services (San Antonio, TX, USA).
2.1 Study Design
This was a single-center, open-label study in healthy subjects with three sequential treatment periods (Fig. 1).
Study schematic. Sequence of the trial with three treatment periods: Period 1 = single-dose MCC only; Period 2 = multiple doses of viloxazine ER (900 mg/day); Period 3 = combination of viloxazine ER (900 mg) and single-dose MCC (MCC was administered 4.5 h after viloxazine ER). ER extended release, MCC modified Cooperstown cocktail, PK pharmacokinetic
All medications were orally administered with 240 mL of water. Apart from this, to minimize variability in absorption, fluid was restricted from 1 h before to 1 h after dosing, and participants were prohibited from eating for at least 4 h after dosing. In the morning of Period 1 (Day 1), following a minimum 10-h overnight fast, subjects received a single dose of a modified Cooperstown cocktail (MCC) consisting of caffeine 200 mg, dextromethorphan 30 mg, and midazolam 0.025 mg/kg, followed by 48 h of pharmacokinetic blood sampling (see Sect. 2.4) [13]. In Period 2 (Days 3–5), subjects received viloxazine ER 900 mg, administered once daily in the morning, for three days. In Period 3 (Day 6), following breakfast, subjects received viloxazine ER 900 mg in the morning, then the same MCC as in Period 1, administered 4.5 h after viloxazine ER to allow the time of the maximum plasma concentration (Tmax) of viloxazine and the probe substrates to coincide.
Viloxazine ER 900 mg (greater than the maximal dose studied in adults with ADHD [600 mg]) was used in adults to achieve a Cmax comparable with that observed in children 6–11 years of age taking viloxazine ER 400 mg (Cmax 7.45 μg/mL; the maximum recommended daily dose in children with ADHD). This was thought to maximize the potential effect of viloxazine ER on CYP enzyme activity, increase the relevance of the study across the populations in which viloxazine is used, and maximize the potential for drug interaction. MCC doses were selected based on previous studies [14,15,16].
2.2 Subjects
Healthy subjects between 18 and 55 years of age were recruited for the trial. Inclusion criteria required subjects to be nonsmokers, have CYP2D6 EM (i.e., normal enzyme activity, EM activity score = 1.5–2.0) or PM (no or very low enzyme activity, PM activity score of 0) genotype [11], and have a body mass index (BMI) of 18–30 kg/m2 (inclusive). Females of childbearing potential were required to have a negative serum pregnancy test prior to receiving study medications and to practice abstinence or use acceptable birth control throughout the study. Exclusion criteria included CYP2D6 ultra-rapid, extensive intermediate or intermediate metabolizers; current presence or history of seizures, cardiac disorders, narrow-angle glaucoma (due to concern with midazolam use) or other significant systemic disease; evidence of infections with HIV or hepatitis B or C; positive alcohol or drug screen, or alcohol or drug abuse (within the past year); routine consumption of caffeine (more than two servings/day) or being prone to caffeine withdrawal headaches; clinically relevant laboratory test results, vital sign, or electrocardiogram (ECG) abnormalities; use of prescription medication (other than topical or hormonal agents) or herbal, food supplement or nonprescription medication other than acetaminophen (past 14 days or within five half-lives, whichever was longer); recent use (within 30 days of screening) of investigational drugs or drugs known to notably induce or inhibit hepatic drug metabolism; being pregnant or breast-feeding; having an allergy to viloxazine-caffeine, dextromethorphan, or midazolam-based products; or any other qualifier that had the potential to interfere with study participation as determined by the investigator. The use of caffeine was also prohibited within 72 h of study Day 1.
2.3 Genotyping
Potential subjects underwent genotype testing for 2D6 and other isoenzyme metabolizer status within 18 months of study Day 1. DNA extraction (using a buccal swab) and CYP2D6 genotype testing were performed by Worldwide Clinical Trials Bioanalytical Services (Austin, TX, USA).
2.4 Sample Collection
Each subject had a total of 57 blood draws (approximately 6 mL/draw in Periods 1 and 3, and 4 mL/draw in Period 2) collected during the study. Blood was collected in Period 1 to analyze the plasma concentrations of CYP substrates and metabolites; sample collection occurred over 48 h beginning on Day 1 at the following 22 time points: time 0 (predose) and 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, 8, 10, 12, 16, 20, 24, 30, 36, and 48 h postdose, with the 48-h postdose blood sample completed prior to first administration of viloxazine ER on the morning of Day 3 (i.e., prior to the start of Period 2). Blood was collected in Period 2 for viloxazine pharmacokinetic analysis, including one trough (predose) blood draw on Day 4, and 12 samples over 24 h on Day 5, at time 0 (predose) and 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 18, and 24 h postdose, with the 24-h postdose blood draw completed prior to viloxazine ER dosing on Day 6 (prior to the start of Period 3). In Period 3 (Day 6), blood was collected for CYP substrates and metabolite pharmacokinetic analysis at the same 22 time points relative to MCC dosing as on Day 1; thus the 0 h (predose) sample on Day 6 was collected approximately 4.5 h following viloxazine ER dosing, immediately prior to the MCC dosing for Period 3. Each pharmacokinetic blood sample was collected in a K2-EDTA tube and gently inverted. Blood samples were then centrifuged at approximately 4°C, and the resulting plasma was aliquoted into two polypropylene screw-cap tubes and frozen at −20°C or lower pending shipment for analysis.
2.5 Bioanalytical Methods
Plasma concentrations of viloxazine, caffeine, dextromethorphan, midazolam, and respective MCC metabolites paraxanthine, dextrorphan, and 1-hydroxymidazolam, were determined by a validated liquid chromatographic and tandem mass spectrometer detection method. Bioanalytical methods for viloxazine plasma concentrations have been previously published [17, 18]. Plasma bioanalysis for caffeine, dextromethorphan, and midazolam, and their respective metabolites, was performed at Worldwide Clinical Trials (Austin, TX, USA) and is described in the electronic supplementary material (ESM). The analytical range for viloxazine was 0.0100–10.0 μg/mL. The analytical ranges for MCC substrates and metabolites were caffeine 50.0–15,000 ng/mL, dextromethorphan 0.0300–30.0 ng/mL, and midazolam 0.200–40.0 ng/mL. All concentrations are reported as freebase.
2.6 Pharmacokinetic and Statistical Analyses
2.6.1 Analysis Populations
The safety population included all subjects who received at least one dose of study medication (i.e., MCC on Day 1). The pharmacokinetic population included all subjects from the safety population who had an adequate pharmacokinetic profile for viloxazine, caffeine, dextromethorphan, or midazolam in the appropriate treatment periods, with no major protocol deviations that could impact pharmacokinetic data (e.g., emesis). The bioavailability population included all subjects from the pharmacokinetic population with an EM phenotype for the specific CYP enzyme under analysis (i.e., PMs were excluded) and who completed all three treatment periods with adequate pharmacokinetic profiles, meaning sufficient pharmacokinetic profiles to calculate Cmax, the area under the plasma concentration-time curve from time 0 to the last quantifiable concentration (AUCt), and area under the plasma concentration-time curve from time 0 extrapolated to infinity (AUC∞) for the substrate of interest from Periods 1 and 3, with no evidence of a major protocol deviation that may have impacted pharmacokinetic data.
2.6.2 Data Analysis
Pharmacokinetic parameters were derived based on concentration-time data from Periods 1 and 3 for MCC substrates and metabolites. Steady-state pharmacokinetic parameters were derived for viloxazine based on concentration-time data from Period 2. Data were analyzed by noncompartmental methods in Phoenix™ WinNonlin® (version 6.3; Certara LP, Princeton, NJ, USA). Statistical analyses were performed in SAS® (version 9.4; SAS Institute Inc., Cary, NC, USA) using the MIXED procedure, and Phoenix™ WinNonlin® using the Linear Mixed Effects module. The statistical analysis results from SAS® were verified against results from WinNonlin®. During the pharmacokinetic analysis, plasma concentrations that were below the limit of quantification (BLQ) were treated as zero from time 0 to the time at which the first quantifiable concentration was observed; embedded and terminal BLQ measures were treated as ‘missing’. AUC0–∞ values resulting from >20% extrapolation were excluded from all summary statistics, analyses, and comparisons. Pharmacokinetic results for viloxazine, MCC substrates, and MCC metabolites were summarized by analyte and period using descriptive statistics. Actual sample times were used for pharmacokinetic and statistical analyses, and mean concentration-time profiles are presented using scheduled collection time. Concentration-time data and pharmacokinetic parameters were further summarized by phenotype (PM vs. EM) when applicable.
2.6.3 Relative Bioavailability Analysis of the Impact of Viloxazine Extended-Release (ER) on the Pharmacokinetics of Caffeine, Dextromethorphan, and Midazolam
The impact of viloxazine ER administration on MCC substrates was evaluated using analysis of variance (ANOVA) on the log-transformed pharmacokinetic parameters Cmax, AUCt, and AUC∞. Log-transformed data were back-exponentiated to the original scale to obtain the least squares geometric mean ratios (GMRs) and 90% confidence intervals (CIs) for the comparison of each MCC substrate administered with viloxazine ER versus each MCC substrate without viloxazine. The same statistical analysis was conducted for the respective MCC metabolites. The absence of an impact of viloxazine ER on MCC substrates and metabolites was claimed if the 90% CIs for these ratios were fully contained within predefined no-difference limits of 80.00–125.00% for Cmax, AUCt, and AUC∞. The coefficient of variation (CV%) for the GMR was calculated as (sqrt(exp(residual for the log-transformed data)−1)) × 100.
2.6.4 Steady-State Attainment Analysis
A steady-state attainment analysis of viloxazine plasma concentration was performed for viloxazine ER treatment based on the pharmacokinetic analysis population using a mixed-effect ANOVA model with contrasts. The analysis was performed on log-transformed trough concentrations on Days 4, 5, and 6. The first contrast compared the mean trough concentration on Day 4 with the pooled mean trough concentration over Days 5 and 6. The second contrast compared the mean trough concentration on Day 5 with the mean trough concentration on Day 6. The first comparison that shows a nonsignificant p-value at α ≥ 0.05 indicates attainment of steady state by that day. If all comparisons are significant, then steady state is not achieved during the study period.
2.6.5 Relative Bioavailability Analysis of the Impact of Cytochrome P450 (CYP) 2D6 Extensive Metabolizer and Poor Metabolizer Polymorphisms on Viloxazine Pharmacokinetics
The CYP2D6 PM versus EM comparative pharmacokinetic population assessed the impact of CYP2D6 genetic polymorphisms on steady-state viloxazine plasma concentrations with viloxazine ER administration in Period 2. Student’s t test for two independent samples assessed pharmacokinetic parameter differences between PMs versus EMs.
2.6.6 Safety Monitoring and Assessments
Safety assessments included physical examination, vital signs, clinical laboratory tests (i.e., serum chemistry, hematology, and urinalysis), and the Columbia-Suicide Severity Rating Scale (C-SSRS) suicidality assessment. Vital signs were assessed at screening, entry, and end of study (EOS). Blood pressure and heart rate were taken after the subject was in a sitting or supine position for a minimum of 5 min. Blood pressure and heart rate were also measured predose and 30 min and 1, 2, 3, and 4 h (± 5 min) following midazolam administration on Days 1 and 6. Clinical laboratory tests were obtained at screening, entry, and EOS. A single 12-lead ECG was performed after the subject had been supine for at least 10 min at screening and EOS. The C-SSRS assessment was administered at screening and EOS. Adverse events (AEs) were classified into standardized terminology from the verbatim description (Investigator term) and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1 and summarized by System Organ Class and Preferred Term. AEs were monitored over the course of treatment beginning from the first dose of study medication; thus, all reported AEs were treatment emergent. Safety and tolerability data are summarized using descriptive statistics.
3 Results
3.1 Subject Disposition and Demographics
Thirty-seven healthy subjects (19 males, 18 females) were enrolled and included in the safety and pharmacokinetic populations. One subject withdrew consent and discontinued the study in Period 2 (Day 5); therefore, 36 subjects were included in the CYP2D6 PM versus EM comparative pharmacokinetic and bioavailability populations. Subjects were (mean ± standard deviation [SD]) 38.3 ± 9.3 years of age (range 22–54) and had a BMI of 26.0 ± 2.3 kg/m2; 64.9% were White and 29.7% were Black or African American; 81.1% were CYP2D6 EMs, and 18.9% were PMs (Table 1).
3.2 Impact of Viloxazine ER on the Pharmacokinetics and Bioavailability of CYP Substrates
Pharmacokinetic parameters for caffeine, dextromethorphan, and midazolam are provided in Table 2, and plasma concentrations over time are shown in Figs. 2, 3, 4a (linear scale) and Figs. 2, 3, 4b (semi-log scale); pharmacokinetic parameters for paraxanthine, dextrorphan, and 1-hydroxymidazolam are provided in the ESM.
3.2.1 Caffeine (CYP1A2)
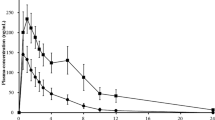
Relative to a single dose of MCC alone, administration of viloxazine ER with MCC did not markedly impact the Tmax (1.5 h after the combination vs. 1 h after MCC) or Cmax of caffeine but did increase total caffeine exposure (AUC) and terminal elimination half-life (t½). The GMR% and 90% CI are displayed in Table 3. Although the 90% CIs for caffeine Cmax were fully contained within the predetermined no-difference limits of 80.00–125.00%, the 90% CIs for AUCt and AUC∞ were completely above these limits (Fig. 2c), indicating that viloxazine ER substantially increases systemic caffeine exposure, albeit due to exclusion of values resulting from extrapolation of > 20%; only three subjects were included in the AUC∞ calculation for caffeine.
Viloxazine ER increased total exposure of CYP1A2 substrate, as shown by mean caffeine concentration-time profiles after administration of MCC alone (Period 1) or after combination of viloxazine ER and MCC (Period 3) on a linear and b semi-logarithmic scales and c caffeine bioavailability, presented as a percentage ratio of the viloxazine ER + MCC combination relative to MCC alone (geometric means ± 90% CIs). Yellow shaded area between the dotted lines represents the predetermined no-difference limits of 80–125%. *n = 34, except AUC∞ n = 3. AUCt area under the concentration-time curve from 0 to the last measurable time, AUC∞ area under the concentration-time curve from 0 to infinity, CIs confidence intervals, Cmax maximum measured plasma concentration, CYP cytochrome P450, ER extended release, MCC modified Cooperstown cocktail
3.2.2 Dextromethorphan (CYP2D6)
Relative to MCC alone, administration of viloxazine ER with MCC resulted in a 1-h prolongation of median dextromethorphan Tmax (4 h after the combination vs. 3 h after MCC alone) as well as a modest increase in Cmax and dextromethorphan AUC (Table 2). The 90% CIs for dextromethorphan Cmax, AUCt, and AUC∞ were completely above the predetermined no-difference limits of 80.00–125.00% (Fig. 3c), indicating that viloxazine ER increases systemic dextromethorphan exposure, but to a lesser degree than its impact on caffeine.
Viloxazine ER modestly increased systemic dextromethorphan (a CYP2D6 substrate) exposure, as shown by mean concentration-time profiles after administration of MCC alone (Period 1) or after combination of viloxazine ER and MCC (Period 3) on a linear scales, b semi-logarithmic scales, and c dextromethorphan bioavailability, presented as a percentage ratio of the viloxazine ER + MCC combination relative to MCC alone (geometric means ± 90% CIs). Yellow shaded area between the dotted lines represents the predetermined no-difference limits of 80–125%. *n = 29, except AUC∞ n = 27. AUCt area under the concentration-time curve from 0 to the last measurable time, AUC∞ area under the concentration-time curve from 0 to infinity, CIs confidence interval, Cmax maximum measured plasma concentration, CYP cytochrome P450, ER extended release, MCC modified Cooperstown cocktail
3.2.3 Midazolam (CYP3A4)
Relative to MCC alone, administration of viloxazine ER with MCC had a minimal impact on median midazolam Tmax (1 h after the combination vs. 0.5 h after MCC) and Cmax but modestly increased midazolam AUC (Table 3). The 90% CIs for midazolam Cmax were fully contained within the predetermined no-difference limits of 80.00–125.00%, but AUCt and AUC∞ were above these limits (Fig. 4c).
Viloxazine ER modestly increased systemic midazolam (a CYP3A4 substrate) exposure and bioavailability, as shown in mean midazolam concentration-time profiles after administration of MCC alone (Period 1) or after combination of viloxazine ER and MCC (Period 3) on a linear and b semi-logarithmic scales, and c midazolam bioavailability, presented as a percentage ratio of the viloxazine ER + MCC combination relative to MCC alone (geometric means ± 90% CIs). Yellow shaded area between the dotted lines represents the predetermined no-difference limits of 80–125%. *n = 35. AUCt area under the concentration-time curve from 0 to the last measurable time, AUC∞ area under the concentration-time curve from 0 to infinity, CIs confidence intervals, Cmax maximum measured plasma concentration, CYP cytochrome P450, ER extended release, MCC modified Cooperstown cocktail
3.3 Impact of CYP2D6 Polymorphisms on the Pharmacokinetics and Bioavailability of Viloxazine
Pharmacokinetic parameters for viloxazine are listed in Table 4 and plasma concentrations over time are shown in Fig. 5a (linear scale) and Fig. 5b (semi-log scale) for CYP2D6 PMs and EMs during Period 2. Relatively tight variability among PMs (approximately 10% increase in CV% vs. EMs) (Table 4) suggests the sample size (n = 7) was sufficient to provide a reliable estimate of the impact of CYP2D6 genetic polymorphisms. Comparisons among Days 4, 5, and 6 (shown in the ESM Table S6) show viloxazine steady state was achieved on the second day of dosing (Day 4; p values > 0.05).
CYP2D6 polymorphisms showed minimal impact on systemic viloxazine exposure and bioavailability, as shown by mean viloxazine concentration-time profiles for CYP2D6 PMs and EMs after administration of viloxazine ER on a linear and b semi-logarithmic scales, and c viloxazine bioavailability, presented as a percentage ratio of CYP2D6 PMs relative to CYP2D6 EMs (geometric means ± 90% CIs). Yellow shaded area between the dotted lines represents the predetermined no-difference limits of 80–125%. AUC24 area under the concentration-time curve from 0 to 24 h, CIs confidence intervals, Cmax maximum measured plasma concentration, Cmin minimum measured plasma concentration, CYP cytochrome P450, EMs CYP2D6 extensive metabolizers, ER extended release, PMs CYP2D6 poor metabolizers
There was no difference in median steady-state viloxazine Tmax between CYP2D6 PMs and EMs (5.00 h for both groups) (Table 4). For all three pharmacokinetic parameters, the upper-bound CIs were beyond the predetermined no-difference limits of 125.00% (Fig. 5c). However, the mean increase in peak and total exposures for PMs was within the pharmacokinetic variability of EMs, signifying the differences are not likely to be clinically relevant (Fig. 5a).
3.4 Safety
Overall, the dosage of viloxazine ER 900 mg/day (which is higher than the 200–600 mg/day studied for adult ADHD) administered for four consecutive days was well tolerated when administered alone and with MCC. Fifty-three AEs were reported by 24 (64.9%) subjects over the course of the study. Of these subjects, 8 (21.6%), 13 (35.1%), and 18 (50.0%) reported AEs following dosing in Periods 1 (MCC), 2 (viloxazine ER), and 3 (MCC + viloxazine ER), respectively; the most commonly reported AE was insomnia (n = 16), which was reported only with the combined use of MCC + viloxazine ER (0%, 0%, and 44.4%, respectively), followed by somnolence (n = 5), which was reported only with viloxazine ER administered alone (0%, 13.5%, and 0%, respectively), and dizziness (n = 5), which was reported by one subject each with MCC alone and viloxazine ER alone, and three subjects with combined use (2.7%, 2.7%, 8.3%). The majority (90.6%) of AEs were assessed by the Investigators as mild in severity, and the remainder were judged to be moderate (9.4%). No severe or serious AEs were reported, and no AEs resulted in subject discontinuation. The AEs assessed as moderate were insomnia (n = 2; Period 3), nausea (n = 2, 1 subject in Period 2 and another in Period 3), and dizziness (n = 1, Period 3). No AEs were associated with clinical laboratory test results, ECG, or physical examination assessments. Two subjects had increases in blood pressure that were reported as AEs: one subject had blood pressures of 153/108 and 184/100 mmHg at the 3-h assessment following dosing with MCC during Period 1, and a second subject had recorded blood pressures of 186/97 mmHg and 181/86 mmHg at the 2-h assessment (following dosing with MCC) during Period 3. Both events resolved without intervention within 1 h of onset and were judged as mild in severity and possibly related to treatment. Based on the C-SSRS assessment, no subjects displayed suicidal ideation or suicidal behavior during the study. Treatment-emergent AEs are shown in ESM Table S7, and blood pressure and heart rate are shown in ESM Fig. S1.
4 Discussion
4.1 Overview
This study assessed the ability of viloxazine ER to meaningfully affect CYP1A2, 2D6, and 3A4 enzyme function in healthy adults. Based on US FDA guidelines [7] for defining the strength of a drug’s capacity for CYP inhibition, our results show viloxazine ER to be a strong inhibitor of CYP1A2 and a weak inhibitor of CYP2D6 and 3A4. Specifically, viloxazine ER increased exposure (AUC) of the CYP1A2 index substrate caffeine by 4.4- to 5.8-fold (Fig. 2), the CYP2D6 substrate dextromethorphan by 1.9-fold (~90%) [Fig. 3], and the CYP3A4 substrate midazolam 1.7-fold (~70%) relative to MCC alone (Fig. 4). Despite these increases in exposure, viloxazine ER did not increase the maximal measured concentration (Cmax) of single-dose caffeine or midazolam and produced only a modest increase in Cmax (~50%) of dextromethorphan.
These data are consistent with a published in vitro study showing viloxazine to be a strong, reversible inhibitor of CYP1A2 and a weak inhibitor of CYP2B6, 2D6, and 3A4/5 [19]. The in vitro study also showed viloxazine produced no meaningful inhibition at CYP2C8, 2C9, or 2C19 and no impact on CYP induction at clinically relevant doses [6]. Building on the in vitro work, the present study provides a clinical context for understanding potential sources of variability in concomitant medication exposure when using viloxazine ER in a clinical setting and how this variability might impact treatment outcomes. Specifically, the results suggest potential for clinically relevant drug interactions with viloxazine ER and drugs that are primarily metabolized by CYP1A2, and, to a lesser extent, drugs that are metabolized by CYP2D6 or 3A4. Observations of an increase in AEs of insomnia and a decrease in AEs of somnolence when viloxazine ER was coadministered with MCC, likely resulting from inhibition of caffeine metabolism, would appear to support this conclusion. Notably, the use of viloxazine ER in combination with MCC in this study appeared generally safe and well tolerated, with no reported severe or serious AEs and no discontinuations resulting from AEs, even despite the 900-mg/day viloxazine ER dosage, which is higher than the currently recommended maximum dosage for treatment of ADHD in adults (600 mg/day) [3]. However, we note that the study was a single-dose design, and therefore, the results presented do not evaluate the accumulation of index substrates (particularly caffeine) with repeated dosing and potential consequent effects on tolerability or AEs. Additionally, simulations were not done to estimate drug accumulation over multiple doses.
4.2 Influence of CYP2D6 Polymorphisms on Systemic Viloxazine Exposure
A secondary objective of the present study was to assess the impact of CYP2D6 genetic polymorphisms on the pharmacokinetic profile of viloxazine. Our results suggest a low likelihood for CYP2D6 polymorphisms to meaningfully impact viloxazine exposure, as CYP2D6 PMs had increases in viloxazine Cmax, Cmin, and AUC24 values that were 21%, 33%, and 26% higher than EMs, respectively, with upper-bound 90% CIs falling only slightly above the predetermined no-difference limit of 80.00–125.00% (Fig. 5). The increase in peak and total exposures for CYP2D6 PMs generally fell within the pharmacokinetic variability of CYP2D6 EMs, and the differences observed in PMs are not considered to be clinically relevant.
Whether or not changes in CYP enzyme function will result in clinically meaningful changes in drug efficacy or safety is influenced by many factors, such as the fraction of drug metabolized by a given pathway; genetic variation in enzyme function; the ability to shift metabolism to compensatory pathways; the drug’s therapeutic index; the dose of medication administered; and the potential influence of concomitantly administered food, drug, or herbal preparations on primary and alternative elimination pathways.
Viloxazine is hepatically metabolized to 5-hydroxyviloxazine with subsequent glucuronidation to its primary metabolite 5-hydroxyviloxazine-glucuronide; approximately 22% of the dose is excreted unchanged [6]. Like it is for many psychiatric medications, CYP2D6 is a primary pathway for viloxazine hydroxylation; however, in the case of viloxazine, CYP1A2, 2B6, 2C9, 2C19, and 3A4 also play a minor role, and in total, 5-hydoxyviloxazine metabolites account for only about 50% of the metabolized fraction of the drug [6]. Previous research has shown that the overall fraction of a drug metabolized by CYP2D6 can predict whether genetic polymorphisms for this enzyme will result in substantial variability in drug exposure [20]. For drugs with < 60% CYP2D6-mediated metabolism in vivo (such as viloxazine), the difference in AUCs between CYP2D6 PMs and EMs was modest (< 2.5 fold), whereas for major 2D6 substrates (> 60% 2D6 involvement), the exposure differences were between 3.5- and 53-fold larger [20].
Consistent with this research, CYP2D6 PMs in the present study demonstrated a < 1.5-fold increase in viloxazine exposure relative to EMs. Conversely, the nonstimulant ADHD drug atomoxetine, which relies more heavily on CYP2D6 for metabolism, is more heavily influenced by CYP2D6 genetic polymorphisms [21, 22] and shows ~ 8- to 10-fold higher atomoxetine exposure in CYP2D6 PMs compared with EMs [23]. Lower reliance on CYP2D6 for drug metabolism may also make viloxazine less susceptible to drug interaction when administered with CYP2D6 inhibitors. Overall, our study data suggest that reduction in CYP2D6 enzyme function or availability by itself is unlikely to result in clinically significant variability in viloxazine exposure. Therefore, no dose reduction or routine testing for CYP2D6 genetic polymorphism is recommended when viloxazine ER is used. Additionally, there is no need to genotype patients who are naïve to viloxazine.
4.3 Clinical Relevance
Individuals with ADHD may be treated with combination therapy, either to better control ADHD symptoms or to treat psychiatric comorbidities [24,25,26,27,28,29]. Because viloxazine is a weak inhibitor of CYP2D6 and CYP3A4, it is not anticipated to impact the pharmacokinetics of drugs metabolized by these pathways to a clinically meaningful extent under most circumstances. Exemplifying this, a pharmacokinetic study in healthy adults showed no evidence of clinically relevant drug interaction when viloxazine ER was coadministered with lisdexamfetamine [17]. While lisdexamfetamine is not itself metabolized by any CYP enzymes, its primary metabolite, d-amphetamine, is metabolized, at least in part, by CYP2D6 [30]. Administration of clinically relevant single doses of lisdexamfetamine (50 mg) alone and in combination with viloxazine ER (700 mg) showed no relevant increase in d-amphetamine, with Cmax, AUCt, and AUC∞ all within the predetermined no-difference limits of 80.00–125.00% [17]. The lack of impact on d-amphetamine, versus the modest impact on dextromethorphan pharmacokinetics seen in the present study, may be accounted for by the fraction of each drug metabolized by CYP2D6 (while d-amphetamine is presumed to be metabolized via multiple routes, dextromethorphan elimination uses the CYP2D6 pathway almost exclusively) as well as the larger dose of viloxazine ER used in this study (900 mg/day × 4 days vs. a single 700-mg dose in the lisdexamfetamine study) [17]. Similarly, a drug-drug interaction study between viloxazine ER and methylphenidate (another stimulant used in ADHD treatment) also showed no significant drug interactions [18].
When viloxazine is coadministered with paroxetine, a selective serotonin reuptake inhibitor and a strong CYP2D6 inhibitor, only modest changes to viloxazine pharmacokinetics were observed (increase in AUC < 35% and no changes in Cmax) that are unlikely to have a clinically relevant impact on efficacy or safety [19]. Viloxazine ER effects on CYP3A4 were below the range established by the FDA that would require additional drug-drug interaction studies, such as those with oral contraceptives.
Conversely, because viloxazine is a strong inhibitor of CYP1A2, clinically meaningful interactions with drugs primarily metabolized by this enzyme are likely, particularly for drugs with narrow therapeutic indices and without compensatory elimination pathways. Indeed, the few potentially serious drug interactions involving viloxazine that have been reported during its otherwise long track record of safe use since the 1970s have involved drugs with narrow therapeutic indices that predominantly use singular metabolic pathways. For instance, in a 1986 case report, theophylline, a bronchodilator predominantly metabolized by CYP1A2, showed significantly decreased clearance, with a doubling of serum concentrations and signs of toxicity three days following viloxazine treatment initiation. These effects were quickly reversed upon cessation of viloxazine treatment [31]. A subsequent drug interaction study in eight healthy volunteers found viloxazine (300 mg/day) significantly increased theophylline plasma concentrations and decreased apparent clearance, likely resulting from viloxazine’s strong inhibition of CYP1A2 [32]. Theophylline is known to have a narrow therapeutic window with saturable metabolism, and as a result, its use requires careful monitoring, particularly when administered in tandem with drugs affecting CYP1A2 [33, 34].
Similarly, a drug interaction study by Pisani et al. showed the potential for carbamazepine intoxication with viloxazine coadministration [35]. Carbamazepine is an anticonvulsant medication that also has a narrow therapeutic index [34, 36]. At the time the drug interaction study with carbamazepine was conducted (pre-1994), viloxazine was among the few available antidepressants considered to be non-epileptogenic [37, 38] and was therefore evaluated in seven adults with epilepsy and depression to characterize the potential for drug interactions between the two products. Individuals receiving stable treatment with carbamazepine were administered viloxazine at doses totaling 300 mg/day for three weeks. Viloxazine coadministration increased carbamazepine concentrations, although symptoms of carbamazepine intoxication quickly normalized following viloxazine discontinuation [35]. Although carbamazepine is said to be metabolized primarily by CYP3A4 [39], its metabolism is also affected by polymorphisms in CYP1A2, and it is known to interact with ciprofloxacin, which, like viloxazine, is a strong inhibitor of CYP1A2 yet a weak inhibitor of CYP3A4. Although clinical monitoring for drug interactions in the context of polypharmacy is generally prudent, such monitoring is particularly important for sensitive CYP1A2 substrates and CYP1A2 substrates with a narrow therapeutic range.
While viloxazine ER is a relatively new viloxazine formulation, the original, immediate-release version has a long history of use in Europe dating from the 1970s and was typically administered in one to four divided doses totaling 100–600 mg/day without any major safety concerns [37, 40, 41]. Contemporarily, no major safety concerns have been reported in drug interaction studies after single doses of viloxazine ER in healthy adults (dosing 700 mg/day) either alone or in combination with methylphenidate [18], lisdexamfetamine [17], or paroxetine [19], nor after chronic dosing in children (100–400 mg/day) [42,43,44], adolescents (200–600 mg/day) [45, 46], or adults (200–600 mg/day) [47] in well-controlled studies, leading to viloxazine ER approval for the treatment of ADHD in these populations [43,44,45,46,47].
Although doses above 600 mg/day have not been examined in pediatric populations, in healthy adults, the maximum tolerated doses of viloxazine ER in phase I testing were 2100 mg/day after a single dose and 1800 mg/day after multiple doses [48]. Additionally, a recently published study using single doses of viloxazine ER 1800 mg for two consecutive days found no demonstrable increase in the risk of cardiac arrhythmias, altered ECG parameters, or major safety concerns [49]. These data offer preliminary evidence suggesting viloxazine ER has a wide therapeutic index and would be unlikely to result in serious toxicity following modest elevations in plasma concentration.
Although viloxazine ER significantly increased systemic caffeine exposure in the present study, caffeine is known to have a wide therapeutic index and rarely causes serious toxicity [50]. Because most individuals self-regulate their caffeine intake based on subjective and physiological effects, caffeine intoxication is rare [51]. Given the numerous intrinsic and extrinsic sources of variability in caffeine exposure, at typical levels of caffeine consumption, individuals are likely to be able to safely regulate their intake while also receiving treatment with viloxazine ER [51, 52]. Notably, caffeine use was allowed in the phase III pediatric and adult ADHD treatment studies, where the majority (over 85%) of adult subjects treated with viloxazine ER also used caffeine [47]. However, clinicians should be aware of the possibility that viloxazine interactions with CYP1A2 could lead to caffeine-related AEs.
5 Conclusions
We report increases in systemic caffeine exposure (~ 4.8 fold increase over baseline) without an impact on Cmax, and modest increases in dextromethorphan (~ 1.9 fold) and midazolam (~ 1.7 fold) exposure after dosing with viloxazine ER in healthy adults. These data suggest that viloxazine ER functions as a strong CYP1A2 inhibitor and a weak CYP2D6 and 3A4 inhibitor [7]. We also report minimal impact of CYP2D6 polymorphisms on systemic viloxazine exposure, as viloxazine AUC increased by only 25% in PMs relative to EMs. Furthermore, all treatments appeared safe and well tolerated, with no severe or serious AEs and no AEs that led to study discontinuation. Clinical monitoring is generally advised with polypharmacy. Monitoring may be important with viloxazine ER and CYP1A2 substrates, particularly those known to have a narrow therapeutic index.
References
Findling RL, Candler SA, Nasser AF, Schwabe S, Yu C, Garcia-Olivares J, et al. Viloxazine in the management of CNS disorders: a historical overview and current status. CNS Drugs. 2021;35(6):643–53.
Edinoff AN, Akuly HA, Wagner JH, Boudreaux MA, Kaplan LA, Yusuf S, et al. Viloxazine in the treatment of attention deficit hyperactivity disorder. Front Psychiatry. 2021;12: 789982.
Qelbree (Viloxazine Extended-Release Capsules) [prescribing information]. Rockville: Supernus Pharmaceuticals, Inc.; 2022.
Yu C, Garcia-Olivares J, Candler S, Schwabe S, Maletic V. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285–300.
Robinson CL, Parker K, Kataria S, Downs E, Supra R, Kaye AD, et al. Viloxazine for the treatment of attention deficit hyperactivity disorder. Health Psychol Res. 2022;10(3):38360.
Yu C. Metabolism and in vitro drug-drug interaction assessment of viloxazine. Xenobiotica. 2020;50(11):1285–300.
Clinical Drug Interaction Studies—Cytochrome P450 enzyme- and transporter-mediated drug interactions: guidance for industry. Silver Spring: Center for Drug Evaluation and Research; US Food and Drug Administration; US Department of Health and Human Services; 2020.
Lynch T, Price AL. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76(3):391–6.
Ogu CC, Maxa JL. Drug interactions due to cytochrome P450. Baylor University Medical Center Proceedings; 2000: Taylor & Francis; 2000. pp. 421–3.
van der Weide J, Hinrichs JW. The influence of cytochrome P450 pharmacogenetics on disposition of common antidepressant and antipsychotic medications. Clin Biochem Rev. 2006;27(1):17–25.
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83(2):234–42.
Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. A review of the important role of CYP2D6 in pharmacogenomics. Genes (Basel). 2020;11(11):1295.
Streetman DS, Bleakley JF, Kim JS, Nafziger AN, Leeder JS, Gaedigk A, et al. Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the “Cooperstown cocktail.” Clin Pharmacol Ther. 2000;68(4):375–83.
Goh BC, Reddy NJ, Dandamudi UB, Laubscher KH, Peckham T, Hodge JP, et al. An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5+1 cocktail in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88(5):652–9.
Ermer J, Corcoran M, Martin P. Lisdexamfetamine dimesylate effects on the pharmacokinetics of cytochrome P450 substrates in healthy adults in an open-label, randomized, crossover study. Drugs R D. 2015;15(2):175–85.
Armani S, Ting L, Sauter N, Darstein C, Tripathi AP, Wang L, et al. Drug interaction potential of osilodrostat (LCI699) based on its effect on the pharmacokinetics of probe drugs of cytochrome P450 enzymes in healthy adults. Clin Drug Investig. 2017;37(5):465–72.
Faison SL, Fry N, Adewole T, Odebo O, Wang Z, Maletic V, et al. Pharmacokinetics of co-administered viloxazine extended-release (SPN-812) and lisdexamfetamine in healthy adults. J Clin Psychopharm. 2021;41(2):155–62.
Faison SL, Fry N, Adewole T, Odebo O, Wang Z, Maletic V, et al. Pharmacokinetics of co-administered viloxazine extended-release (SPN-812) and methylphenidate in healthy adults. Clin Drug Investig. 2020;41:149–59.
Wang Z, Kosheleff AR, Adeojo LW, Odebo O, Adewole T, Qin P, et al. Impact of paroxetine, a strong CYP2D6 inhibitor, on SPN-812 (viloxazine extended-release) pharmacokinetics in healthy adults. Clin Pharmacol Drug Dev. 2021;10(11):1365–74.
Gibbs JP, Hyland R, Youdim K. Minimizing polymorphic metabolism in drug discovery: evaluation of the utility of in vitro methods for predicting pharmacokinetic consequences associated with CYP2D6 metabolism. Drug Metab Dispos. 2006;34(9):1516–22.
Yu G, Li G-F, Markowitz JS. Atomoxetine: a review of its pharmacokinetics and pharmacogenomics relative to drug disposition. J Child Adolesc Psychopharmacol. 2016;26(4):314–26.
Fijal BA, Guo Y, Li SG, Ahl J, Goto T, Tanaka Y, et al. CYP2D6 predicted metabolizer status and safety in adult patients with attention-deficit hyperactivity disorder participating in a large placebo-controlled atomoxetine maintenance of response clinical trial. J Clin Pharmacol. 2015;55(10):1167–74.
Michelson D, Read HA, Ruff DD, Witcher J, Zhang S, McCracken J. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(2):242–51.
Adler LA, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep. 2006;8:409–15.
Chen Q, Hartman CA, Haavik J, Harro J, Klungsøyr K, Hegvik T-A, et al. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: a population-based cross-sectional study. PLoS ONE. 2018;13(9): e0204516.
Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten Defic Hyperact Disord. 2015;7(1):27–38.
Michielsen M, Comijs HC, Semeijn EJ, Beekman AT, Deeg DJ, Kooij JS. The comorbidity of anxiety and depressive symptoms in older adults with attention-deficit/hyperactivity disorder: a longitudinal study. J Affect Disord. 2013;148(2–3):220–7.
Molife C, Bernauer MJ, Farr AM, Haynes VS, Kelsey D. Combination therapy patterns and predictors of ADHD in commercially insured and Medicaid populations. Postgrad Med. 2012;124(5):7–22.
Pohl GM, Van Brunt DL, Ye W, Stoops WW, Johnston JA. A retrospective claims analysis of combination therapy in the treatment of adult attention-deficit/hyperactivity disorder (ADHD). BMC Health Serv Res. 2009;9:95.
Bach MV, Coutts RT, Baker GB. Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives. Xenobiotica. 1999;29(7):719–32.
Laaban JP, Dupeyron JP, Lafay M, Sofeir M, Rochemaure J, Fabiani P. Theophylline intoxication following viloxazine induced decrease in clearance. Eur J Clin Pharmacol. 1986;30:351–3.
Perault MC, Griesemann E, Bouquet S, Lavoisy J, Vandel B. A study of the interaction of viloxazine with theophylline. Ther Drug Monit. 1989;11(5):520–2.
Journey JD, Bentley TP. Theophylline toxicity. StatPearls. Online: StatPearls Publishing, LLC; 2020.
Product-Specific Recommendations for Generic Drug Development [online] US Food and Drug Administration; US Department of Health and Human Services; 2015.
Pisani F, Fazio A, Oteri G, Perucca E, Russo M, Trio R, et al. Carbamazepine-viloxazine interaction in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1986;49:1142–5.
Greenberg RG, Melloni C, Wu H, Gonzalez D, Ku L, Hill KD, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39(5):232.
Ban TA, McEvoy JP, Wilson WH. Viloxazine: a review of the literature. Int Pharmacopsychiatr. 1980;15:118–23.
Edwards JG, Glen-Bott M. Does viloxazine have epileptogenic properties? J Neurol Neurosurg Psychiatry. 1984;47:960–4.
Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, et al. Human liver carbamazepine metabolism: role of CYP3A4 and CYP2C8 in 10, 11-epoxide formation. Biochem Pharmacol. 1994;47(11):1969–79.
Pinder RM, Brogden RN, Speight TM, Avery GS. Viloxazine: a review of its pharmacological properties and therapeutic efficacy in depressive illness. Drugs. 1977;13:401–21.
Kabes J, Dostal T, Nahunek K, Svestka J, Molcan J, Caplová T, et al. Multicentre study with viloxazine (Vivalan®) in depressed patients. Int Pharmacopsychiatr. 1980;15:228–39.
Johnson JK, Liranso T, Saylor K, Tulloch G, Adewole T, Schwabe S, et al. A phase II double-blind, placebo-controlled, efficacy and safety study of SPN-812 (extended-release viloxazine) in children with ADHD. J Atten Disord. 2020;24(2):348–58.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Chowdhry F, et al. A phase 3, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended release) in the treatment of ADHD in school-age children. Clin Ther. 2020;42(8):1452–66.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Busse GD, et al. Once-daily SPN-812 200 and 400 mg in the treatment of ADHD in school-aged children: a phase III randomized, controlled trial. Clin Therapeut. 2021;43(4):684–700.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Busse GD, et al. A phase 3, placebo-controlled trial of once-daily viloxazine extended-release capsules in adolescents with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2021;41(4):370–80.
Nasser A, Liranso T, Adewole T, Fry N, Hull JT, Chowdhry F, et al. A phase 3 placebo-controlled trial of once-daily 400-mg and 600-mg SPN-812 (viloxazine extended-release) in adolescents with ADHD. Psychopharm Bul. 2021;51(2):43–64.
Nasser A, Hull JT, Chaturvedi SA, Liranso T, Odebo O, Kosheleff AR, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897–915.
Research CfDEa. Integrated Review. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/211964Orig1s000IntegratedR.pdf. Accessed 10 Apr 2023.
Nasser A, Faison SL, Liranso T, Adewole T, Busse GD, Fava M, et al. Evaluation of the effect of SPN-812 (viloxazine extended release) on QTc interval in healthy adults. J Clin Psychiatry. 2020;81(6):e1–6.
Arens A, Benowitz NL. Caffeine. In: Olson RK, editor. Poisoning & drug overdose. 7th ed. New York: McGraw Hill Education; 2018.
Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev. 2018;70(2):384–411.
Fulton JL, Dinas PC, Carrillo AE, Edsall JR, Ryan EJ, Ryan EJ. Impact of genetic variability on physiological responses to caffeine in humans: a systematic review. Nutrients. 2018;10(10):1373.
Acknowledgements
The authors would like to thank Toyin Adewole, Shamia Faison, and Stefan Schwabe for their contributions to the study, and Worldwide Clinical Trials (the Clinical Research Organization for the study) for their work on study conduct, bioanalysis, data and pharmacokinetic analysis. They would also like to thank Jennifer Koch and Andrea Formella for project management and editorial assistance. Editorial and writing assistance was provided by AlphaBioCom, a Red Nucleus company, and funded by Supernus Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study and the open access fee were funded and paid for by Supernus Pharmaceuticals, Inc.
Conflict of interests
Zhao Wang and Zulane Maldonado-Cruz are employees of Supernus Pharmaceuticals. Tesfaye Liranso, Alisa Kosheleff and Azmi Nasser were employees of Supernus Pharmaceuticals at the time of conduct of the study.
Availability of data and material
The data are not available in a repository, but reasonable requests can be directed to the corresponding author (Zhao Wang) at zwang@supernus.com.
Code availability
Not applicable.
Ethics approval
This trial was conducted in accordance with the Helsinki Declaration and the International Council for Harmonisation Note for Guidance on Good Clinical Practice. The trial conduct was reviewed and approved by IntegReview IRB (22 September 2017).
Consent to participate
Informed consent was obtained from all individual subjects included in the study.
Consent for publication
Subjects signed informed consent regarding publishing their data.
Author contributions
Conceptualization: AN, TL. Methodology: AN, TL. Data analysis: ZW, TL, AN, ZM-C. Writing: AK, AN, ZW.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Z., Liranso, T., Maldonado-Cruz, Z. et al. Impact of Viloxazine Extended-Release Capsules (Qelbree®) on Select Cytochrome P450 Enzyme Activity and Evaluation of CYP2D6 Genetic Polymorphisms on Viloxazine Pharmacokinetics. Clin Drug Investig 44, 303–317 (2024). https://doi.org/10.1007/s40261-024-01356-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01356-0