Abstract
Samples of Li4Ti5O12−y solid solutions are synthesized by one-step solid-state carbothermal reduction reaction using Li2CO3, anatase, and carbon black under a nitrogen atmosphere. The underlying formation mechanism that leads to Li4Ti5O12−y solid solutions is proposed. The formation mechanism of the Li4Ti5O12−y solid solution is investigated by in situ variable temperature X-Ray diffraction (VT-XRD) and thermogravimetric analysis/differential scanning calorimetry (TGA-DSC). First, some Ti4+ centers are converted to Ti3+ (TiO2-TiO2−x) because of the presence of carbon black. Secondly, Li2CO3 reacts with TiO2−x (anatase) to form Li2TiO3. Thirdly, Li2TiO3 reacts with TiO2−x to form the Li4Ti5O12−y solid solution, while anatase starts to transform into rutile at the same time. Rutile reacts with Li2TiO3 to form Li4Ti5O12−y at higher temperatures. The presence of Ti3+ not only improves the electrical conductivity but also improves the ionic conductivity. As a result, the as-prepared material exhibits good rate capability and cycling stability with 99.3% capacity retention after 200 cycles.
Similar content being viewed by others
Introduction
Spinel Li4Ti5O12 (LTO) has already been employed as anode material in many fields, such as hybrid electric vehicles (HEVs), plug-in hybrid vehicles (PHEVs), and electric vehicles (EVs)1,2,3,4. As an alternative to carbon anodes, Li4Ti5O12 has a long flat charge/discharge voltage platform (1.55 V vs. Li/Li+), which is higher than the reduction potentials of most electrolyte solutions, thus avoiding the formation of solid-electrolyte interphase (SEI) layer (which usually occurs below 1.0 V vs. Li/Li+) and improving safety5,6,7. LTO is known as a “zero strain material” because the change of Li4Ti5O12 volume is small during the lithium-ion insertion and extraction process, which results in a long-term cycling life8,9,10. Furthermore, a high coulombic efficiency, long cycling performance, excellent safety, and a relatively high reversible capacity of 175 mA h g−1 make Li4Ti5O12 a promising anode material. However, the empty Ti 3d state with a band gap of ca. 2 eV still results in poor rate capability11,12,13,14. So far, considerable effort has been made to improve the reaction kinetics of LTO. Among these methods, the use of a conductive surface coating is the most common method. Carbon-based coatings are the most widely used type, including glucose15, sucrose16 soybean oil17, pitch18, poly(3,4-ethylenedioxythiophene) (PEDOT)19, and citric acid20. The organic carbon source undergoes a series of chemical reactions with increasing temperature in the carbonization process. The H, O, and N fraction in the carbon source decrease, the carbon content is continuously enriched, and pure carbon is formed in the end. A conductive coating can improve the surface conductivity, which can directly affect the electrochemical performance of materials. Wang et al.21 hypothesized that the carbonization not only provides a carbon coating layer but also partially reduces Ti4+ to Ti3+ at the contact surface of carbon and LTO. The mixed Ti4+/Ti3+ can improve the electrochemical performance of materials. Thus, a proper surface modification is a promising way to improve the electrochemical behavior of materials.
Li4Ti5O12 is a composite oxide of the AB2X4 series, such as LiMn2O4. LTO is a part of the spinel solid solution Li1+xTi2−xO4 (0 ≤ x ≤ 1/3). According to the structure of Li4Ti5O12, the formula can be described as Li8a [Li1/3Ti5/3]16d [O4]32e. The subscripts indicate the number of equivalent sites with Wyckoff symbols for the space group Fd-3m22. During the Li+-ion insertion/extraction process of Li4Ti5O12, the chemical composition and electrochemical properties of the crystal lattice change continuously with Li+-ion migration. When Li+ ions are inserted to the structure of spinel Li4Ti5O12, the foreign Li+ ions occupy the octahedral 16c sites of the lattice, which are close to the tetrahedral 8a sites, and the original lithium ions of the 8a sites also migrate to the octahedral 16c sites23. Finally, all the 16c sites are occupied by lithium ions and a rock salt phase of [Li2]16c [Li1/3Ti5/3]16d [O4]32e is formed, which results in a transition from insulator Li4Ti5O12 to Li7Ti5O12 with good conductivity with increasing lithium-ion content in the lattice24. Li7Ti5O12 has a good electrical conductivity (10−2 S cm−1), higher than that of Li4Ti5O12 (10−13 S cm−1). The most important factor is that the spinel host structure accommodates lithium ions without a significant change to the lattice constant25,26. This is an interesting phenomenon and has attracted much attention. Wangemaker et al.27 reported that the use of Li4Ti5O12−y solid solutions could improve the Li+-ion mobility, which can speed up the charge/discharge process. Recently, Qiu et al.28 prepared blue-colored hydrogenated Li4Ti5O12 by treating commercial Li4Ti5O12 at 500 °C under a 40 bar hydrogen atmosphere. Li4Ti5O12 exhibits a high discharge capacity and superior rate capability. Nie et al.29 prepared Ti3+ self-doped Li4Ti5O12 nanosheets via a facile solvothermal approach combined with a hydrogenation treatment. The Li4Ti5O12 nanosheets exhibit excellent rate capability and good cycling stability. In addition, spinel LiTi2O4, which belongs to the AB2X4 series, has a significantly higher electrical conductivity of 5.56 × 106 S cm−1 compared to Li4Ti5O1230. However, they concluded that the presence of Ti3+ has a great effect on the electrical conductivity of Li4Ti5O12. The formation mechanism of Ti3+ and the relationship between Ti3+ and electrochemical performance of materials were not studied in detail.
In this study, we prepared Li4Ti5O12−y solid solutions using carbon black, and the formation mechanism of Li4Ti5O12 in the presence of carbon (carbon black) was investigated. Moreover, the effect of Ti3+ on the electrochemical performance of the Li4Ti5O12−y solid solutions has been investigated systematically. The electrochemical performance of the prepared Li4Ti5O12−y solid solution was also studied.
Experimental
Material Preparation
Samples of Li4Ti5O12−y solid solutions were synthesized by a one-step carbon thermal reduction method. Stoichiometric amounts of Li2CO3 and TiO2 (anatase) were used as lithium and titanium sources, respectively. A 5% excess of Li2CO3 was added to the reactants because of the ease of evaporation of the lithium source at high temperatures. Carbon black was not used as the carbon source but as a reductant. Typically, 0.79 g Li2CO3, 2.02 g TiO2, and different content of carbon black were thoroughly mixed in agate mortar, and then heated at 850 °C in a tube furnace under a nitrogen atmosphere for 10 h. The carbon black loadings used were 0, 1, 2, and 3 wt.%, of the total weight of Li2CO3 and TiO2, and the obtained materials were labeled as P-LTO, 1-LTO, 2-LTO, and 3-LTO, respectively.
Material Characterization
In situ variable temperature X-Ray diffraction (VT-XRD) was carried out on a PANalytical X’Pert powder diffractometer with Cu Kα radiation (λ = 1.5405 Å), equipped with an Anton Parr HTK 1200 N high-temperature heating apparatus for the identification of phases and phase transitions. For the in situ high temperature VT-XRD study, the Li4Ti5O12 mixture was pressed into a square tablet with an area of 100 mm2 and thickness of 2 mm. Then, the tablet was heated in a N2 atmosphere from room temperature to 600 °C at a heating rate of 10 °C min−1, and the first XRD pattern was collected. Then, the sample was heated to 900 °C, pausing for 30 min at every 50 °C interval to collect the XRD data from 20 to 50° (2θ) (Fig. SI 1). The XRD patterns are labeled to distinguish the patterns collected at each stage during heating process. For example, the XRD pattern at 600 °C is denoted 600-1 and the pattern kept for 30 min is denoted 600-2, respectively. Thermaogravimetric analysis (TGA-DSC) was conducted on a STA409C/PCPFEIFFER VACUUM TGA-7 analyzer (NETZSCH-Ger tebau GmbH, Germany) in a nitrogen atmosphere with a flow rate of 30 mL min−1 from 40 to 900 °C at a heating rate of 3 °C min−1. The phase identification was performed by XRD (Panalytical Incorporated, Netherlands) from 15° to 50°. The morphologies of the samples were obtained using high-resolution scanning electron microscopy (HR-SEM, SU 8010, Hitachi, Ltd., Japan). UV-Vis diffuse reflectance spectra of the samples were recorded over the range of 200–800 nm in absorption mode using a Thermo Nicolet Evolution 500 UV-Vis spectrophotometer equipped with an integrating sphere attachment. The X-ray photoelectron (XPS) spectra were obtained with ESCALAB250 XPS (Thermo Fisher Scientific, USA) at 2 × 10−9 mbar. Al Ka (1486.6 eV) was used as the X-ray source at a 15-keV anode voltage, and all binding energies were referenced to the C1s peak (284.8 eV) arising from adventitious carbon.
Electrochemical Measurements
Coin cells (CR2025) were assembled to test the electrochemical performance of the obtained samples for comparison. The working electrode was prepared by coating a slurry of active material (80 wt.%), carbon black (electronic conductive additive, 10 wt.%), and polyvinylidene fluoride (PVDF, binder, 10 wt.%) in N-methyl pyrrolidone (NMP) solution onto a copper foil. The obtained electrodes were then dried at 110 °C overnight under vacuum. The half cells were assembled in an Ar-filled glove box. Celgard 2400 and lithium were used as the separator and counter electrode, respectively. 1 M LiPF6 in ethylene carbonate (EC), dimethyl carbonate (DMC), and ethylene methyl carbonate (EMC) (1: 1: 1 by volume) was used as electrolyte. The galvanostatic charge/discharge characteristics of the cells were analyzed in a voltage range of 1.0–2.5 V (vs. Li/Li+) at various rates using a LAND CT2001 battery tester. The specific capacity was calculated based on the active mass of the electrode.
Cyclic voltammetry (CV) curves were performed on a Zennium/IM6 electrochemical workstation (Zahner, Germany) in the voltage range of 1.0–2.5 V (vs. Li/Li+) at different scan rates. Electrochemical impedance spectroscopy (EIS) measurements were obtained on the same workstation with an oscillating voltage of 5 mV over frequency ranging from 10−2 to 105 Hz.
Results and Discussion
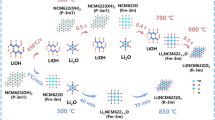
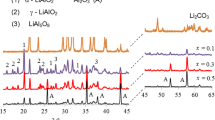
The in situ VT-XRD measurements were used to analyze the formation mechanism of LiTi4O5 during the carbothermal solid-state reduction reaction. The in situ VT-XRD patterns obtained on heating the sample from room temperature to 900 °C in a 2θ range of 15–50° are shown in Fig. 1. The in situ VT-XRD patterns of the mixture (25-1, Li2CO3, anatase, and carbon black) before heating are consistent with the corresponding crystal structure in the inorganic database (ICSD). No peaks of carbon black were observed because it is amorphous, and there is little rutile in the reactant mixture. Figure 1 shows that the intensity of Li2CO3 (JCPDS CARD No. 01-072-1216) and anatase (JCPDS CARD No. 00-002-0387) decreased significantly at 600 °C. The peaks at 20.17°, 35.8° and 43.5° which correspond to the (0 2 0), (-1 3 1) and (-1 3 3) planes of intermediate Li2TiO3 (JCPDS CARD No. 00-033-0831) appeared. The intensity of peaks corresponding to Li2TiO3 increased with the decrease in the Li2CO3 reactant until Li2CO3 was consumed at 600-2. The peaks at 35.5°, 43.2°, and 47.3° corresponding to the (3 1 1), (4 0 0), and (3 3 1) planes of Li4Ti5O12 (JCPDS CARD No. 00-049-0207) were observed at 700 °C, which implies that Li4Ti5O12 product was generated from 700 °C (700-1). In fact, the two peaks at 18° corresponding to (1 1 1) planes of Li4Ti5O12 and Li2TiO3 were not distinguishable because of the overlapping of these two phases. After heating to 800 °C for 30 min, the anatase peaks disappeared completely, and the intensity of the peaks of the rutile phase (JCPDS CARD No. 01-086-0147) increased rapidly at the same time (800-1). The increased intensity of rutile peaks is due to the transformation of anatase to rutile. In fact, anatase began to turn into rutile at 700 °C (700-1), but the phase transition process occurred rapidly at 800 °C. After anatase disappeared, the peak intensity corresponding to rutile decreased at 850 °C (850-1), together with the decrease of Li2TiO3. Rutile is more stable than anatase and it requires more time and a higher temperature to react with Li2TiO3. This explains why researchers often use anatase.
To confirm the formation mechanism of Li4Ti5O12 during the solid state reaction further, the reactant mixture (Li2CO3, anatase, and carbon black) was examined by TGA-DSC from room temperature to 900 °C (Fig. 2). Under hypoxic conditions, to maintain electrical neutrality, the reduction of Ti4+ to Ti3+ is accompanied by the formation of oxygen vacancies31.
It is worth noting that Chen et al.32 reported oxygen-deficient Li4Ti5O12−y by annealing the product under an N2 atmosphere at 500 °C. This approach takes advantage of the defective mesoporous structure with oxygen vacancies and Ti3+-O-Ti4+ pairs to achieve higher electrical conductivity and better electrochemical performance of LTO.
In the TGA curves, the first weight loss is 0.12% at 200 °C, and this is due to the dehydration process of the reactant. The second weight loss is 16.44 wt.% at 450–710 °C, corresponding to a broad exothermic peak at 600–710 °C in the DSC curve, which corresponds well to the theoretical weight loss of CO2 from Li2CO3 (15.6 wt.%). Here, ball milling can decrease the temperature of CO2 loss (<400 °C)33,34. Therefore, there are different reaction temperatures under different conditions. The reaction equation from 450–710 °C are shown in Eq. (2).
There is no weight loss in the TGA curve from 710–760 °C, but there is a small broad endothermic peak in the DSC curve. This DGA-DSC curve corresponds to the in situ VT-XRD patterns in Fig. 1, where intermediate Li2TiO3 reacts with anatase and carbon black to generate the target product, Li4Ti5O12−y.
A third tiny exothermic peak from 760–810 °C was observed in the DSC curve with no corresponding weight loss in the TGA curve. This temperature range corresponds to the phase transition of anatase to rutile in the in situ VT-XRD patterns (Fig. 1). The equation is shown in Eq. (4).
The final small exothermic peak appeared between 810–870 °C, but there is no weight loss. According to the in situ VT-XRD patterns shown in Fig. 1, the equation for the reaction of Li2TiO3 with rutile and carbon black to generate the target product Li4Ti5O12−y is given by Eq. (5).
There are two exothermic peaks in DSC curve with no weight loss during the cooling process. These peaks are marked as peaks 5 and 6 at the same temperature range as peaks 4 and 3 respectively. This suggests that the two pairs of peaks correspond to the same reaction. No obvious exothermic peak weight loss was observed below 750 °C.
Based on the above results, the formation mechanism of solid solution Li4Ti5O12−y can be summarized by Eqs (1–5). In addition, the schematic of the formation mechanism is shown in Fig. SI 2.
To investigate the effects of carbon black on the electrochemical performance of LTO, a series of comparative experiments were conducted. Figure 3 presents the XRD patterns of the P-LTO, 1-LTO, 2-LTO, and 3-LTO, respectively. XRD measurements were conducted to examine the crystal structure of the four prepared samples. All samples showed typical diffraction peaks corresponding to the planes of LTO crystals (JCPDS no. 00-049-0207). No diffraction peaks of carbon or impurity phases were detected, suggesting that the presence of carbon black in the formation Li4Ti5O12 did not significantly affect the bulk crystalline structure of the LTO. As demonstrated, the phase structures of the samples are the same. However, the colors of the samples are quite different. P-LTO is white, whereas the others (1-LTO, 2-LTO, and 3-LTO) are blue.
The morphologies of P-LTO and 2-LTO were investigated by SEM. As shown in Fig. SI 3a, the P-LTO particles are irregularly shaped cubes with a size range of about 1–2 μm. Compared with the P-LTO sample, 2-LTO particles (Fig. SI 3b) shows regular cubic geometry and relatively uniform size.
Typically, the color change of LTO during the reaction process is attributed to oxygen vacancies, oxygen deficiency, and surface disorder35,36. This can be demonstrated by spectroscopic analysis using UV-vis diffraction spectroscopy, as shown in Fig. SI 4. Both the white and blue LTO absorb UV light, which is determined by the inherent bandgap. The UV-vis spectrum of the P-LTO is characterized by the strong absorption of UV radiation in the range 200–300 nm, and no absorption was observed in the visible light region (400–800 nm). This explains why the P-LTO is white. Visible light absorption can be attributed to the creation of mid-gap states between the conduction band and valence band. The blue samples have similar absorption profiles in the UV region, although these are different from that of P-LTO and exhibit a new and remarkable absorption in the visible region, especially in the red and infrared regions, subsequently yielding the blue color and suggesting the existence of Ti3+. Therefore, oxygen-deficient LTO can be regarded as a solid solution of Ti3+ and Ti4+. The 2-LTO and 3-LTO samples show similar absorption profiles in the visible light region. The 1-LTO sample also showed significant visible light absorption. Calcination with carbon black led to the formation of oxygen vacancies in the LTO lattice. Here, acetylene black served as a reductant to induce the partial reduction of Ti sites (Ti4+ to Ti3+).
XPS analysis was carried out to investigate the Ti-ion valence state of the P-LTO and 2-LTO samples further. The XPS data were corrected by taking into account the binding energy of the C1s line of residual carbon at 284.6 eV. As shown in the spectra of P-LTO (Fig. 4a), there are two peaks centered at 458.7 and 457.8 eV, corresponding to the characteristic peaks of Ti4+ and Ti3+ species. No trivalent Ti was observed in P-LTO. Accordingly, the XPS spectra of Fig. 4b suggest mixed valence Ti3+/Ti4+ in the 2-LTO sample with a carbon additive. The carbon additive provides a reductive atmosphere and induces the partial reduction of Ti4+ to Ti3+, which would lead to an increase in number of electrons and improvement in the electrical conductivity. Because of the larger ionic radius of Ti3+ (0.67 Å) compared to Ti4+ (0.605 Å)37, the presence of Ti3+ will affect the lattice parameter and make Li+-ion migration easier. The higher electrical conductivity results in improved interfacial charge transfer kinetics, which can result in good rate performance.
TGA measurement was carried out from room temperature to 800 °C under air atmosphere to calculate the residual carbon content. As shown in Fig. SI 5, the first weight loss below 200 °C could be attributed to the moisture of the samples. The slight weight rise around 130–230 °C can be attributed to the oxidation of Ti (III). The content of residual carbon can be easily estimated based on the weight loss caused by the carbon oxidation in the range of 300–550 °C. The residual carbon content of 1-LTO, 2-LTO and 3-LTO are 0.33%, 0.78% and 2.48%, respectively. It can be clearly seen that the amount of residual carbon of 3-LTO is seriously excessive. Compared with active material Li4Ti5O12, the carbon additive provides a negligible amount of capacitance during the charge and discharge process, so if there is an excessive amount of carbon additive in the active material, its specific capacitance inevitably decreases.
To investigate the electrochemical kinetics, CV measurements were conducted at a scan rate of 0.5 mV s−1 in the voltage range of 1.0–2.5 V (Fig. SI 6). A single pair of oxidation and reduction peaks (around 1.5 and 1.6 V), which corresponds to the Ti4+/Ti3+ redox couple38, was observed for all the samples. The 2-LTO exhibits a pair of obvious oxidation and deoxidization peaks at 1.52 and 1.62 V, corresponding to the charge and discharge plateaus. The P-LTO, 1-LTO, and 3-LTO samples have pairs of oxidation and reduction peaks at 1.51 and 1.65, 1.48 and 1.69, and 1.52 and 1.65 V, respectively. The potential difference (φa-φb) between the anodic and cathodic peaks can reflect the degree of polarization of the electrode. The potential difference of the 2-LTO electrode (0.1 V) is lower than that of others and has a better overlap of the CV curves for 10 cycles, which suggests that 2-LTO electrode has the best reversibility of the redox reaction.
To investigate the influence of Ti3+ on the kinetic performance further, the CV curves of the four samples were obtained at different scan rates (0.1, 0.2, 0.5, and 1 mV s−1), as shown in Fig. 5. The peak currents and the potential difference increased with increasing scan rate. All the peak current densities of blue LTO (1-LTO, 2-LTO, and 3-LTO) are higher than those of P-LTO; in particular, 2-LTO retains a stable shape redox peaks with increasing scan rate. These results indicate that Ti3+ accelerates the lithium-ion insertion and extraction process, particularly affecting the process of lithium-ion insertion.
The relationship between the redox peak current (ip) and the square root of the scan rate (v1/2) is shown in Fig. 6. There is a linear relationship because the diffusion rate limits the process of lithium-ion insertion/extraction. For the semi-infinite and finite diffusion, the Randles-Sevcik equation was used to calculate the relationship between the redox peak current and the square root of the scan rate.
Here, n is the number of electrons in the redox reaction, A is the area of the electrode surface (cm2), C0 is the molar concentration of lithium ions (mol cm−3), DLi+ is the diffusion coefficient of lithium ions, and v is the scan rate of the potential (V s−1). As indicated in Table 1, 2-LTO has a larger diffusion coefficient than the other samples, which indicates that the presence of Ti3+ can effectively improve the lithium ion diffusion.
The cycling performance of the four samples at 0.5 C is displayed in Fig. 7a. The four samples have good cycling stability. The 2-LTO electrode showed an improved cycling performance compared with other electrodes. After an initial discharge capacity of 126.7, 135.4, 154.2, and 147.4 mA h g−1, the P-LTO, 1-LTO, 2-LTO, and 3-LTO samples achieved capacity retentions of 97.5%, 98.2%, 99.3%, and 96% after 200 cycles, respectively. Figure 7b compares the rate capability of the P-LTO, 1-LTO, 2-LTO, and 3-LTO samples at different current rates. The current density was increased gradually from 0.2 C to 5 C and returned to 0.2 C again. The cells were run for 10 cycles at each of the C rates. The P-LTO sample exhibited good discharge capacities and cycling stabilities at a low current rate of 0.2 C. However, its capacity dropped significantly with increasing current rate, whereas the samples with carbon additive exhibited relatively higher capacities. The discharge capacities of P-LTO are 127.3, 101.7, 70.1, 41.6, and 27.5 mA h g−1 at 0.2 C, 0.5 C, 1 C, 2 C and 5 C, respectively. The samples with carbon black additive exhibited better electrochemical performance than P-LTO. Among all samples, the 2-LTO sample exhibited the highest discharge capacity and best cycling stability, which is consistent with the results of CV measurements at 0.5 mV s−1 (Fig. SI 6). When the current density returned to 0.2 C, a stable capacity of 163.9 mA h g−1 was obtained without significant capacity fading. The excessive carbon black decreases the capacity of the 3-LTO sample. These results indicate that the presence of Ti3+ in Li4Ti5O12 can improve the rate capability, which agrees well with the previous results.
The linear scanning voltammetry (LSV) measurement was carried out to test the electronic conductivity of the samples. Figure SI 7 shows that the electrical conductivity of P-LTO, 1-LTO, 2-LTO, and 3-LTO samples were 1.1 × 10−6, 6.6 × 10−5, 2.2 × 10−4 and 1.1 × 10−4 S cm−1, respectively, which is higher than the pure Li4Ti5O12 (10−9 S cm−1) reported in the literature. This is mainly due to the ion radius of Ti3+ (0.67 Å) which is larger than that of Ti4+ (0.605 Å), and the increased radius of Ti3+ can change the lattice parameters of the Li4Ti5O12 material, making insertion and extraction of lithium ions easier, thus enhance the rate performance of Li4Ti5O12. To a certain extent, the electronic conductivity of Li4Ti5O12 increased with the increasing fraction of carbon black, when the amount of carbon black exceeded 2 wt. %, the electronic conductivity of the sample begins to decrease. It means excessive carbon exists in the sample in the form of impurities, which leads to bad electronic conductivity and poor electrochemical performance.
To investigate the differences in electrochemical performance, EIS measurements were carried out for the samples at a voltage of 1.55 V (vs. Li/Li+). The Nyquist plots for the cells are fitted to the equivalent circuit shown in Fig. 8. The EIS curves are composed of a depressed semicircle at the high- to intermediate-frequency range and a line in the low-frequency range. Usually, the charge transfer resistance (Rct) in the high middle-frequency region is related to lithium-ion charge transfer at the material interface, whereas the low-frequency Warburg region is related to the lithium-ion diffusion coefficients of the material. A higher exchange current density (i0 = RT/nFRct) of the material results in improved high rate performance. The Rct values of P-LTO, 1- LTO, 2-LTO, and 3-LTO are 38.8, 34.6, 31.7 and 54.8 Ω, respectively. Based on the above analysis, 2-LTO exhibited the best electrochemical characteristics and the lowest resistances, and, thus, the best cycling and rate performance.
Conclusion
The formation mechanism of solid solution Li4Ti5O12−y prepared by a one-step solid-state reduction reaction was investigated by in situ VT-XRD and TGA-DSC measurements. First, TiO2 loses some oxygen (O) and is converted to TiO2−x under a reductive atmosphere. Then, Li2CO3 reacts with TiO2−x (anatase) to form intermediate Li2TiO3−x. Li2TiO3 reacts with anatase in the presence of carbon black to form Li4Ti5O12−y at 700 °C. Anatase starts to transform into rutile above 700 °C, and Li2TiO3−x continues to react with TiO2−x (rutile) and carbon black, generating Li4Ti5O12−y at higher temperatures. In this method, carbon black served as a reductant and reduced the surface Ti4+ to Ti3+. The surface modification can improve the electrical conductivity and Li+ diffusion coefficient and improve rate capacity and cycling stability significantly. Many researchers have modified the surface of Li4Ti5O12 particles with carbon layers, which they claim can improve the electrical conductivity. However, most of them did not mention the changes in the contact surface between the carbon layer and the Li4Ti5O12 particles. Our findings reveal that the carbon layer can reduce the surface Ti4+ of Li4Ti5O12 particles to Ti3+ in the carbonization process, and to some extent can improve the electrical conductivity. We believe that these results provide a solid foundation for optimizing the synthetic method and further developing Li4Ti5O12 as an anode material for lithium-ion batteries.
References
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652 (2008).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Tarascon, J.-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2011).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2009).
Liu, J. et al. Microwave-assisted hydrothermal synthesis of nanostructured spinel Li4Ti5O12 as anode materials for lithium ion batteries. Electrochim. Acta 63, 100–104 (2012).
Lu, X. et al. Lithium storage in Li4Ti5O12 spinel: the full static picture from electron microscopy. Adv. Mater. 24, 3233–3238 (2012).
Zhang, Z. et al. Hydrothermal synthesis of Li4Ti5O12 microsphere with high capacity as anode material for lithium ion batteries. Ceram. Int. 39, 2695–2698 (2013).
Ohzuku, T., Ueda, A. & Yamamoto, N. Zero-Strain Insertion Material of Li[Li1/3Ti5/3]O4 for Rechargeable Lithium Cells. J. Electrochem. Soc. 142, 1431–1435 (1995).
Haetge, J., Hartmann, P., Brezesinski, K., Janek, J. & Brezesinski, T. Ordered large-pore mesoporous Li4Ti5O12 spinel thin film electrodes with nanocrystalline framework for high rate rechargeable lithium batteries: relationships among charge storage, electrical conductivity, and nanoscale structure. Chem. Mater. 23, 4384–4393 (2011).
Thackeray, M. Structural considerations of layered and spinel lithiated oxides for lithium ion batteries. J. Electrochem. Soc. 142, 2558–2563 (1995).
Ouyang, C., Zhong, Z. & Lei, M. Ab initio studies of structural and electronic properties of Li4Ti5O12 spinel. Electrochem. Commun. 9, 1107–1112 (2007).
Park, K.-S., Benayad, A., Kang, D.-J. & Doo, S.-G. Nitridation-driven conductive Li4Ti5O12 for lithium ion batteries. J. Am. Chem. Soc. 130, 14930–14931 (2008).
Brousse, T. et al. All oxide solid-state lithium-ion cells. J. Power Sources 68, 412–415 (1997).
Lippens, P.-E., Womes, M., Kubiak, P., Jumas, J.-C. & Olivier-Fourcade, J. Electronic structure of the spinel Li4Ti5O12 studied by ab initio calculations and X-ray absorption spectroscopy. Solid State Sci. 6, 161–166 (2004).
Li, X. et al. In-situ carbon coating to enhance the rate capability of the Li4Ti5O12 anode material and suppress the electrolyte reduction decomposition on the electrode. Electrochim. Acta 190, 69–75 (2016).
Wang, Y. et al. Solid-state synthesis of graphite carbon-coated Li4Ti5O12 anode for lithium ion batteries. Ionics 20, 1377–1383 (2014).
Kong, M. Z., Wang, W. L., Park, J.-Y. & Gu, H.-B. Synthesis and electrochemical properties of a carbon-coated spinel Li4Ti5O12 anode material using soybean oil for lithium-ion batteries. Mater. Lett. 146, 12–15 (2015).
Jung, H.-G., Kim, J., Scrosati, B. & Sun, Y.-K. Micron-sized, carbon-coated Li4Ti5O12 as high power anode material for advanced lithium batteries. J. Power Sources 196, 7763–7766 (2011).
Wang, X. et al. PEDOT coated Li4Ti5O12 nanorods: Soft chemistry approach synthesis and their lithium storage properties. Electrochim. Acta 129, 283–289 (2014).
Kuo, Y.-C. & Lin, J.-Y. One-pot sol-gel synthesis of Li4Ti5O12/C anode materials for high-performance Li-ion batteries. Electrochim. Acta 142, 43–50 (2014).
Wang, Y. et al. Synthesis and electrochemical performance of nano-sized Li4Ti5O12 with double surface modification of Ti (III) and carbon. J. Mater. Chem. 19, 6789–6795 (2009).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys Rev 136, B864 (1964).
Yi, T.-F., Xie, Y., Zhu, Y.-R., Zhu, R.-S. & Shen, H. Structural and thermodynamic stability of Li4Ti5O12 anode material for lithium-ion battery. J. Power Sources 222, 448–454 (2013).
Yi, T.-F. et al. Structure and electrochemical performance of niobium-substituted spinel lithium titanium oxide synthesized by solid-state method. J. Electrochem. Soc. 158, A266–A274 (2011).
Kitta, M., Akita, T., Tanaka, S. & Kohyama, M. Characterization of two phase distribution in electrochemically-lithiated spinel Li4Ti5O12 secondary particles by electron energy-loss spectroscopy. J. Power Sources 237, 26–32 (2013).
Kitta, M., Akita, T., Tanaka, S. & Kohyama, M. Two-phase separation in a lithiated spinel Li4Ti5O12 crystal as confirmed by electron energy-loss spectroscopy. J. Power Sources 257, 120–125 (2014).
Wagemaker, M. et al. A kinetic two-phase and equilibrium solid solution in spinel Li4+ xTi5O12. Adv. Mater. 18, 3169–3173 (2006).
Qiu, J. et al. Blue hydrogenated lithium titanate as a high-rate anode material for lithium-ion batteries. J. Mater. Chem. A 2, 6353–6358 (2014).
Nie, S., Li, C., Peng, H., Li, G. & Chen, K. Ti3+ self-doped Li4Ti5O12 nanosheets as anode materials for high performance lithium ion batteries. Rsc Adv. 5, 23278–23282 (2015).
Colbow, K., Dahn, J. & Haering, R. Structure and electrochemistry of the spinel oxides LiTi2O4 and Li43Ti53O4. J. Power Sources 26, 397–402 (1989).
Shi, Y., Zhang, D., Chang, C., Huang, K. & Holze, R. Enhanced electrochemical performance of oxygen-deficient Li4Ti5O12−y anode material induced by graphene oxide. J. Alloy Compd. 639, 274–279 (2015).
Chen, X., Guan, X., Li, L. & Li, G. Defective mesoporous Li4Ti5O12−y: An advanced anode material with anomalous capacity and cycling stability at a high rate of 20 C. J. Power Sources 210, 297–302 (2012).
Yuan, T., Cai, R., Ran, R., Zhou, Y. & Shao, Z. A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J. Alloy Compd. 505, 367–373 (2010).
Veljković, I., Poleti, D., Karanović, L., Zdujić, M. & Branković, G. Solid state synthesis of extra phase-pure Li4Ti5O12 spinel. Sci. Sinter. 43, 343–351 (2011).
Shin, J.-Y., Joo, J. H., Samuelis, D. & Maier, J. Oxygen-deficient TiO2−δ nanoparticles via hydrogen reduction for high rate capability lithium batteries. Chem. Mater. 24, 543–551 (2012).
Qiu, J. et al. Hydrogenation synthesis of blue TiO2 for high-performance lithium-ion batteries. J Phys. Chem. C 118, 8824–8830 (2014).
Jung, H.-G. et al. Microscale spherical carbon-coated Li4Ti5O12 as ultra high power anode material for lithium batteries. Energ. Environ. Sci. 4, 1345–1351 (2011).
Yi, T.-F. et al. Enhanced electrochemical performance of a novel Li4Ti5O12 composite as anode material for lithium-ion battery in a broad voltage window. Ceram. Int. 41, 2336–2341 (2015).
Acknowledgements
This research was supported by the Leading Human Resource Training Program of Regional Neo industry through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2016H1D5A1909732). This work was also supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program (10080293, Development of carbon-based non-phenolic electrode materials with 3,000 m2/g grade surface area for energy storage device) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Contributions
G.J.Y. designed and carried out the experiments, collected and analyzed the data, and wrote the manuscript. S.J.P. contributed to discussion of the interpretation of the results and managed the overall execution of the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, G., Park, SJ. The formation mechanism of Li4Ti5O12−y solid solutions prepared by carbothermal reduction and the effect of Ti3+ on electrochemical performance. Sci Rep 9, 4774 (2019). https://doi.org/10.1038/s41598-019-41206-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41206-0
- Springer Nature Limited