Abstract
Background and Objectives
Patients with migraine and prior preventive treatment failures have a significant burden on quality of life and disability. The CONQUER study evaluated the effects of galcanezumab on patient functioning, disability, and health status in episodic or chronic migraine with a previous failure of two to four migraine preventive medication categories.
Methods
Patients with two to four preventive migraine treatment category failures received galcanezumab 120 mg/month (240-mg loading dose) or placebo subcutaneously, for 3 months (double-blind period). In the 3-month open-label period, all patients received galcanezumab irrespective of the treatment received in the double-blind period. Changes in Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ), Migraine Disability Assessment (MIDAS), and European Quality of Life-5 Dimensions-5 Levels (EQ-5D-5L) scores were assessed.
Results
A total of 462 patients were randomized to receive galcanezumab (N = 232) or placebo (N = 230). At month 3, improvement in the MSQ Role-Function-Restrictive score from baseline was significantly greater for galcanezumab (23.19 ± 1.34) vs placebo (10.66 ± 1.33) [p ≤ 0.0001]. Significant improvements in remaining MSQ domains and total MSQ scores were observed (p < 0.0001) during the double-blind period. MIDAS total scores were significantly (p ≤ 0.0001) reduced with galcanezumab (− 21.10 + 3.32) vs placebo (− 3.30 + 3.28). EQ-5D-5L visual analog scale scores improved for galcanezumab (3.40 + 1.31) vs placebo (− 0.09 + 1.29; p = 0.028). During the open-label period, quality of life continued to improve for galcanezumab, with patients previously assigned to placebo reaching similar results. During both study periods, similar findings were reported in subpopulations with episodic migraine and chronic migraine.
Conclusions
Galcanezumab significantly improved functioning and reduced disability in patients with episodic migraine and chronic migraine and two to four migraine preventive treatment category failures.
Clinical Trial Registration
NCT03559257, registration date: 6 June, 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Galcanezumab improves quality of life and reduces disability in patients with episodic or chronic migraine, with a previous failure of two to four migraine preventive medication categories. |
Changes in quality of life for galcanezumab from baseline to month 3 were statistically significantly greater than placebo in the double-blind period of this randomized clinical trial. |
Improvements in functioning and reduction in disability for the galcanezumab treatment arm continued through month 6 of the open-label period, with those previously assigned to placebo achieving similar results during these 3 months of active treatment. |
1 Introduction
Recent research has quantified the cumulative burden of migraine associated with increasing preventive treatment failures [1, 2]. In a recent global online cross-sectional study, 11,266 adults with migraine were included, who had ≥ 4 monthly migraine days each month in the 3 months preceding the survey [1]. Of the included patients, 87.5% were taking preventive treatments. A majority of the previously treated patients experienced treatment failure (77.9%), of which two or more treatment failures were experienced by 87.5% of patients. These patients with two or more treatment failures reported impacts on their personal (70%), social (82%), and professional lives (up to 75%). The majority of patients (78%) reported spending an average of 21 h/month in isolation/darkness, a third (32%) of patients were fearful of having another migraine episode, and more than half (64%) required external support to carry out daily tasks [1]. The subgroup of patients with migraine and two or more available preventive treatment failures is of particular interest owing to this evidence of decreased quality of life (QoL) and increased disability and economic burden among patients with inadequately managed migraine [1, 2].
Prior to the recent emergence of novel migraine preventive treatments, the standard of care posed challenges including high discontinuation rates because of a lack of efficacy and/or safety/tolerability reasons [3]. The current American Headache Society (AHS) treatment consensus statement for adults with migraine recommends initiating treatment with monoclonal antibodies that target calcitonin gene-related peptide (CGRP) or its receptor in patients experiencing ≥ 4 migraine headache days per month, unable to tolerate the treatment with two or more non-anti-CGRP preventive medications (including topiramate, divalproex sodium/valproate sodium, beta-blockers, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and other treatments recommended by the AHS guidelines) because of side effects or inadequate response to treatment after 8 weeks [4]. The European Headache Federation guidelines have similar recommendations for the use of CGRP antagonists, i.e., a history of failure of two or more available preventive treatments due to inadequate efficacy, tolerability, comorbidities, or poor compliance [5]. Both the AHS consensus statement and European Headache Federation guidelines recommend improvements in patient functioning and health-related QoL, and reductions in disability as preventive treatment goals [4, 5].
Galcanezumab is a humanized IgG4 monoclonal antibody that binds CGRP and prevents its biological activity without blocking the CGRP receptor [6]. Galcanezumab has been shown to significantly improve patient functioning and decrease migraine-related disability in patients with episodic migraine (EM) and chronic migraine (CM) in clinical trials and in an observational study, in addition to improving total pain burden [7,8,9,10,11]. Primary results of the CONQUER trial demonstrated the efficacy and safety of galcanezumab in patients with EM or CM with a previous failure of two to four migraine preventive medication categories [12]. This paper reports secondary outcomes of the CONQUER trial, i.e., the effects of galcanezumab on patient functioning, disability, and health status in the CONQUER trial population.
2 Methods
2.1 Study Design
CONQUER (NCT03559257) [13] was a phase IIIb, randomized, double-blind, placebo-controlled trial of galcanezumab in adult patients with EM and CM with a previous failure of two to four migraine preventive medication categories in the past 10 years because of insufficient efficacy and/or safety/tolerability reasons [12]. The study consisted of four periods: screening (3–30 days); a prospective baseline period to determine eligibility (30–40 days); a double-blind treatment (3 months); and an optional open-label treatment (3 months). During the double-blind period, the patients were randomized 1:1 to receive galcanezumab 120 mg/month (loading dose 240 mg) or placebo, subcutaneously. During the open-label period, patients previously assigned to galcanezumab continued with galcanezumab 120 mg/month (plus one injection of placebo to maintain blinding at month 3) subcutaneously and those previously assigned to placebo received galcanezumab subcutaneously (loading dose of 240 mg: two injections of 120 mg each at month 3) followed by a dose of 120 mg/month [12, 14]. Mulleners et al. [12] reported the details regarding the CONQUER study design, patient population, and primary findings including more information on preventive treatment failures, acute medication use, and the mean changes in the number of monthly headache days. The failed treatments included propranolol or metoprolol, topiramate, valproic acid, amitriptyline, flunarizine, candesartan, botulinum toxin A or B, and medication locally approved for the prevention of migraine.
The study protocol was approved by the ethical review board at each site and all participants provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and other applicable laws and regulations.
2.2 Patient Population
Patients aged 18–75 years, with a diagnosis of migraine (for ≥ 1 year) per the International Headache Society International Classification of Headache Disorders, Third Edition guidelines [15] and, documentation of two to four migraine preventive medication category failures in the past 10 years were included. The exclusion criteria, and previously administered medication categories have been described elsewhere [12].
2.3 Outcome Measures
Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ v2.1) is a self-administered health status instrument with a 4-week recall period that was developed to address the physical and emotional impact on functioning that is of specific concern to individuals with migraine [16, 17]. The instrument has 14 items in three domains: (1) Role Function-Restrictive (RF-R); (2) Role Function-Preventive (RF-P); and (3) Emotional Function (EF). The RF-R domain consists of seven items that measure the impact of migraine on work or daily activities, relationships with family and friends, leisure time, productivity, concentration, energy, and tiredness. The RF-P domain consists of four items that measure the impact of migraine on social and daily activities The EF domain consists of three items that assess the emotional impact of migraine. Response options range from 1 (none of the time) to 6 (all of the time); after the raw score for a domain (or the total) is computed, the score is linearly converted to a 0–100 scale, 100 indicating full functionality [16, 18]. Table S1 of the Electronic Supplementary Material (ESM) presents a brief description of the MSQ items.
Responder definition thresholds for MSQ v2.1 domains have been determined using triangulation analysis for patient populations with EM and CM [19, 20]. These thresholds identify clinically meaningful changes in the functional and emotional impact of migraine. The domain change thresholds are: ≥ 25.71 for RF-R, ≥ 20.00 for RF-P, and ≥ 26.67 for EF in patients with EM; ≥ 17.14 for RF-R, ≥ 20.00 for RF-P, and ≥ 26.67 for EF in patients with CM [19, 20]. Descriptive categories based on score ranges have recently been derived for the MSQ v2.1 RF-R domain to facilitate the interpretation of scores at any point in time. The categories were based on the relationship of RF-R scores with patient-reported migraine severity, disability, and frequency of migraine attacks. The validated RF-R score categories are: 85–100 (not impaired/minimally impaired); 75–84 (mildly impaired); 55–74 (moderately impaired); 40–54 (severely impaired); and < 40 (extremely impaired) [21].
The Migraine Disability Assessment Scale (MIDAS) is a patient-rated measure of headache-related disability over a 3-month period. The five items in this instrument reflect the number of days reported as missing, or with reduced productivity at work or home, school, and social events, with a score range of 0–270 [22, 23]. Higher scores indicate more disability: 0–5, little/no disability; 6–10, mild disability; 11–20, moderate disability; 21–40, severe disability; and ≥ 41, very severe disability [23].
The EQ-5D-5L questionnaire is a patient-rated scale of current (today) general health status at the time of questionnaire completion [24, 25]. It consists of two parts, a health state index score and a Visual Analog Scale (VAS) score that captures a patient’s self-perception of current health state. The first part assesses five dimensions including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with five response options from no problems to extreme problems. The VAS ranges from 0 to 100 (from worst health to best health you can imagine).
2.4 Assessments
Patients completed questionnaires/scales via an electronic patient-reported outcome tablet device, which was used as the source data. The MSQ v2.1 domain and total scores were assessed at baseline and at each month during the double-blind and open-label periods, MIDAS total scores and EQ-5D-5L total scores were assessed at baseline, month 3, and month 6. All assessments were completed during the visits to the physician’s office. Additionally, post hoc analyses included determining the percentage of patients achieving responder definition thresholds for MSQ v2.1 domains at months 3 and 6, and the percentage of patients in each of the MSQ v2.1 RF-R score and the MIDAS disability score categories at baseline, month 3, and month 6.
2.5 Statistical Analysis
Using the SAS Enterprise Guide 7.2 (SAS Institute, Cary, NC, USA), the analyses were conducted for the intent-to-treat population and subpopulations with EM and CM. The intent-to-treat population was defined as all patients who were randomized and received one or more doses of the study drug. Changes from baseline in MSQ v2.1 scores to each post-baseline visit were analyzed using a mixed-model repeated-measures model, which included effects of treatment, baseline migraine headache days frequency category, pooled country, month and treatment-by-month interaction as fixed categorical covariates; and baseline-by-month interaction as continuous covariates. Changes from baseline to the last observation carried forward endpoint in MIDAS and EQ-5D-5L scores were analyzed using an analysis of covariance, which included effects of treatment, baseline migraine headache days frequency category, and pooled country as well as a continuous fixed covariate of baseline. The mean change from baseline in the MSQ-RF-R domain score at month 3 was included as a key secondary efficacy endpoint. A gatekeeping sequence was used to control the type I error for the primary endpoint of reduction in migraine headache days [12] and MSQ-RF-R testing.
Change from baseline in the EQ-5D-5L health index state at month 3 and month 6 was determined using the UK and US population-based index values. In brief, the country-specific value set measures how health is valued by people in a specific country [26, 27]. All treatment effects were evaluated based on a two-sided significance level of 0.05. As the patients completed each scale in its entirety or not at all, there was no imputation for missing values.
3 Results
3.1 Baseline and Demographic Characteristics
A total of 462 patients (galcanezumab, N = 232; placebo, N = 230) were randomized during the double-blind period. This included 269 (58.20%) patients with EM and 193 (41.80%) patients with CM. Of the 462 patients, 451 (97.62%) [galcanezumab, N = 225; placebo, N = 226] completed the double-blind period: 266/269 (98.90%) with EM and 185/193 (95.85%) with CM. Almost all patients who completed the double-blind treatment entered the open-label period (449/451, 99.60%; previous galcanezumab, N = 225; previous placebo, N = 224) and 432 (96.21%) completed the open-label period (previous galcanezumab, N = 217; previous placebo, N = 215).
Baseline characteristics were similar between galcanezumab and placebo groups in the total population as well as in the subpopulations (EM or CM, Table 1). In the total population, mean age was 45.80 years and the majority of patients were white (81.65%) and female (85.93%). Patients in the galcanezumab and placebo groups were of similar age (45.87 years and 45.67 years), and a similar proportion of patients were white (81.70% and 81.61%). The proportion of female patients included in the placebo group was higher than the proportion included in the galcanezumab group (87.83% vs 84.05%). Mean monthly migraine headache days was 13.23 in the total population, 9.34 in the subpopulation with EM, and 18.65 in the subpopulation with CM. For the subpopulation with CM, mean monthly migraine headache days with abortive medication use and mean duration of migraine illness were higher than the subpopulation with EM. At baseline, total MSQ score, MIDAS total score, and EQ-5D-5L score did not differ significantly between treatment groups (Table 1). In our study population, the prior preventive medications used by > 20% patients were topiramate (76.00%), amitriptyline (56.10%), propranolol (36.71%), valproic acid (34.84%), botulinum toxin type A (22.93%), and metoprolol (20.34%). Table S2 of the ESM presents the proportion of patients not responding to the prior preventive medications in the total population and the subgroups with EM or CM. Acute medications used in double-blind and open-label phases are presented in Table S3 of the ESM.
3.2 Double-Blind Period
3.2.1 MSQ v2.1 Score
In the total study population, at month 3, patient functioning improved significantly for galcanezumab vs placebo as measured by the least-squares (LS) mean change from baseline (standard error) in the MSQ RF-R domain scores: 23.19 (1.34) vs 10.66 (1.33) [p ≤ 0.0001], respectively (Fig. 1A). Similar results for galcanezumab vs placebo were observed for the RF-P domain, EP domain, and the total MSQ score (p ≤ 0.0001 for RF-P, EF, and MSQ total scores). For the total and individual domain scores, galcanezumab showed significantly greater reductions vs placebo as early as at month 1 (p ≤ 0.0001), and also at month 3 (p < 0.0001) (Fig. 1A–D).
Least-squares (LS) mean change (standard error [SE]) from baseline to month 6. (A) Role Function-Restrictive (RF-R), (B) Role Function-Preventive (RF-P), (C) Emotional Function (EF), and (D) Migraine-Specific Quality of Life Questionnaire (MSQ) version 2.1 total scores. Values presented for total placebo (PBO) and total galcanezumab (GMB) populations for double-blind and open-label periods. In the open-label period, all patients were treated with GMB, including the patients who received PBO in the double-blind period. EM episodic migraine, **p < 0.0001 for comparison between total PBO and total GMB populations; #values for the GMB population in the open-label period
At month 3, a greater increase in the proportion of patients categorized as having minimal/no or mild functional impairment per the MSQ RF-R was observed with galcanezumab group vs placebo: EM (galcanezumab: 2.92–46.67%; placebo: 6.06–24.98%) and CM (galcanezumab: 1.05–30.68%; placebo: 5.10–12.63%) (Table 2). Table S4 of the ESM presents the shift from one MSQ category at baseline to another at month 3. At month 3, the proportions of patients with EM achieving MSQ responder definition thresholds were significantly greater in the galcanezumab group vs the placebo group for RF-R (p < 0.01), RF-P (p < 0.01), and EF (p < 0.05) domains. These findings were consistent in the subpopulation with CM (Table 3).
3.2.2 Migraine Disability Assessment Scale Total Scores
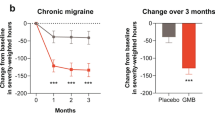
In the total population, at month 3, a significantly greater reduction in disability occurred for galcanezumab vs placebo as measured by the LS mean change from baseline (standard error) in MIDAS total scores (galcanezumab: 50.61 [46.00] to 29.80 [43.91], LS mean change: −21.10 [3.32]; placebo 50.20 [45.35] to 47.64 [56.00], LS mean change: −3.30 [3.28], p ≤ 0.0001). These findings were consistent across patient subpopulations with EM (p < 0.01) and CM (p < 0.05) (Fig. 2).
Least-squares (LS) mean change from baseline in Migraine Disability Assessment (MIDAS) total scores at (A) month 3 and (B) month 6. In the open-label period, all patients were treated with galcanezumab (GMB), including the patients who received placebo (PBO) in the double-blind period. CM chronic migraine, EM episodic migraine, *p < 0.01; **p ≤ 0.0001; #p < 0.05 vs PBO. The error bars represent standard error
From baseline to month 3, a greater increase in the proportion of patients categorized as having little/no to mild disability per the MIDAS (Table 2) was observed in the galcanezumab group for EM (galcanezumab: 18.25–48.89%; placebo: 12.12–22.83%) and CM (galcanezumab: 9.47–30.68%; placebo: 11.22–13.69%). Table S5 of the ESM presents the shifting from one MIDAS category at baseline to another at month 3.
3.2.3 EQ-5D-5L Health Index and VAS Scores
In the total population, galcanezumab showed numerically greater improvements in the health index scores compared with placebo at month 3 from baseline: EQ-5D-5L (UK): galcanezumab: 0.795 to 0.821, LS mean difference = 0.017, placebo: 0.812 to 0.809, LS mean difference = − 0.001 (galcanezumab vs placebo, p = 0.1630); EQ-5D-5L (USA): galcanezumab: 0.842 to 0.861, LS mean difference = 0.013, placebo: 0.852 to 0.850, LS mean difference = − 0.002 (galcanezumab to placebo, p = 0.1267). These findings were consistent for galcanezumab vs placebo in the subpopulations with EM (UK: p = 0.2056; USA: p = 0.1651) and CM (UK: p = 0.5013; USA: p = 0.4378), Table S6 of the ESM. Tables S7–S9 of the ESM present the proportion of patients’ responses to EQ-5D domains.
In the total population, at month 3, a statistically significant improvement in EQ-5D-5L VAS scores for galcanezumab vs placebo was observed: LS mean change from baseline (standard error): 3.38 (1.31) vs −0.086 (1.29) [p = 0.0277]. Improvement in VAS scores with galcanezumab vs placebo was also observed in patient subpopulations with EM and CM; however, the improvement was not statistically significant (EM: p = 0.0549; CM, p = 0.3546) (Fig. 3).
Least-squares (LS) mean change from baseline in the EQ-5D-5L Visual Analog Scale scores at (A) month 3 and (B) month 6. In the open-label period, all patients were treated with galcanezumab (GMB), including the patients who received placebo (PBO) in the double-blind period. CM chronic migraine, EM episodic migraine, #p < 0.05 vs PBO. The error bars represent standard error
3.3 Open-Label Period: Health-Related QoL Outcomes
During the open-label period (month 4 to month 6), improvements in patient functioning as measured by MSQ scores and individual domains were maintained for the prior galcanezumab group. Upon initiation of galcanezumab treatment during the open-label period, patients previously assigned to the placebo group improved or caught up with the prior galcanezumab group (Fig. 1A–D). These findings were consistent across the subpopulations with EM and CM. At month 6, the proportions of patients with minimal/no or mild functional impairment per the RF-R were 58.02% and 41.86% for the prior galcanezumab treatment group, and 67.20% and 33.71% for the prior placebo group, for EM and CM, respectively (Table 2).
The prior placebo group showed an increase in the percentage of patients achieving the MSQ responder definition thresholds for each domain at month 6 of the open-label period vs at month 3. In the subpopulation with CM, significant improvement was observed for the group previously randomized to galcanezumab when compared with the prior placebo group for the RF-R domain (odds ratio 2.08, 95% confidence interval 1.10, 3.95, p < 0.05). Response rates in RF-P and EF domains were similar for the two groups at month 6 in the subpopulation with CM (Table 3).
At month 6, the proportion of patients categorized as having little/no or mild disability per the MIDAS was 55.72% and 38.10% for the prior galcanezumab treatment group, and 59.68% and 29.21% for the prior placebo group, for EM and CM respectively (Table 2). Improvements in the MIDAS total score for the previous galcanezumab group were maintained and greater numerical improvement for CM was observed at month 6.
No statistically significant differences in EQ-5D-5L Health State Index scores (UK and USA) were observed between the previous galcanezumab treatment group vs the previous placebo group at month 6 in the overall population (health index UK, p = 0.6108; health state index USA, p = 0.5477). This finding was consistent for EM (health index UK, p = 0.4357; health state index USA, p = 0.4889) and CM (health index UK, p = 0.1927; health state index USA, p = 0.1565). Upon initiation of galcanezumab treatment, patients previously assigned to the placebo group had no significant differences in health state perceptions per the VAS as the prior galcanezumab group in the overall population (p = 0.5377), the subpopulation with EM (p = 0.3812) and the subpopulation with CM (p = 0.8849) (Fig. 3).
4 Discussion
The overall results for this study indicate that galcanezumab improves patient functioning, reduces disability, and improves patient self-perceptions of health state in a population with a previous failure of two to four migraine preventive medication categories, and with a statistically significant separation from placebo during the 3-month double-blind period of the CONQUER trial. The MSQ RF-R demonstrated significant separation in galcanezumab vs placebo groups as early as month 1, which continued through month 3. At month 3, significantly more patients achieved MSQ responder definition thresholds for galcanezumab vs placebo. In addition, the proportion of patients categorized as having minimal to no functional impairment on the RF-R domain of MSQ v2.1 was three times greater for the galcanezumab group than the placebo group at month 3 (EM: 26.7% vs 7.1%; CM: 14.8% vs 5.3%). There were significant improvements in the mean MSQ total score, RF-P, and EF domain scores, which were also greater for galcanezumab than placebo as early as month 1. A significantly greater reduction in headache-related disability was observed for galcanezumab vs placebo as measured by MIDAS. The total population had very severe disability at baseline per the average MIDAS score (~50), with the galcanezumab-treated arm experiencing a greater than 20-point reduction at month 3, compared with a less than 5-point reduction for placebo. Statistical separation was not observed for the EQ-5D-5L health index score; however, it was observed for the patients’ self-perception of health status measured by the VAS. The improvements observed during the double-blind period were consistent for the subpopulations with EM and CM. During the 3-month open-label period, the prior placebo group caught up to the previous galcanezumab group across the various patient-reported outcomes over all the populations in this study. In addition, the galcanezumab group continued to demonstrate incremental improvements from month 3 to month 6 across the various outcome measures.
These findings are important because patients with migraine live for years with disability, including functional or physical, emotional, social, and psychological impairment [28] and have low scores on the migraine-related patient-reported outcome measures [16, 17]. A specific migraine subpopulation with a greater burden includes those with a failure of two or more preventive treatment categories [1, 2, 29]. In the current study, patient-reported outcome measures did reflect severe functional impairment per the MSQ and very severe disability per the MIDAS at baseline. Compared with placebo, treatment with galcanezumab resulted in significant improvements in patient functioning on MSQ v2.1 total and domain scores and significantly larger reductions in disability, in the total population, and in the subpopulations with EM or CM. The results presented here are important for understanding how patient functioning and disability due to migraine changes with galcanezumab in a population with a previous failure of 2–4 migraine preventive medication categories. These data may facilitate clinician and patient decisions as they strive to meet the treatment goals of improving patient functioning, improving health-related QoL, and reducing disability [4, 5].
In our study, approximately 50% of the subpopulation with EM had little/no or mild disability at month 3 in the galcanezumab group, while the proportion was approximately 20% for the placebo group. The additional 3-month open-label period provided further insight into the subpopulation with CM. This subpopulation included a greater proportion of patients in the original galcanezumab group (treated for 6 months) who achieved RF-R responder definition thresholds at month 6 compared with the prior placebo group (treated with galcanezumab for 3 months), indicating additional improvements in patient functioning when this population continues treatment beyond 3 months. This demonstrates that galcanezumab can be an effective treatment in the patients who have experienced medication treatment failure with two to four preventive migraine treatment categories. The findings of our study align with the most recent AHS consensus statement and European Headache Federation guidelines, both of which recommend initiating preventive therapies targeting the CGRP pathway in patients with an inability to tolerate or with an inadequate response to two or more commonly prescribed oral preventive treatments [4, 5]. The results presented here for a population with two or more preventive category failures may help support the next treatment steps for patients with considerable clinical need.
The results presented in this study are consistent with the significant improvements seen in the CONQUER trial in the frequency of monthly migraine headache days as well as ≥50% and ≥ 75% response rates with galcanezumab vs placebo [12]. Least-squares mean changes in migraine monthly headache days from baseline to month 3 between galcanezumab and placebo groups were − 3.1, − 2.6, and − 3.7 days for the total population and subpopulations with EM and CM [12]. The changes reported for the RF-R domain score of MSQ v2.1 are broadly consistent with those reported in the subgroup analyses of the EVOLVE-1 and-2 (patients with EM) and REGAIN (patients with CM) studies [30, 31]. However, unlike the present study, the previous studies excluded patients with a history of three or more efficacy-related treatment failures. This study also evaluated the responder definition threshold for all three MSQ domains, the RF-R categorical analyses, and the changes in the EQ-5D-5L.
Changes in QoL outcome measures with other CGRP antagonists, namely erenumab and fremanezumab, in patients with migraine, and multiple preventive treatment failures have been reported. In the FOCUS study, fremanezumab vs placebo resulted in significantly greater improvements vs placebo at 4 weeks in MSQOL (p < 0.0001) in patients with EM and CM and with up to four treatment failures, as well as significant reductions in the MIDAS at month 3 and improved EQ-5D-VAS scores [32]. The LIBERTY study for erenumab, which included patients with EM, used a different QoL measure, the Migraine Physical Function Impact Diary (MPFID) and showed significant improvements with erenumab vs placebo at each of 3 months of double-blind treatment in MPFID domains of physical impairment and everyday activities [33].
The FOCUS, LIBERTY, and CONQUER trials have differences in their trial designs. FOCUS requirements for past treatment failures were specific to preventive treatment classes with failures because of a lack of improvement after ≥ 3 months of therapy at a stable dose, discontinuations because of adverse events/intolerability, or contraindications in the past 10 years for patients with EM and CM [32]. LIBERTY requirements for past medication treatment failures were specific to certain preventive treatments with failures due to efficacy after 2–3 months at acceptable doses, adverse events/intolerability, or contraindications in the past 5 years, in EM only [33]. CONQUER requirements for past treatment failures were specific to preventive treatment categories due to inadequate efficacy (maximum tolerated dose for ≥2 months), and adverse events/intolerability in the past 10 years in patients with EM and CM. In the CONQUER trial, contraindication to medication was not accounted as preventive treatment failure [12].
There are limitations to our research. First, the generalizability of the findings in the subpopulations with EM and CM needs to be explored, which would require larger scale real-world studies. Second, we did not address the failure of four or more treatment categories in our study, which occurs in some clinical cases. Third, in this study we determined the health-related QoL outcomes as monotherapy and not when used concurrently with other preventive medications. Fourth, there are limitations with the EQ-5D-5L for a disease state such as migraine. This instrument was collected during clinic site visits, not specifically on days with or without a migraine headache. Given that the EQ-5D-5L captures the current QoL state (reported for ‘today’), random errors for this outcome may have been introduced, which in turn affects the sensitivity of the instrument in this study. Other limitations include that this study was not specifically powered for these secondary objectives to evaluate health-related QoL and disability; these electronic patient-reported outcomes have various look-back periods, which could introduce recall bias.
5 Conclusions
Results from the CONQUER trial show that galcanezumab treatment leads to significant improvements in patient functioning and reductions in disability compared with placebo in patients with a history of medication failure with two to four preventive migraine treatment categories. These results are consistent with the main findings of the previous migraine efficacy studies relating to galcanezumab. Improvements in functioning were observed as early as month 1 and lasted through all 3 months of double-blind treatment. During the open-label period, patients previously receiving placebo showed improvements in functioning and reduction in disability with galcanezumab. These results were consistent in the subpopulations with EM and CM across both study periods.
Change history
07 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40261-022-01123-z
References
Martelletti P, Schwedt TJ, Lanteri-Minet M, Quintana R, Carboni V, Diener HC, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115.
Lucas CP-RP, Watson DP, Gaul C, Ramsden E, Ritter S, et al. A real‐world analysis of the burden of migraine in patients with prior treatment failure: evidence from the BECOME Study. In: 13(th) European Headache Federation Congress 2019: abstracts: May 30(th) to June 1(st) 2019. Athens, Greece. J Headache Pain. 2019;20(Suppl 1):109.
Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33.
American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18.
Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, et al. Correction to: European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):58.
Tepper SJ. CGRP and headache: a brief review. Neurol Sci. 2019;40(Suppl. 1):99–105.
Forderreuther S, Zhang Q, Stauffer VL, Aurora SK, Lainez MJA. Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: data from the phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. J Headache Pain. 2018;19(1):121.
Ford JH, Tassorelli C, Leroux E, Wang S, Ayer D, Nichols R, et al. Changes in patient functioning and disability: results from a phase 3, double-blind, randomized, placebo-controlled clinical trial evaluating galcanezumab for chronic migraine prevention (REGAIN). Qual Life Res. 2021;30:105–15.
Jedynak J, Eross E, Gendolla A, Rettiganti M, Stauffer VL. Shift from high-frequency to low-frequency episodic migraine in patients treated with galcanezumab: results from two global randomized clinical trials. J Headache Pain. 2021;22(1):48.
Vernieri F, Altamura C, Brunelli N, Costa CM, Aurilia C, Egeo G, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study). J Headache Pain. 2021;22(1):35.
Ailani J, Andrews JS, Rettiganti M, Nicholson RA. Impact of galcanezumab on total pain burden: findings from phase 3 randomized, double-blind, placebo-controlled studies in patients with episodic or chronic migraine (EVOLVE-1, EVOLVE-2, and REGAIN trials). J Headache Pain. 2020;21(1):123.
Mulleners WMKB, Láinez MJ, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b tria. Lancet Neurol. 2020;19(10):814–25.
ClinicalTrials.gov. A study of galcanezumab (LY2951742) in adults with treatment-resistant migraine (CONQUER). https://clinicaltrials.gov/ct2/show/NCT03559257. Accessed 25 Aug 2020.
Reuter U, Lucas C, Dolezil D, Hand AL, Port MD, Nichols RM, et al. Galcanezumab in Patients with Multiple Previous Migraine Preventive Medication Category Failures: Results from the Open-Label Period of the CONQUER Trial. Adv Ther. 2021;38(11):5465–83.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Martin BC, Pathak DS, Sharfman MI, Adelman JU, Taylor F, Kwong WJ, et al. Validity and reliability of the Migraine-Specific Quality of Life Questionnaire (MSQ Version 2.1). Headache. 2000;40(3):204–15.
Jhingran P, Davis SM, LaVange LM, Miller DW, Helms RW. MSQ: Migraine-Specific Quality-of-Life Questionnaire. Further investigation of the factor structure. Pharmacoeconomics. 1998;13(6):707–17.
Bagley CL, Rendas-Baum R, Maglinte GA, Yang M, Varon SF, Lee J, et al. Validating migraine-specific quality of life questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52(3):409–21.
Speck RM, Shalhoub H, Wyrwich KW, Yu R, Ayer DW, Ford J, et al. Psychometric validation of the role function restrictive domain of the Migraine Specific Quality-of-Life Questionnaire Version 2.1 electronic patient-eeported outcome in patients with episodic and chronic migraine. Headache. 2019;59(5):756–74.
Speck R, Yu R, Ford J, Ayer D, Akkala S, Wyrwich K. Responder definition thresholds of the Migraine-Specific Quality of Life Questionnaire Version 2.1 domains for use in patients with episodic and chronic migraine. In: 26th Annual Conference of the International Society for Quality of Life Research. Qual Life Res. 2019 Oct;28(Suppl. 1):1–190.
Speck RM, Kudrow D, Christie S, Ayer D, Ford J, Bushnell D. The migraine-specific quality of life questionnaire, role function restrictive domain: defining clinically meaningful categories of functional impairment severity (P-0226). Cephalalgia. 2021; 41(1S) 1–228.
Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl. 1):S20–8.
Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–15.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
EuroQol G. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
EuroQoL Group. EQ-5D-5L. https://euroqol.org/wp-content/uploads/2018/02/EQ-5D-5L_Crosswalk_Value_Sets.xls. Accessed 3 Sep 2020.
EuroQol Group. Valuation of EQ-5D. 2020. https://euroqol.org/eq-5d-instruments/valuation-of-eq-5d/. Accessed 3 Sep 2020.
Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache. 2018;58(Suppl. 1):17–32.
Kim SY, Park SP. The role of headache chronicity among predictors contributing to quality of life in patients with migraine: a hospital-based study. J Headache Pain. 2014;2(15):68.
Ruff DD, Ford JH, Tockhorn-Heidenreich A, Sexson M, Govindan S, Pearlman EM, et al. Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure. Cephalalgia. 2019;39(8):931–44.
Ruff DD, Ford JH, Tockhorn-Heidenreich A, Stauffer VL, Govindan S, Aurora SK, et al. Efficacy of galcanezumab in patients with episodic migraine and a history of preventive treatment failure: results from two global randomized clinical trials. Eur J Neurol. 2020;27(4):609–18.
Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394:1030–40.
Reuter UGP, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7.
Acknowledgments
The authors extend their gratitude to the patients and their families and caregivers for participating in the CONQUER trial. The authors also thank Karan Sharma from Eli Lilly Services India Pvt. Ltd. for providing medical writing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly and Company.
Conflict of interest/Competing interests
SJT received grants for research (no personal compensation) from Allergan, Amgen, Eli Lilly, Lundbeck, Neurolief, Novartis, Satsuma, and Zosano; served as a consultant and/or on advisory boards (honoraria) for Aeon, Align Strategies, Allergan/Abbvie, Alphasights, Amgen, Aperture Venture Partners, Aralez Pharmaceuticals Canada, Axsome Therapeutics, Becker Pharmaceutical Consulting, BioDelivery Sciences International, Biohaven, ClearView Healthcare Partners, CRG, Currax, Decision Resources, DeepBench, Eli Lilly, Equinox, ExpertConnect, GLG, Guidepoint Global, Healthcare Consultancy Group, Health Science Communications, HMP Communications, Impel, Lundbeck, M3 Global Research, Magellan Rx Management, Medicxi, Navigant Consulting, Neurolief, Nordic BioTech, Novartis, Pulmatrix, Reckner Healthcare, Relevale, SAI MedPartners, Satsuma, Slingshot Insights, Spherix Global Insights, Sudler and Hennessey, Synapse Medical Communications, Teva, Theranica, Thought Leader Select, Trinity Partners, XOC, and Zosano; received a salary from Dartmouth-Hitchcock Medical Center, American Headache Society, and Thomas Jefferson University; and received CME honoraria from the American Academy of Neurology, American Headache Society, Cleveland Clinic Foundation, Diamond Headache Clinic, Elsevier, Forefront Collaborative, Hamilton General Hospital, Ontario, Canada, Headache Cooperative of New England, Henry Ford Hospital, Detroit, Inova, Medical Learning Institute Peerview, Medical Education Speakers Network, Miller Medical Communications, North American Center for CME, Physicians’ Education Resource, Rockpointe, ScientiaCME, and WebMD/Medscape. JA has received honoraria for consulting from Amgen, Abbvie, Biohaven, Eli Lilly and Company, Lundbeck, Teva, Impel, Satsuma, Theranica, Axsome, and Vorso; received stock options from CtrlM for consulting; honoraria for speaking engagements from Abbvie, Amgen, Biohaven, Eli Lilly, Lundbeck, and Teva; provided advising and editorial services and received honoraria from Current Pain and Headache Reports, SELF, Neurology Live, and Medscape; and engaged in CME with Avent, Pri-Med, Forefront, Medscape, Clinical Care Options, Academy for Continued Healthcare Learning, Answers in CME , and NeurologyLive. JA’s institution has received funding for clinical trials for her work as a principal investigator from Allergan/Abbvie, Biohaven, Eli Lilly and Company, Satsuma, and Zosano. JHF, RMN, LQL, PK, and AT-H hold stocks, and are employees at Eli Lilly and Company. ALH is an employee at IQVIA.
Ethics approval
The CONQUER (NCT03559257) trial protocol was approved by the institutional review boards of each participating center before initiation of patient enrolment. The study adhered to the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, Council for International Organizations of Medical Sciences, and applicable local regulations.
Consent to participate
All patients provided informed consent before enrollment in the trial.
Consent for publication
The article does not contain any individual person’s data in any form. Therefore, consent for publication was not required.
Availability of data and material
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Code availability
Not applicable.
Authors’ contributions
ALH contributed to the analysis of the data. SJT, JA, JHF, LQL, PK, ALH, and ATH contributed to interpretation of the data All the authors contributed to the writing, critical revision, and final approval of the manuscript.
Additional information
The original online version of this article was revised: Affiliation details for Stewart J. Tepper and Janet H. Ford were incorrect.
The original online version of this article was revised: Affiliation details for Stewart J. Tepper and Janet H. Ford were incorrect and Zip Code for affiliation 4 and 5 should be 46285.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tepper, S.J., Ailani, J., Ford, J.H. et al. Effects of Galcanezumab on Health-Related Quality of Life and Disability in Patients with Previous Failure of 2–4 Migraine Preventive Medication Categories: Results from a Phase IIIb Randomized, Placebo-Controlled, Multicenter Clinical Trial (CONQUER). Clin Drug Investig 42, 263–275 (2022). https://doi.org/10.1007/s40261-021-01115-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-021-01115-5