Abstract
Pure Li4Ti5O12 with high crystallinity was successfully synthesized by a solvothermal process. The effects of initial Li/Ti ratio and post-heating temperature on the phase evolution, particle morphology and electrochemical properties were systematically investigated. Excess lithium, compared to the theoretical value in Li4Ti5O12, was required to get pure Li4Ti5O12 due to the condensation reaction. Low Li/Ti ratio led to the appearance of secondary phase rutile TiO2, while high heat-treatment temperature easily resulted in particle agglomeration of Li4Ti5O12 powder. The existence of rutile TiO2 decreased the specific capacity, and the particle agglomerate had a strong negative effect on the rate capability of electrode. The sample synthesized at the optimized condition exhibited a stable specific capacity of 150 mAh/g and a good rate performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries have attracted much attention as important energy supply in portable electronic devices, hybrid electrical vehicles and electrical vehicles because of their high power and energy density [1–3]. At present, new electrode materials exhibiting excellent rate capability and high safety performance are urgently demanded to meet the requirement of electrical vehicles. The spinel lithium titanate Li4Ti5O12 is being considered as an ideal anode material in lithium-ion batteries due to its unique characteristics, including very flat charge/discharge voltage plateaus and a small structural change during charge/discharge processes. The zero-strain insertion characteristic provides material with an excellent cycling performance [4, 5], while that of the flat operating voltage at 1.55 V (versus Li+/Li) can avoid the deposition of dendritic metallic lithium, therefore a high operational safety can be expected [6, 7]. Despite the high Li deintercalation/intercalation potential, it can, in principle, be coupled with high-voltage cathodes such as LiNi0.4Mn1.6O4 to provide a cell with an operating voltage of approximately 3 V [8].
However, Li4Ti5O12 is an insulator, its rate capability is greatly limited by its inherently low lithium-ion diffusivity and electronic conductivity. Typical approaches to resolve this problem include employing nanoparticles to reduce the diffusion length of lithium ions, and increase the contact area between the electrode and the electrolyte [9–11], doping Li4Ti5O12 with aliovalent cation (Al3+, Ga3+, Co3+, Mg2+, Ta5+) [12–14] in Li and Ti sites to produce mixed valence of Ti3+/Ti4+, and thus increase the electronic conductivity, and incorporating directly the conductive second phase (carbon, Ag and so on) [7, 15, 16].
Actually, the particle size and the crystalline ordering degree have strong impacts on the electrochemical properties of electrode. Small-sized active material can not only reduce the lithium-ion diffusion distance, but also increases the contact area with conductive reagent and electrolyte solution, thus can decrease the local current density and mitigate the electrode polarization. The high crystallinity is believed to be beneficial to the good cycling stability of electrode [9]. Compared to the doped materials, the pure material is easier to be synthesized and handled in practical operations. Many methods, including conventional solid-state reaction [12–14], sol–gel method [6, 17, 18], solvothermal technique [19–22], combustion synthesis [23], rheological phase reaction [11] and other synthesis routes, have been exploited to prepare Li4Ti5O12 materials. Among them, solvothermal technique with simple and flexible controls has spurred considerable interests. Although Li4Ti5O12 powders prepared by solvothermal method have been investigated extensively [19–22], the work concerning the effect of the synthesis parameters on the electrochemical properties is very limited. Considering that the practical composition of the synthesized material via solvothermal route is usually different from the nominal composition, in this work, the effect of initial Li/Ti ratio in starting solution on the phase purity and the electrochemical properties was investigated. The influence of the post-heat-treatment temperature on the electrochemical performance of Li4Ti5O12 electrode was also addressed. The synthesized Li4Ti5O12 exhibited excellent rate capability and cycling performance, showing the solvothermal synthesis is a promising method to obtain high-performance Li4Ti5O12 anode material.
Experimental
Materials synthesis
The spherical precursors of Li4Ti5O12 powders were synthesized by solvothermal method using lithium acetate (LiAc, AR ≥99.0 %, Beijing Yili Fine Chemicals Co., Ltd.) and tetrabutyl titanate [Ti(O(CH2)3CH3)4, denoted as Ti(OR)4, AR ≥99.0 %, Beijing Jinlong Chemical Reagent Co., Ltd.) as Li and Ti cation sources, respectively. The molar ratios of the mixtures were fixed at different proportions (Li/Ti ratio = 0.8–1.4). Ti(OR)4 was dissolved in ethanol under magnetic stirring, and then LiAc was added into the mixtures with further stirring to obtain a homogeneous dispersion system. The concentration of Ti(OR)4 in ethanol was 1.4 × 10−4 mol/ml. The transparent solution was then transferred into a 100 ml teflon-lined stainless steel autoclave and kept at 180 °C for 24 h. After cooling down to room temperature, a milky white precursor was prepared. The produced powder was washed and filtered with ethanol to eliminate the unreacted reagents and the partial organic compounds. The precipitate was dried at 80 °C in air for 3 h. To obtain well-crystallized Li4Ti5O12, the precursor was calcined at 800 °C for 2 h in air with a heating rate of 5 °C/min. At last, the effect of heat-treatment temperature on the particle morphology and electrochemical properties was investigated. The precursor with the optimal Li/Ti ratio based on the above results was subjected to calcination at temperatures of 400, 600, and 800 °C, respectively.
Characterization
Phase purity and crystallinity of the synthesized samples were identified by means of powder X-ray diffraction (XRD) performed on a Rigaku D/MAX-A diffractometer with Cu Kα radiation source (λ = 1.54056 Å) in the range of 10° ≤ 2θ ≤ 90°, while the morphology and size distribution of precursors and post-treated powders were observed on a LEO-1450 scanning electron microscope (SEM). The actual molar ratio of Li/Ti in the precursor was determined by inductively coupled plasma atomic emission spectrometer (ICP-AES) (IRIS Intrepid II XSP). The thermal behavior of the precursor powders was examined by a thermogravimetry–differential thermal analysis (TG–DTA) instrument (Netzsch STA 449 C) with a heating rate of 10 °C/min from room temperature to 900 °C under air.
Electrochemical measurement
Half-cells were used to evaluate the electrochemical performance. Celgard 2400 microporous membrane was used as separator, a lithium foil as negative electrode, and 1 M LiPF6 dissolved in a mixture of ethyl methyl carbonate (EMC), dimethyl carbonate (DMC) and ethylene carbonate (EC) with a volume ratio of 1:1:1 as the electrolyte. The working electrode was made from the mixture of active material Li4Ti5O12, acetylene black (AB) and polyvinylidene fluoride (PVdF) in a weight ratio of 85:10:5. The slurry of the mixture was uniformly pasted on aluminum foil, dried and cut into disks. Then the working electrode was dried under vacuum at 120 °C for 24 h before electrochemical evaluation. Cell assembly was carried out in a glove box filled with high-purity argon where the oxygen and water vapor contents were each <1 ppm.
The galvanostatic charging–discharging test was employed to evaluate the cycle stability and electrochemical capacity of the samples by a computer-controlled Land CT 2001A battery test system. The cell was cycled at different current densities, and the cut-off voltage for charging and discharging processes was 1.0–2.5 V, respectively. The specific capacity was calculated based on the whole weight of the synthesized samples, including Li4Ti5O12 and possible impurity TiO2. AC electrochemical impedance spectroscopy was measured by a Solartron 1260/1287 (UK) impedance analyzer in the frequency range from 1 MHz to 0.01 Hz at the state of fully lithiated. Experiments were carried out at room temperature.
Results and discussion
Effect of Li/Ti molar ratio
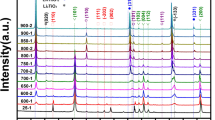
Due to the complicated coordination process of tetrabutyl titanate [Ti(OR)4] with LiAc in the solvothermal condition, the chemical composition of the resultant is usually different from the nominal composition. To prepare high-purity Li4Ti5O12, it is essential to control the starting molar ratio of Li/Ti in the reaction mixtures. Different solutions with Li/Ti ratio = 0.8, 1.0, 1.2 and 1.4 were solvothermally treated in an autoclave, and the precipitates after heat treatment were subjected to the phase identification by XRD. The results are shown in Fig. 1. The samples with Li/Ti molar ratios of 0.8, 1.0, 1.2, 1.4 are named as LTO0.8, LTO1.0, LTO1.2 and LTO1.4, respectively. For all samples, diffraction peaks indexed on the cubic spinel phase Li4Ti5O12 with Fd\(\overline{ 3}\)m space group (JCPDS No. 49-0207) are observed. However, some additional diffraction peaks corresponding to rutile TiO2 with P42/mnm space group (JCPDS No. 21-1276) are also detected at the same time except for sample LTO1.4. Although the theoretical Li/Ti ratio of Li4Ti5O12 is 0.8, sample LTO0.8 shows strong TiO2 peaks, demonstrating that a significant amount of lithium remained in the solution. With increasing Li/Ti ratio, the relative peak intensity of Li4Ti5O12 increases gradually, while that of TiO2 decreases remarkably and finally tends to disappear. When the molar ratio of Li/Ti reaches 1.4, the single-phase spinel type Li4Ti5O12 without any impurity is obtained.
Compared to the theoretical ratio, the excess lithium required to obtain pure single-phase Li4Ti5O12 in the solvothermal synthesis route is related to the reaction mechanism of Ti(OR)4 and LiAc. Under solvothermal condition, part of tetrabutyl titanate Ti(OR)4 may first resolve in ethanol and take alcoholysis reaction to form Ti(OR)4 − x(OH) x , as expressed by reaction (1). The Ti(OR)4 − x(OH) x monomers then condense with LiAc to produce Ti(OR)4 − x(OH)x − y(OLi) y via reaction (2). Different condensation reactions occur among the clusters of Ti(OR)4 − x(OH)x − y(OLi) y . The condensation may occur between Ti–(OH) groups, producing H2O as by-product, while the reaction may also occur between Ti–OH and Ti–OLi producing LiOH, corresponding to reaction (3) and (4), respectively. The product of the solvothermal reaction was composed of mainly Li–Ti–O amorphous material with some remained organic radicals that already show the basic lattice structure of Li4Ti5O12, as shown in Fig. 6 (sample LTO), and small amount of LiOH as by-product. Therefore, part of the lithium remains in the solution. If Li source LiAc is not excess in the starting materials than the theoretically required amount, then the attached lithium in the solid particles after condensation reactions (3) and (4) will be inadequate to form Li4Ti5O12, leading to the generation of trace of TiO2 after heat treatment, as evidenced in Fig. 1.
Analyzed by means of ICP-AES, the actual Li/Ti molar ratio in the precursor of sample LTO1.4 is 1.0, indicating that some lithium remained in solution, which is in good agreement with reaction (4). Considering that the Li/Ti is 0.8 in Li4Ti5O12, the high Li/Ti (1.0) in the precursor of LTO1.4 implies that some lithium is lost during calcination [17, 24, 25].
To investigate the thermal decomposition behavior of the precursor, TG–DTA examination was performed on the precursor of sample LTO1.4. The result is shown in Fig. 2. In the TG curve, the total mass loss obtained in the temperature range from room temperature to 900 °C is approximately 25.64 %. The first step of mass loss about 19.06 % occurred between room temperature and 180 °C, corresponding to the endothermic peak at 86.7 °C in the DTA curve, is due to the removal of adsorbed ethanol and water molecules. The second step of mass loss occurred in 180–600 °C, associating with the exothermic peak at 271.9 °C, is attributed to the loss of the organics and the formation of Li4Ti5O12 phase. When the temperature is above 600 °C, no major weight loss was examined, indicating that the decomposition of organic groups was completed.
The particle morphologies of Li4Ti5O12 precursors with different molar ratios of Li/Ti are shown in Fig. 3. The samples are all composed of well-dispersed spherical particles with some small particles adhering to their surface. With increasing Li/Ti ratio, the number of small particles decreases remarkably, the average particle size reduces from 3 to 2 μm and the particle distribution tends to be more uniform.
After heat treatment at 800 °C in air, the powders exhibit even smaller particle size of about 1–1.5 μm and smoother particle surface, as presented in Fig. 4. This is considered to be mainly resulted from the decomposition of organic groups on the particle surface of Li4Ti5O12 precursor.
With Li4Ti5O12/Li half-cell, the cycling performances of samples LTO0.8, LTO1.0, LTO1.2 and LTO1.4 at 0.2 C were examined, and the results are shown in Fig. 5a. All the synthesized active materials of LTO0.8, LTO1.0, LTO1.2 and LTO1.4 display a stable cycling performance. The specific capacity of these samples increases with increasing Li/Ti ratio, and sample LTO1.4 exhibits the highest specific capacity among these samples. The existence of rutile TiO2 is apparently detrimental to lithium storage capacity of the samples. This is in good agreement with the literature results that only small amounts of lithium ions can be intercalated in rutile TiO2 at room temperature [26, 27].
The initial discharge–charge potential curves of samples LTO0.8, LTO1.0, LTO1.2 and LTO1.4 are shown in Fig. 5b. All the samples exhibit only one typical discharge/charge potential plateau of Li4Ti5O12 representing a two-phase reaction between Li4Ti5O12 and Li7Ti5O12 [28], no plateau corresponds to the lithiation/delithiation process of rutile TiO2 [29]. The results indicate that the impurity rutile TiO2 in the samples does not have electrochemical activity in this condition.
Effect of heat-treatment temperature
Considering that the electrochemical performances of Li4Ti5O12 are closely related with its crystallinity, a further heat-treatment investigation was conducted. Precursor of sample LTO1.4, showing pure phase and high specific capacity after 800 °C-treatment, was selected to subject the heat-treatment test to clarify the effect of treating temperature on the structure and electrochemical performance of synthesized Li4Ti5O12. The precursor and the samples heat treated at 400, 600 and 800 °C are denoted as LTO, LTO4, LTO6 and LTO8, accordingly. Figure 6 shows the XRD patterns of these samples. They are all identified with a pure cubic phase Li4Ti5O12. The precursor features amorphous structure. The appearance of messy background and the broad peaks with weak intensities indicate the poor crystallinity of the formed precursor. The peak intensities of the spinel phase significantly enhance when the heat-treatment temperature increases, exhibiting the improvement of crystallinity. When the heat-treating temperature is up to 600 °C, good crystallinity and pure spinel phase Li4Ti5O12 have been identified from the XRD data. The lattice parameters calculated according to the XRD data are 8.3395 (3), 8.3661 (9), 8.3695 (9) Å for LTO4, LTO6 and LTO8, respectively, which are in good agreement with previous reported values [6, 30, 31]. The average crystallite sizes calculated from the full width at half maximum (FWHM) of peak (111) are 11.47, 44.70 and 50.04 nm for LTO4, LTO6 and LTO8, respectively. The crystallite size increases with the increase of heat-treatment temperature. The results demonstrate that pure Li4Ti5O12 powders with high crystallinity, small crystallite size can be successfully synthesized by solvothermal method. Additionally, the synthesized Li4Ti5O12 powders display high dispersity and good sphericity without particle agglomeration, which are beneficial for both of electrochemical performance and practical electrode preparation. In most previous studies, spinel Li4Ti5O12 was synthesized by solid-state reaction at 800–1000 °C for 5–24 h [12–14]. Compared with the conventional solid-state technique, the calcination temperature of the solvothermally synthesized products was greatly decreased and the dwelling time was significantly shortened.
The morphologies of the Li4Ti5O12 powders after heat treatment are shown in Fig. 7. With increasing temperature, the particle size decreases slightly and the particle surface becomes much smoother. However, the 800 °C-treated sample LTO8 shows an obvious particle agglomeration, several small particles stick together to form a large one, showing a poor dispersity.
To investigate the influence of heat-treatment temperature on the electrochemical performance, the cycling performances of the samples LTO4, LTO6 and LTO8 were examined. As shown in Fig. 8, sample LTO4 displays extremely high irreversible capacity compared to other samples, mainly resulting from the remained organic groups on the particle surface due to its lower heating temperature. All samples show good cycling stability since the second cycle, while sample LTO6 exhibits the highest electrochemical capacity of 150 mAh/g among all the samples. To understand the dependence of electrochemical capacity of LTO on the heating temperature, several aspects should be taken into account. The first is the crystallinity of powders. High crystallinity is usually beneficial to the good electrochemical performance of Li4Ti5O12 negative electrode [9]. On the other hand, particle agglomeration is unfavorable for the diffusion of lithium ions due to the elongated diffusion distance. The characteristics of good crystallinity and small particle size of sample LTO6 guarantee its high specific capacity. Due to the obvious particle agglomeration, sample LTO8 exhibits the lowest specific capacity, even worse than sample LTO4. Besides the long diffusion path of lithium ions of the agglomerated particles that impedes the kinetics of lithium intercalation into the LTO8 host structure, the lowered specific surface area should be taken into consideration because it can reduce the contact area between the electrode and the AB, and therefore deteriorate the electronic conduction. Furthermore, the lowered specific surface area can result in the increase in actual current density on particle surface, and thus cause a large polarization of the electrode, which is another reason for the low specific capacity of sample LTO8 [6, 31–33].
Figure 9 illustrates the rate capabilities of LTO6 and LTO8 electrodes. The discharge capacity of both samples decreases gradually with increasing current density. Nevertheless, the sample LTO6 maintains a higher reversible capacity at the same current density as compared to the sample LTO8, indicating the good rate capability of sample LTO6. At lower current density of 0.2 C, both the samples reveal relatively good capacity characteristics due to the sufficient insertion/extraction of lithium ions. High current density makes their specific capacity different. The capacity ratio of C2 C/C0.2 C is 0.65 and 0.44 for samples LTO6 and LTO8, respectively. The good rate capability as well as the high specific capacity of sample LTO6 makes it a promising material for a better commercial application.
AC impedance measurement was performed on LTO6 and LTO8 electrodes, the results are shown in Fig. 10. Each curve is comprised of a depressed semi-circle in the high-medium frequency region and an oblique straight line in the low frequency region. The semi-circle in the high-medium frequency region is mainly related to the charge-transfer process, while the inclined line in the low frequency region is attributed to the Warburg impedance that reflects lithium-ion diffusion behavior in the Li4Ti5O12 electrode [34]. Sample LTO6 displays a significantly lower resistance of charge transfer than that of sample LTO8, which is certainly associated with the larger specific surface area of active material LTO6. These results can interpret well the experimental results in Figs. 8 and 9.
Conclusion
Pure and well-crystallized Li4Ti5O12 powders were synthesized from Ti(O(CH2)3CH3)4 and LiAc by solvothermal route involving a further heat treatment at relatively low temperature with short dwelling time. The Li/Ti molar ratio in starting materials and post-heat-treatment temperature have strong impacts on the electrochemical performance of Li4Ti5O12 anode material. Excess lithium, compared to the theoretical value in Li4Ti5O12, is required for the synthesis of pure-phase Li4Ti5O12 in this preparation condition. Low Li/Ti ratio (<1.4 in atom) easily results in the coexistence of the secondary phase TiO2, while high treatment temperature leads to particle agglomeration of Li4Ti5O12 powders. The former causes the decrease in specific capacity, but the latter deteriorates the rate capability of electrode. The 600 °C-treated sample LTO6 with starting LiAc/Ti(OR)4 = 1.4 exhibits the highest specific capacity and best rate performance due to its high purity, good crystallinity and excellent dispersity. At 0.5 C, LTO6 displays a stable reversible capacity of ca. 150 mAh/g. EIS measurement reveals that LTO6 has lower charge-transfer resistance compared to the 800 °C-treated LTO8, which is mainly attributable to the high specific surface area and small particle size of sample LTO6.
References
Tarascon, J.-M., Armand, M.: Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001)
Sato, K., Noguchi, M., Demachi, A., Oki, N., Endo, M.: A mechanism of lithium storage in disordered carbons. Science 264, 556–558 (1994)
Scrosati, B., Garche, J.: Lithium batteries: status, prospects and future. J. Power Sources 195, 2419–2430 (2010)
Ohzuku, T., Ueda, A., Yamamoto, N.: Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 142, 1431–1435 (1995)
Ariyoshi, K., Yamato, R., Ohzuku, T.: Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries. Electrochim. Acta 51, 1125–1129 (2005)
Jiang, C., Ichihara, M., Honma, I., Zhou, H.: Effect of particle dispersion on high rate performance of nano-sized Li4Ti5O12 anode. Electrochim. Acta 52, 6470–6475 (2007)
Yuan, T., Cai, R., Shao, Z.: Different effect of the atmospheres on the phase formation and performance of Li4Ti5O12 prepared from ball-milling-assisted solid-phase reaction with pristine and carbon-precoated TiO2 as starting materials. J. Phys. Chem. C 115, 4943–4952 (2011)
Patoux, S., Daniel, L., Bourbon, C., Lignier, H., Pagano, C., Cras, F.L., Jouanneau, S., Martinet, S.: High voltage spinel oxides for Li-ion batteries: from the material research to the application. J. Power Sources 189, 344–352 (2009)
Guerfi, A., Sévigny, S., Lagacé, M., Hovington, P., Kinoshita, K., Zaghib, K.: Nano-particle Li4Ti5O12 spinel as electrode for electrochemical generators. J. Power Sources 119–121, 88–94 (2003)
Prakash, A.S., Manikandan, P., Ramesha, K., Sathiya, M., Tarascon, J.-M., Shukla, A.K.: Solution-combustion synthesized nanocrystalline Li4Ti5O12 as high-rate performance Li-ion battery anode. Chem. Mater. 22, 2857–2863 (2010)
Yin, S.Y., Song, L., Wang, X.Y., Zhang, M.F., Zhang, K.L., Zhang, Y.X.: Synthesis of spinel Li4Ti5O12 anode material by a modified rheological phase reaction. Electrochim. Acta 54, 5629–5633 (2009)
Huang, S., Wen, Z., Zhu, X., Lin, Z.: Effects of dopant on the electrochemical performance of Li4Ti5O12 as electrode material for lithium ion batteries. J. Power Sources 165, 408–412 (2007)
Chen, C.H., Vaughey, J.T., Jansen, A.N., Dees, D.W., Kahaian, A.J., Goacher, T., Thackeray, M.M.: Studies of Mg-substituted Li4 − xMgxTi5O12 spinel electrodes (0 ≤ x ≤ 1) for lithium batteries. J. Electrochem. Soc. 148, A102–A104 (2001)
Wolfenstine, J., Allen, J.L.: Electrical conductivity and charge compensation in Ta doped Li4Ti5O12. J. Power Sources 180, 582–585 (2008)
Cheng, L., Yan, J., Zhu, G.N., Luo, J.Y., Wang, C.X., Xia, Y.Y.: General synthesis of carbon-coated nanostructure Li4Ti5O12 as a high rate electrode material for Li-ion intercalation. J. Mater. Chem. 20, 595–602 (2010)
Huang, S., Wen, Z., Zhang, J., Yang, X.: Improving the electrochemical performance of Li4Ti5O12/Ag composite by an electroless deposition method. Electrochim. Acta 52, 3704–3708 (2007)
Sorensen, E.M., Barry, S.J., Jung, H.-K., Rondinelli, J.R., Vaughey, J.T., Poeppelmeier, K.R.: Three-dimensionally ordered macroporous Li4Ti5O12: effect of wall structure on electrochemical properties. Chem. Mater. 18, 482–489 (2006)
Bach, S., Pereira-Ramos, J.P., Baffier, N.: Electrochemical properties of sol–gel Li4/3Ti5/3O4. J. Power Sources 81–82, 273–276 (1999)
Fattakhova, D., Petrykin, V., Brus, J., Kostlánová, T., Dědeček, J., Krtil, P.: Solvothermal synthesis and electrochemical behavior of nanocrystalline cubic Li–Ti–O oxides with cationic disorder. Solid State Ion. 176, 1877–1885 (2005)
Kostlánová, T., Dědeček, J., Krtil, P.: The effect of the inner particle structure on the electronic structure of the nano-crystalline Li–Ti–O spinels. Electrochim. Acta 52, 1847–1856 (2007)
Li, J., Tang, Z., Zhang, Z.: Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12. Electrochem. Commun. 7, 894–899 (2005)
Lee, S.C., Lee, S.M., Lee, J.W., Lee, J.B., Lee, S.M., Han, S.S., Lee, H.C., Kim, H.J.: Spinel Li4Ti5O12 nanotubes for energy storage materials. J. Phys. Chem. C 113, 18420–18423 (2009)
Yuan, T., Wang, K., Cai, R., Ran, R., Shao, Z.: Cellulose-assisted combustion synthesis of Li4Ti5O12 adopting anatase TiO2 solid as raw material with high electrochemical performance. J. Alloys Compd. 477, 665–672 (2009)
Zhang, B., Liu, Y., Huang, Z., Oh, S., Yu, Y., Mai, Y.-W., Kim, J.-K.: Urchin-like Li4Ti5O12-carbon nanofiber composites for high rate performance anodes in Li-ion batteries. J. Mater. Chem. 22, 12133–12140 (2012)
Huang, S., Wen, Z., Gu, Z., Zhu, X.: Preparation and cycling performance of Al3+ and F− co-substituted compounds Li4AlxTi5 − xFyO12 − y. Electrochim. Acta 50, 4057–4062 (2005)
Koudriachova, M.V., Harrison, N.M., de Leeuw, S.W.: First principles predictions for intercalation behavior. Solid State Ion. 175, 829–834 (2004)
Zachau-Christiansen, B., West, K., Jacobsen, T., Atlung, S.: Lithium insertion in different TiO2 modifications. Solid State Ion. 28–30, 1176–1182 (1988)
Liu, J., Li, X., Yang, J., Geng, D., Li, Y., Wang, D., Li, R., Sun, X., Cai, M., Verbruggeb, M.W.: Microwave-assisted hydrothermal synthesis of nanostructured spinel Li4Ti5O12 as anode materials for lithium ion batteries. Electrochim. Acta 63, 100–104 (2012)
Chen, J.S., Lou, X.W.: The superior lithium storage capabilities of ultra-fine rutile TiO2 nanoparticles. J. Power Sources 195, 2905–2908 (2010)
Colin, J.F., Godbole, V., Novák, P.: In situ neutron diffraction study of Li insertion in Li4Ti5O12. Electrochem. Commun. 12, 804–807 (2010)
Jung, H.G., Kim, J., Scrosati, B., Sun, Y.K.: Micron-sized, carbon-coated Li4Ti5O12 as high power anode material for advanced lithium batteries. J. Power Sources 196, 7763–7766 (2011)
Zhang, N., Liu, Z., Yang, T., Liao, C., Wang, Z., Sun, K.: Facile preparation of nanocrystalline Li4Ti5O12 and its high electrochemical performance as anode material for lithium-ion batteries. Electrochem. Commun. 13, 654–656 (2011)
Aricò, A.S., Bruce, P., Scrosati, B., Tarascon, J.-M., van Schalkwijk, W.: Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 4, 366–377 (2005)
Yoshikawa, D., Kadoma, Y., Kim, J.-M., Ui, K., Kumagai, N., Kitamura, N., Idemoto, Y.: Spray-drying synthesized lithium-excess Li4 + xTi5 − xO12 − δ and its electrochemical property as negative electrode material for Li-ion batteries. Electrochim. Acta 55, 1872–1879 (2010)
Acknowledgments
This work was financially supported by National Basic Research Program of China (2013CB934003), "863" program (2013AA050902), National Nature Science Foundation of China (21273019) and the Foundamental Research Funds for the Central Universities (FRF-MP-13-002B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yang, Q., Zhao, H., Wang, J. et al. Synthesis parameter dependence of the electrochemical performance of solvothermally synthesized Li4Ti5O12. Mater Renew Sustain Energy 3, 24 (2014). https://doi.org/10.1007/s40243-014-0024-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-014-0024-7