Abstract
Background
Hesitation about using biosimilars still exists among healthcare professionals (HCPs), despite extensive experience with their use. Globally, several health organisations and societies from various specialties have issued biosimilar position statements to guide the use of biosimilars in their specialties. However, it is uncertain how similar or different their positions or recommendations are or whether these positions have evolved with the increased experience and availability of new evidence.
Objectives
The study aimed to describe and assess the recommendations of published position statements regarding several aspects of biosimilars across specialties and determine whether these positions have changed with the emergence of new evidence.
Methods
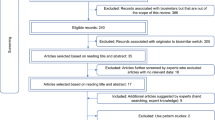
We systematically searched for published position statements of biosimilars in online databases and included statements written in English. The search was from the inception of the databases until May 2023. Two reviewers independently extracted the data. Only position statements that included recommendations to guide the use of biosimilars in clinical practice and were issued by health organisations and societies, including expert panels, were included. We synthesised recommendations on five aspects: prescribing practice, extrapolation of indication, interchangeability, treatment initiation with biosimilars in biologic-naïve patients, and pharmacovigilance.
Results
The review included 25 papers involving eight specialties, 16 of which were from European countries, 1 from an international organisation representing 49 countries, and 6 from various countries. The papers were published between 2009 and 2020, with 19 published between 2015 and 2020. Of the five aspects of biosimilars assessed, nearly half (11 of 25) of the papers at the time they were published did not base their positions on a scientific or evidence-based approach. Only 4 of the 25 position papers were identified as revisions of their previous papers. With increasing experience in biosimilars and the emergence of new evidence, about 60% (16 of 25) of the papers contained outdated recommendations, particularly on two aspects. They were extrapolations of indications and interchangeability (including switching). The recommendations for most papers for three other aspects were still appropriate. These were prescribing biosimilars by their brand name and active ingredient, initiating treatment with biosimilars in biologic-naïve patients, and monitoring the long-term safety of biosimilars through pharmacovigilance. For four of the revised papers, their position evolved from opposing indication extrapolation for biosimilars to accepting it, while the position of two papers shifted from not recommending biosimilar switching to permitting the practice. Meanwhile, most papers were against automatic substitution by pharmacists because the evidence for this practice was still limited.
Conclusions
Across specialties, the variability among the position statements is seen for extrapolation of indications for biosimilars and interchangeability (including switching). This requires a revision, considering the latest evidence and growing experience with the use of biosimilars in extrapolated indications and with switching.



Similar content being viewed by others
References
Barbier L, Vulto AG. Interchangeability of biosimilars: overcoming the final hurdles. Drugs. 2021;81:1897–903. https://doi.org/10.1007/s40265-021-01629-4.
Rathore AS, Stevenson JG, Chhabra H, Maharana C. The global landscape on interchangeability of biosimilars. Expert Opin Biol Ther. 2022;22:133–48. https://doi.org/10.1080/14712598.2021.1889511.
Cohen HP, McCabe D. The importance of countering biosimilar disparagement and misinformation. BioDrugs. 2020. https://doi.org/10.1007/s40259-020-00433-y.
Lyman GH, Balaban E, Diaz M, Ferris A, Tsao A, Voest E, et al. American Society of Clinical Oncology statement: biosimilars in oncology. J Clin Oncol. 2018;36:1260–5. https://doi.org/10.1200/JCO.2017.77.4893.
Mysler E, Azevedo VF, Danese S, Alvarez D, Iikuni N, Ingram B, et al. Biosimilar-to-biosimilar switching: what is the rationale and current experience? Drugs. 2021;81:1859–79. https://doi.org/10.1007/s40265-021-01610-1.
Barbier L, Mbuaki A, Simoens S, Vulto A, Huys I. The role of regulatory guidance and information dissemination for biosimilar medicines—the perspective of healthcare professionals and industry. 2019. https://www.ispor.org/docs/default-source/euro2019/20191011ispor-europe-regulatory-guidance-and-info-biosimilarslb-pdf.pdf?sfvrsn=81181f1e_0. Accessed on 12 Oct 2022
Dörner T, Strand V, Cornes P, Gonçalves J, Gulácsi L, Kay J, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016. https://doi.org/10.1136/annrheumdis-2016-209166.
Patrikos D, Boki K, Boumpas D, Vassilopoulos D. Position paper on biosimilars of the Greek Rheumatology Society and Professional Association of Greek Rheumatologists. Mediterr J Rheumatol. 2018;30:82–5. https://doi.org/10.31138/mjr.30.1.82.
Fernandes GS, Sternberg C, Lopes G, Chammas R, Gifoni MAC, Gil RA, et al. The use of biosimilar medicines in oncology—position statement of the Brazilian Society of Clinical Oncology (SBOC). Braz J Med Biol Res. 2018;51:1–7. https://doi.org/10.1590/1414-431X20177214.
Danese S, Fiorino G, Raine T, Ferrante M, Kemp K, Kierkus J, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease—an update. J Crohns Colitis. 2017;11:26–34. https://doi.org/10.1093/ecco-jcc/jjw198.
Torres T, Ferreira A, Ferreira P, Henriques M, Leite L, Magina S, et al. Portuguese position paper on the use of biosimilars in psoriasis. Acta Med Port. 2016;29:574–7. https://doi.org/10.20344/amp.8118.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. 2009. https://doi.org/10.1136/bmj.b2700.
Hillard T, Baber R. Peer review: the cornerstone of scientific publishing integrity. Climacteric. 2021;24(2):107–8. https://doi.org/10.1080/13697137.2021.1882140.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):1–11. https://doi.org/10.1186/s40779-020-00238-8.
de Boeck K, Castellani C, Elborn JS. Medical consensus, guidelines, and position papers: a policy for the ECFS. J Cyst Fibros Eur Cystic Fibrosis Soc. 2014;13:495–8. https://doi.org/10.1016/j.jcf.2014.06.012.
EMA. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed on 14 Sep 2022
Ruiz S. Biosimilars in the EU. Biosimilar Drug Prod Dev. 2017. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed on 15 Sep 2022
Callaghan JO, Barry SP, Bermingham M, Morris JM, Griffin BT. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol. 2019. https://doi.org/10.1007/s00228-018-2542-1.
Feagan BG. The challenges of switching therapies in an evolving multiple biosimilars landscape: a narrative review of current evidence. Adv Ther. 2020;37:4491–518. https://doi.org/10.1007/s12325-020-01472-1.
Afzali A, Furtner D, Melsheimer R, Molloy PJ. The Automatic substitution of biosimilars: definitions of interchangeability are not interchangeable. Adv Ther. 2021;38(5):2077–93. https://doi.org/10.1007/s12325-021-01688-9.
Frazer MB, Bubalo J, Patel H, Siderov J, Cubilla M, De Lemos M, et al. International Society of Oncology Pharmacy Practitioners global position on the use of biosimilars in cancer treatment and supportive care. J Oncol Pharm Pract. 2020. https://doi.org/10.1177/1078155219893441.
Gregory GP, Carrington C, Cheah CY, Hawkes EA, Irving IM, Siderov J, et al. A consensus statement on the use of biosimilar medicines in hematology in Australia. Asia Pac J Clin Oncol. 2020;16:211–21. https://doi.org/10.1111/ajco.13337.
Joshi GP, Benzon HT, Gan TJ, Vetter TR. Consistent definitions of clinical practice guidelines, consensus statements, position statements, and practice alerts. Anesth Analg. 2019;129:1767–70. https://doi.org/10.1213/ane.0000000000004236.
Azevedo VF, de Souza Meirelles E, Kochen JAL, Medeiros AC, Miszputen SJ, Teixeira FV, et al. Recommendations on the use of biosimilars by the Brazilian Society of Rheumatology, Brazilian Society of Dermatology, Brazilian Federation of Gastroenterology and Brazilian Study Group on Inflammatory Bowel Disease-Focus on clinical evaluation of monoclonal antibodies and fusion proteins used in the treatment of autoimmune diseases. Autoimmun Rev. 2015;14:769–73. https://doi.org/10.1016/j.autrev.2015.04.014.
Fonseca VA, Bloomgarden ZT, Dagogo-Jack S, Grunberger G, Einhorn D, Garber AJ, et al. AACE/ACE position statement on the use of follow-on biologics and biosimilars for endocrine diseases. Endocr Pract. 2017;23:1345–9. https://doi.org/10.4158/EP-2017-0052.
Jayagopal V, Drummond R, Nagi D. Association of British Clinical Diabetologists (ABCD) position statement on the use of biosimilar insulin. Br J Diabetes. 2018;18:171–4. https://doi.org/10.15277/bjd.2018.190.
Burri E, Juillerat P, Maillard MH, Manz M, Michetti P, Mottet C, et al. Swiss position statement on the use of biosimilars in inflammatory bowel disease. Swiss Med Wkly. 2019;149: w20148.
Devlin SM, Bressler B, Bernstein CN, Fedorak RN, Bitton A, Singh H, et al. Overview of subsequent entry biologics for the management of inflammatory bowel disease and Canadian Association of Gastroenterology position statement on subsequent entry biologics. Can J Gastroenterol. 2013;27:567–71. https://doi.org/10.4414/smw.2019.20148.
Argüelles-Arias F, Barreiro-de-Acosta M, Carballo F, Joaquín Hinojosa TT. Joint position statement by “Sociedad Española de Patología Digestiva”(Spanish Society of Gastroenterology) and “Sociedad Española de Farmacología”(Spanish Society of Pharmacology) on biosimilar therapy for inflammatory bowel disease. Rev Esp Enferm Dig. 2013;105(1):37–43. https://doi.org/10.4321/s1130-01082013000100006.
Fiorino G, Caprioli F, Daperno M, Mocciaro F, Principi M, Viscido A, et al. Use of biosimilars in inflammatory bowel disease: a position update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig Liver Dis. 2019;51:632–9. https://doi.org/10.1016/j.dld.2019.02.004.
Mularczyk A, Gonciarz M, Bartnik W, Durlik M, Eder P, Ga̧siorowska A, et al. Biosimilar medicines—their use in the treatment of inflammatory bowel diseases. Position statement of the Working Group of the Polish National Consultant in Gastroenterology. Prz Gastroenterol. 2014;9:1–3. https://doi.org/10.5114/pg.2014.40842.
Moayyedi P, Benchimol EI, Armstrong D, Yuan C, Fernandes A, Leontiadis GI. Joint Canadian Association of Gastroenterology and Crohn’s Colitis Canada position statement on biosimilars for the treatment of inflammatory bowel disease. J Can Assoc Gastroenterol. 2020;3:e1-9. https://doi.org/10.1093/jcag/gwz035.
Becker FG, Cleary M, Team RM, Holtermann H, The D, Agenda N, et al. Use of biosimilars in paediatric inflammatory bowel disease: an updated position statement of the paediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2019. https://doi.org/10.1097/MPG.0000000000002141.
Barosi G, Bosi A, Abbracchio MP, Danesi R, Genazzani A, Corradini P, et al. Key concepts and critical issues on epoetin and filgrastim biosimilars: a position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow Transplantation. Haematologica. 2011;96:937–42. https://doi.org/10.3324/haematol.2011.041210.
Gastl G, Geissler D, Geissler K, Lang A, Ludwig H, Müller M, et al. ASHO position paper on biosimilars. Memo. 2009;2:232–3. https://doi.org/10.1007/s12254-009-0162-2.
De Castro NML, Matilla-Fernández MB, Fraga-Fuentes MD, Mangues-Bafalluy I, Asensi-Díez R, Cajaraville-Ordoñana G, et al. Spanish Society of Hospital Pharmacy position paper on biosimilar medicines. Farm Hosp. 2018;42:180–3. https://doi.org/10.7399/fh.10973.
Goncalves J, Matos de Brito P, Batista A, Feio J, Machado F, Aperta J, et al. Position paper from the Portuguese Association of Hospital Pharmacists for biosimilar therapeutic antibodies. J Clin Pharm Ther. 2017;42:239–43. https://doi.org/10.1111/jcpt.12477.
Süle A, Jørgensen F, Horák P, Peppard J, Kohl S. Biosimilar medicines. Eur J Hosp Pharm. 2019;26(2):117–8. https://doi.org/10.1136/ejhpharm-2018-001821.
Fiorino G, Girolomoni G, Lapadula G, Orlando A, Danese S, Olivieri I. The use of biosimilars in immune-mediated disease: a joint Italian Society of Rheumatology (SIR), Italian Society of Dermatology (SIDeMaST), and Italian Group of Inflammatory Bowel Disease (IG-IBD) position paper. Autoimmun Rev Elsevier BV. 2014;13:751–5. https://doi.org/10.1016/j.autrev.2014.02.004.
Tabernero J, Vyas M, Giuliani R, Arnold D, Cardoso F, Casali PG, et al. Biosimilars: a position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open; 2016. https://doi.org/10.1136/esmoopen-2016-000142
Araújo FC, Sepriano A, Teixeira F, Jesus D, Rocha TM, Martins P, et al. The Portuguese Society of Rheumatology position paper on the use of biosimilars—2017 update. Acta Reumatol Port. 2018;42(3):219–28.
Atzeni F, Sebastiani M, Ricci C, Celano A, Gremese E, Iannone F, et al. Position paper of Italian rheumatologists on the use of biosimilar drugs. Clin Exp Rheumatol. 2015;33:1–4.
Tolonen HM, Falck J, Kurki P, Ruokoniemi P, Hämeen K. Is there any research evidence beyond surveys and opinion polls on automatic substitution of biological medicines? A systematic review. BioDrugs. 2021;35:547–61. https://doi.org/10.1007/s40259-021-00493-8.
Casadevall N, Edwards IR, Felix T, Graze PR, Litten JB, Strober BE, et al. Pharmacovigilance and biosimilars: considerations, needs and challenges. Expert Opin Drug Saf. 2013. https://doi.org/10.1517/14712598.2013.783560.
Felix T, Jordan JB, Akers C, Patel B, Drago D. Current state of biologic pharmacovigilance in the European Union: improvements are needed. Expert Opin Drug Saf. 2019;18(3):231–40. https://doi.org/10.1080/14740338.2019.1577818.
Ben-Horin S, Vande Casteele N, Schreiber S, Lakatos PL. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol. 2016;14:1685–96.
Wiland P, Batko B, Brzosko M, Kucharz EJ, Samborski W, Swierkot J, et al. Biosimilar switching—current state of knowledge. Reumatologia. 2018;56:234–42. https://doi.org/10.5114/reum.2018.77975.
Avila-Ribeiro P, Fiorino G, Danese S. The experience with biosimilars of infliximab in inflammatory bowel disease. Curr Pharm Des. 2017;23:6759–69. https://doi.org/10.2174/1381612824666171204095342.
Radin M, Sciascia S, Roccatello D, Cuadrado MJ. Infliximab biosimilars in the treatment of inflammatory bowel diseases: a systematic review. BioDrugs. 2017;31:37–49. https://doi.org/10.2174/1381612824666171204095342.
Dipasquale V, Romano C. Biosimilar infliximab in paediatric inflammatory bowel disease: efficacy, immunogenicity and safety. J Clin Pharm Ther. 2020;45:1228–34. https://doi.org/10.1111/jcpt.13239.
Gascon P, Krendyukov A, Mathieson N, Natek M, Aapro M. Extrapolation in practice: lessons from 10 years with biosimilar filgrastim. BioDrugs. 2019;33:635–45. https://doi.org/10.1007/s40259-019-00373-2.
Ebbers HC, Schellekens H. Are we ready to close the discussion on the interchangeability of biosimilars? Drug Discov Today. 2019;24:1963–7. https://doi.org/10.1016/j.drudis.2019.06.016.
EMA. Biosimilar medicines can be interchanged. 2022. https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged. Accessed on 11 Oct 2022
Kurki P, Barry S, Bourges I, Tsantili P, Wolff-Holz E. Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: a regulatory perspective. Drugs. 2021;81(16):1881–96. https://doi.org/10.1007/s40265-021-01601-2.
Bakalos G, Zintzaras E. Drug discontinuation in studies including a switch from an originator to a biosimilar monoclonal antibody: a systematic literature review. Clin Ther. 2019;41:155-173.e13. https://doi.org/10.1016/j.clinthera.2018.11.002.
Petrie KJ, Rief W. Psychobiological mechanisms of placebo and nocebo effects: pathways to improve treatments and reduce side effects. Annu Rev Psychol. 2019;70:599–625. https://doi.org/10.1146/annurev-psych-010418-102907.
Herndon TM, Ausin C, Brahme NN, Schrieber SJ, Luo M, Andrada FC, Kim C, Sun W, Zhou L, Grosser S, Yim S, Ricci MS. Safety outcomes when switching between biosimilars and reference biologics: a systematic review and meta-analysis. PLoS One. 2023;18(10): e0292231. https://doi.org/10.1371/journal.pone.0292231.
Kristensen LE, Alten R, Puig L, Philipp S, Kvien TK, Antonia M, et al. Non-pharmacological effects in switching medication: the nocebo effect in switching from originator to biosimilar agent. BioDrugs. 2018;32:397–404. https://doi.org/10.1007/s40259-018-0306-1.
Reinivuori T, Kurki P, Chamberlain P. Immunogenicity assessment of biosimilars. Pharm Med. 2018;32(2):103–21. https://doi.org/10.1007/s40290-018-0231-0.
Iskit AB. Key concepts in biosimilar medicines: what physicians must know. North Clin Istanb. 2022;9(1):86–91. https://doi.org/10.14744/nci.2021.84669.
Piccirillo RA, Parish J. Pharmacovigilance principles: the building blocks of benefit-risk assessments. Glob Clin Transl Res. 2022;4:1–7. https://doi.org/10.36316/gcatr.04.0045.
Oza B, Radhakrishna S, Pipalava P, Jose V. Pharmacovigilance of biosimilars—why is it different from generics and innovator biologics? J Postgrad Med. 2019;65(4):227–32. https://doi.org/10.4103/jpgm.JPGM_109_19.
Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–8.
Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol Drug Saf. 2007;16:223–38.
Covic A, Cannata-andia J, Cancarini G, Coppo R, Fraz M, Ronco P, et al. Biosimilars and biopharmaceuticals: what the nephrologists need to know – a position paper by the ERA-EDTA Council. Nephrol Dial Transplant. 2008;23:3731–7.
American Academy of Ophthalmology. The use of biosimilars in ophthalmic practice. 2022 https://www.aao.org/clinical-statement/use-of-biosimilars-in-ophthalmic-practice. Accessed on 12 Dec 2022
Acknowledgements
The authors would like to thank the Director-General of Health Malaysia for his permission to publish this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship or publication of this systematic review.
Conflict of Interest
The authors declare that there is no conflict of interest.
Availability of Data and Material
All relevant materials for this systematic review are incorporated in the article, are fully cited or are supplied as electronic supplementary material.
Ethics Approval
Ethical approval was not applicable for this systematic review.
Consent
Consent was not applicable for this systematic review.
Author Contributions
The conceptualisation and design of this study were undertaken by NMS and ZA. The search was conducted by NMS. The screening process for document inclusion was conducted independently by NMS and ZA. The data were extracted by NMS, subsequently verified by ZA and subjected to a thorough review. The initial draft of the manuscript was prepared by NMS. ZA and AK conducted an extensive assessment of the manuscript with regards to its significant intellectual content. The draft of the manuscript underwent critical revisions by ZA. The final manuscript for publication was read and approved by all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohd Sani, N., Aziz, Z. & Kamarulzaman, A. Use of Biosimilars: A Systematic Review of Published Position Statements and Recommendations from Health Organisations and Societies. BioDrugs (2024). https://doi.org/10.1007/s40259-024-00649-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s40259-024-00649-2