Abstract
Presently on a global scale, one of the major concerns is to find effective strategies to manage the agricultural waste to protect the environment. One strategy that has been drawing attention among the researchers is the development of biocompatible materials from agricultural waste. This strategy implies successful conversion of agricultural waste products (e.g.: cellulose, eggshell etc.) into building blocks for biomaterial development. Some of these wastes contain even bioactive compounds having biomedical applications. The replacement and augmentation of human tissue with biomaterials as alternative to traditional method not only bypasses immune-rejection, donor scarcity, and maintenance; but also provides long term solution to damaged or malfunctioning organs. Biomaterials development as one of the key challenges in tissue engineering approach, resourced from natural origin imparts better biocompatibility due to closely mimicking composition with cellular microenvironment. The “Garbage In, Biomaterials Out (GIBO)” concept, not only recycles the agricultural wastes, but also adds to biomaterial raw products for further product development in tissue regeneration. This paper reviews the conversion of garbage agricultural by-products to the biocompatible materials for various biomedical applications.
Graphical abstract
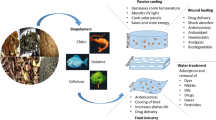
The agro-waste biomass processed, purified, modified, and further utilized for the fabrication of biomaterials-based support system for tissue engineering applications to grow living body parts in vitro or in vivo.





Similar content being viewed by others
References
Singha AS, Thakur VK. Physical, Chemical and Mechanical Properties of Hibiscus sabdariffa Fiber/Polymer Composite. Int J Polym Mater Polym Biomater. 2009;58:217–28 (Taylor & Francis).
Thakur VK, Singha AS, Thakur MK. Biopolymers Based Green Composites: Mechanical, Thermal and Physico-chemical Characterization. J Polym Environ. 2012;20:412–21.
Seadi TA, Holm-Nielsen JB III. 2 Agricultural wastes. Waste Manag Ser. 2004;4:207–15.
Martin-Luengo MA, Yates M, Ramos M, Salgado JL, Aranda RMM, Plou F, et al. Renewable Raw Materials for advanced applications. 2011 World Congr Sustain Technol WCST. 2011. p. 19–22
Guo YP, Yang SF, Zhao JZ, Wang ZC, Zhao MY. Preparation of active carbon with high specific surface area from rice husks. Chem J Chinese Univ-Chinese. 2000;21(3):335–338.
Ling IH, Teo DCL. Lightweight concrete bricks produced from industrial and agricultural solid waste. 2011 World Congr Sustain Technol WCST. IEEE. 2011;148–52.
Surip SN, Bonnia NN, Anuar H, Hassan NA, Yusof NM. Nanofibers from oil palm trunk (OPT): Preparation chemical analysis. 2012 IEEE Symp Bus Eng Ind Appl. 2012;809–12.
Zakari Z, Buniran S, Ishak MI. Nanopores activated carbon rice husk. 2010 Int Conf Enabling Sci Nanotechnol (ESciNano). 2010;1–2.
Jawaid M, Khalil HPSA. EFFECT OF LAYERING PATTERN ON THE DYNAMIC MECHANICAL PROPERTIES AND THERMAL DEGRADATION OF OIL PALM-JUTE FIBERS REINFORCED EPOXY HYBRID COMPOSITE. BioResources. 2011;6:2309–22.
Joshi SV, Drzal LT, Mohanty AK, Arora S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos Part Appl Sci Manuf. 2004;35:371–6.
Kalia S, Kaith BS, Kaur I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym Eng Sci. 2009;49:1253–72.
Namvar F, Jawaid M, Tanir PM, Mohamad R, Azizi S, Khodavandi A, et al. Potential Use of Plant Fibres and their Composites for Biomedical Applications. BioResources. 2014;9:5688–706.
Ogah AO, Afiukwa JN, Nduji AA. Characterization and comparison of rheological properties of agro fiber filled high-density polyethylene bio-composites. Open J Polym Chem. 2014;4(1):12–19.
Murali S, Shrivastava R, Saxena M. Quantification of agricultural residues for energy generation - A case study. J IPHE. 2007;3:27–31.
Kumar A, Kumar N, Baredar P, Shukla A. A review on biomass energy resources, potential, conversion and policy in India. Renew Sustain Energy Rev. 2015;45:530–9.
Tsang YF, Kumar V, Samadar P, Yang Y, Lee J, Ok YS, et al. Production of bioplastic through food waste valorization. Environ Int Elsevier. 2019;127:625–44.
Dittenber DB, GangaRao HVS. Critical review of recent publications on use of natural composites in infrastructure. Compos Part Appl Sci Manuf. 2012;43:1419–29.
Dungani R, Karina M, Subyakto, Sulaeman A, Hermawan D, Hadiyane A. Agricultural waste fibers towards sustainability and advanced utilization: a review. Asian J Plant Sci. 2016;15:42–55 (Asian Network for Scientific Information).
Abdul Khalil HPS, Firoozian P, Bakare IO, Akil HMd, Noor AMd. Exploring biomass based carbon black as filler in epoxy composites: Flexural and thermal properties. Mater Des. 2010;31:3419–25.
Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–33.
Asgher M, Ahmad Z, Iqbal HMN. Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crops Prod. 2013;44:488–95.
Ofori-Boateng C, Lee KT. Sustainable utilization of oil palm wastes for bioactive phytochemicals for the benefit of the oil palm and nutraceutical industries. Phytochem Rev. 2013;12:173–90.
Iqbal HMN, Kyazze G, Keshavarz T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. BioResources. 2013;8:3157–76.
Driemeier C, Santos WD, Buckeridge MS. Cellulose crystals in fibrovascular bundles of sugarcane culms: orientation, size, distortion, and variability. Cellulose. 2012;19:1507–15.
ROWELL RM. Characterization and factors effecting fiber properties. Nat Polym Agrofibers Based Compos [Internet]. Embrapa Instrumentacao Agropecuaria; 2000 [cited 2021 Apr 5]; Available from: https://ci.nii.ac.jp/naid/10019393394/
Azwa ZN, Yousif BF, Manalo AC, Karunasena W. A review on the degradability of polymeric composites based on natural fibres. Mater Des. 2013;47:424–42.
Siqueira G, Bras J, Dufresne A. New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate. Langmuir American Chemical Society. 2010;26:402–11 (American Chemical Society).
Methacanon P, Weerawatsophon U, Sumransin N, Prahsarn C, Bergado DT. Properties and potential application of the selected natural fibers as limited life geotextiles. Carbohydr Polym. 2010;82:1090–6.
John MJ, Thomas S. Biofibres and biocomposites. Carbohydr Polym. 2008;71:343–64.
Thygesen A, Thomsen AB, Daniel G, Lilholt H. Comparison of composites made from fungal defibrated hemp with composites of traditional hemp yarn. Ind Crops Prod. 2007;25:147–59.
Jane J-L, Kasemsuwan T, Leas S, Zobel H, Robyt JF. Anthology of Starch Granule Morphology by Scanning Electron Microscopy. Starch - Stärke. 1994;46:121–9.
Lindeboom N, Chang PR, Tyler RT. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch - Stärke. 2004;56:89–99.
Rosen S, Meade B, Fuglie K, Rada N. International Food Security Assessment, 2014–2024. 2.
Chen Q, Yu H, Wang L, Abdin Z ul, Chen Y, Wang J, et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015;5:67459–74 (The Royal Society of Chemistry).
Liu D, Qi Z, Zhang Y, Xu J, Guo B. Poly(butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties. Starch - Stärke. 2015;67:802–9.
Guna V, Ilangovan M, Anantha Prasad MG, Reddy N. Water Hyacinth: A Unique Source for Sustainable Materials and Products. ACS Sustain Chem Eng. 2017;5:4478–90 (American Chemical Society).
Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–16.
Sah MK, Pramanik K. Soluble-eggshell-membrane-protein-modified porous silk fibroin scaffolds with enhanced cell adhesion and proliferation properties. J Appl Polym Sci [Internet]. 2014 [cited 2021 Apr 6];131. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/app.40138
Reddy N, Yang Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011;29:490–8.
Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007.
Naskar D, Nayak S, Dey T, Kundu SC. Non-mulberry silk fibroin influence osteogenesis and osteoblast-macrophage cross talk on titanium based surface. Sci Rep. 2014;4:4745.
Humenik M, Scheibel T, Smith A. Spider Silk: Understanding the Structure–Function Relationship of a Natural Fiber. In: Howorka S, editor. Prog Mol Biol Transl Sci [Internet]. Academic Press; 2011 [cited 2021 Apr 6]. p. 131–85. Available from: https://www.sciencedirect.com/science/article/pii/B9780124159068000078
Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK, et al. Silk proteins for biomedical applications: Bioengineering perspectives. Prog Polym Sci. 2014;39:251–67.
Gomes S, Leonor IB, Mano JF, Reis RL, Kaplan DL. Natural and genetically engineered proteins for tissue engineering. Prog Polym Sci. 2012;37:1–17.
Sah MK, Pramanik K. Regenerated Silk Fibroin from B. mori Silk Cocoon for Tissue Engineering Applications. Int J Environ Sci Dev. 2010;404–8.
Maziz A, Leprette O, Boyer L, Blatché C, Bergaud C. Tuning the properties of silk fibroin biomaterial via chemical cross-linking. Biomed Phys Eng Express. 2018;4:065012. IOP Publishing
Janani G, Kumar M, Chouhan D, Moses JC, Gangrade A, Bhattacharjee S, et al. Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl Bio Mater. 2019;2:5460–91 (American Chemical Society).
Tu J, Wang H, Li H, Dai K, Wang J, Zhang X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials. 2009;30:4369–76.
Dong J, Sun Q, Wang J-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials. 2004;25:4691–7.
Kim S, Xu J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J Cereal Sci. 2008;47:1–5.
Ghanbarzadeh B, Musavi M, Oromiehie AR, Rezayi K, Razmi Rad E, Milani J. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT - Food Sci Technol. 2007;40:1191–7.
Sousa FFO, Luzardo-Álvarez A, Blanco-Méndez J, Martín-Pastor M. NMR techniques in drug delivery: Application to zein protein complexes. Int J Pharm. 2012;439:41–8.
Khalil AA, Deraz SF, Elrahman SA, El-Fawal G. Enhancement of Mechanical Properties, Microstructure, and Antimicrobial Activities of Zein Films Cross-Linked Using Succinic Anhydride, Eugenol, and Citric Acid. Prep Biochem Biotechnol Taylor & Francis. 2015;45:551–67 (Taylor & Francis).
Shukla R, Cheryan M. Zein: the industrial protein from corn. Ind Crops Prod. 2001;13:171–92.
Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Controlled Release. 2014;189:108–22.
Zhao X, Chen F, Xue W, Lee L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008;22:568–75.
Silva NHCS, Vilela C, Marrucho IM, Freire CSR, Neto CP, Silvestre AJD. Protein-based materials: from sources to innovative sustainable materials for biomedical applications. J Mater Chem B. 2014;2:3715–40 (The Royal Society of Chemistry).
Burgos-Díaz C, Wandersleben T, Marqués AM, Rubilar M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr Opin Colloid Interface Sci. 2016;25:51–7.
Chien KB, Shah RN. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012;8:694–703.
Song F, Tang D-L, Wang X-L, Wang Y-Z. Biodegradable Soy Protein Isolate-Based Materials: A Review. Biomacromolecules American Chemical Society. 2011;12:3369–80.
Sah MK, Rath SN. Soluble eggshell membrane: A natural protein to improve the properties of biomaterials used for tissue engineering applications. Mater Sci Eng C. 2016;67:807–21.
Makareeva E, Leikin S. Chapter 7 - Collagen Structure, Folding and Function. In: Shapiro JR, Byers PH, Glorieux FH, Sponseller PD, editors. Osteogenes Imperfecta [Internet]. San Diego: Academic Press; 2014 [cited 2021 Apr 6]. p. 71–84. Available from: https://www.sciencedirect.com/science/article/pii/B9780123971654000071
Betancourt DE, Baldion PA, Castellanos JE. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int J Biomater. 2019;2019:e5268342 (Hindawi).
Sato M, Asazuma T, Ishihara M, Kikuchi T, Masuoka K, Ichimura S, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64A:248–56.
Rýglová Š, Braun M, Suchý T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromol Mater Eng. 2017;302:1600460.
Govindharaj M, Roopavath UK, Rath SN. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J Clean Prod. 2019;230:412–9.
Dang Q, Liu K, Zhang Z, Liu C, Liu X, Xin Y, et al. Fabrication and evaluation of thermosensitive chitosan/collagen/α, β-glycerophosphate hydrogels for tissue regeneration. Carbohydr Polym. 2017;167:145–57.
Im J, Hyun Choi C, Mun F, Lee J, Kim H, Jung W-K, et al. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv Royal Society of Chemistry. 2017;7:2009–18.
Chandika P, Ko S-C, Oh G-W, Heo S-Y, Nguyen V-T, Jeon Y-J, et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int J Biol Macromol. 2015;81:504–13.
Ioan D-C, Rău I, Tihan GT, Zgârian RG, Ghica MV, Albu Kaya MG, et al. Piroxicam-Collagen-Based Sponges for Medical Applications. Int J Polym Sci. 2019;2019:e6062381 (Hindawi).
Alarcon EI, Udekwu KI, Noel CW, Gagnon LB-P, Taylor PK, Vulesevic B, et al. Safety and efficacy of composite collagen–silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale. 2015;7:18789-98.
Nanda HS, Chen S, Zhang Q, Kawazoe N, Chen G. Collagen Scaffolds with Controlled Insulin Release and Controlled Pore Structure for Cartilage Tissue Engineering. BioMed Res Int; 2014;2014:e623805.
Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34:5937–46.
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–34.
Jia W, Tang H, Wu J, Hou X, Chen B, Chen W, et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials. 2015;69:45–55.
Lin K-F, He S, Song Y, Wang C-M, Gao Y, Li J-Q, et al. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl Mater Interfaces. 2016;8:6905–16 (American Chemical Society).
Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C. 2018;90:713–27.
Chamieh F, Collignon A-M, Coyac BR, Lesieur J, Ribes S, Sadoine J, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814 (Nature Publishing Group).
Winias S, Ernawati DS, Ariani MD, Rahayu RP. Scaffold combination of chitosan and collagen synthesized from chicken feet induces osteoblast and osteoprotegerin expression in bone healing process of mice. Dent J Maj Kedokt Gigi. 2017;50:86.
Liu Y, Gu J, Fan D. Fabrication of High-Strength and Porous Hybrid Scaffolds Based on Nano-Hydroxyapatite and Human-Like Collagen for Bone Tissue Regeneration. Polymers. 2020;12:61 (Multidisciplinary Digital Publishing Institute).
Wei L-G, Chang H-I, Wang Y, Hsu S, Dai L-G, Fu K-Y, et al. A gelatin/collagen/polycaprolactone scaffold for skin regeneration. PeerJ. 2019;7:e6358 (PeerJ Inc.).
Long K, Liu Y, Li W, Wang L, Liu S, Wang Y, et al. Improving the mechanical properties of collagen-based membranes using silk fibroin for corneal tissue engineering. J Biomed Mater Res A. 2015;103:1159–68.
Cao H, Chen M-M, Liu Y, Liu Y-Y, Huang Y-Q, Wang J-H, et al. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces. 2015;136:1098–106.
Guo H, Hong Z, Yi R. Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J Food Sci Wiley Online Library. 2015;80:N1595–601.
Veeruraj A, Arumugam M, Ajithkumar T, Balasubramanian T. Isolation and characterization of drug delivering potential of type-I collagen from eel fish Evenchelys macrura. J Mater Sci Mater Med Springer. 2012;23:1729–38.
Nicklas M, Schatton W, Heinemann S, Hanke T, Kreuter J. Enteric coating derived from marine sponge collagen. Drug Dev Ind Pharm. 2009;35:1384–8 (Taylor & Francis).
Banerjee P, Lenz D, Robinson JP, Rickus JL, Bhunia AK. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab Invest Nature Publishing Group. 2008;88:196–206.
Sastry TP. Collagen thin film glucose biosensor. Asian J Biomed Pharm Sci. 2014;4:11–4.
Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–21.
Bouler JM, Pilet P, Gauthier O, Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017;53:1–12.
Dadhich P, Das B, Pal P, Srivas PK, Dutta J, Ray S, et al. A Simple Approach for an Eggshell-Based 3D-Printed Osteoinductive Multiphasic Calcium Phosphate Scaffold. ACS Appl Mater Interfaces. 2016;8:11910–24 (American Chemical Society).
Jafarkhani M, Fazlali A, Moztarzadeh F, Moztarzadeh Z, Mozafari M. Fabrication and Characterization of PLLA/Chitosan/Nano Calcium Phosphate Scaffolds by Freeze-Casting Technique. Ind Eng Chem Res. 2012;51:9241–9 (American Chemical Society).
Shavandi A, Bekhit AE-DA, Ali A, Sun Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater Chem Phys. 2015;149–150:607–16.
Baykan E, Koc A, Eser Elcin A, Murat Elcin Y. Evaluation of a biomimetic poly(ε-caprolactone)/β-tricalcium phosphate multispiral scaffold for bone tissue engineering: In vitro and in vivo studies. Biointerphases. 2014;9:0290113 (American Vacuum Society).
Tavangar A, Tan B, Venkatakrishnan K. Synthesis of three-dimensional calcium carbonate nanofibrous structure from eggshell using femtosecond laser ablation. J Nanobiotechnology. 2011;9:1.
Baino F, Vitale-Brovarone C. 8 - Bioactive glass and glass–ceramic foam scaffolds for bone tissue restoration. In: Netti PA, editor. Biomed Foams Tissue Eng Appl [Internet]. Woodhead Publishing; 2014 [cited 2021 Apr 6]. p. 213–48. Available from: https://www.sciencedirect.com/science/article/pii/B9780857096968500085
Feng P, Niu M, Gao C, Peng S, Shuai C. A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering. Sci Rep. 2014;4:5599 (Nature Publishing Group).
Lin Y-H, Chiu Y-C, Shen Y-F, Wu Y-HA, Shie M-Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J Mater Sci Mater Med. 2017;29:11.
Somanathan T, Prasad K, Ostrikov K (Ken), Saravanan A, Krishna VM. Graphene Oxide Synthesis from Agro Waste. Nanomaterials. Multidisciplinary Digital Publishing Institute; 2015;5:826–34.
Anantharaman A. Green Synthesis of Calcium Oxide Nanoparticles and Its Applications. 2016;6:5.
Wu S-C, Tsou H-K, Hsu H-C, Hsu S-K, Liou S-P, Ho W-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram Int. 2013;39:8183–8.
Roopavath UK, Sah MK, Panigrahi BB, Rath SN. Mechanochemically synthesized phase stable and biocompatible β-tricalcium phosphate from avian eggshell for the development of tissue ingrowth system. Ceram Int. 2019;45:12910–9.
Neunzehn J, Szuwart T, Wiesmann H-P. Eggshells as natural calcium carbonate source in combination with hyaluronan as beneficial additives for bone graft materials, an in vitro study. Head Face Med. 2015;11:12.
Maleki S, Barzegar-Jalali M, Zarrintan MH, Adibkia K, Lotfipour F. Calcium carbonate nanoparticles; potential applications in bone and tooth disorders. Pharm Sci. Tabriz University of Medical Sciences; 20:175–82.
Siqueira G, Abdillahi H, Bras J, Dufresne A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim Dourado). Cellulose. 2010;17:289–98.
Kalia S, Dufresne A, Cherian BM, Kaith BS, Avérous L, Njuguna J, et al. Cellulose-Based Bio- and Nanocomposites: A Review. Int J Polym Sci. 2011;2011:e837875 (Hindawi).
Akin DE, Condon B, Sohn M, Foulk JA, Dodd RB, Rigsby LL. Optimization for enzyme-retting of flax with pectate lyase. Ind Crops Prod. 2007;25:136–46.
Banik S, Basak MK, Paul D, Nayak P, Sardar D, Sil SC, et al. Ribbon retting of jute—a prospective and eco-friendly method for improvement of fibre quality. Ind Crops Prod. 2003;17:183–90.
Song B, Lin R, Lam CH, Wu H, Tsui T-H, Yu Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew Sustain Energy Rev. 2021;135:110370 (Elsevier).
Johar N, Ahmad I, Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crops Prod. 2012;37:93–9.
Jahan MS, Saeed A, He Z, Ni Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose. 2011;18:451–9.
Qua EH, Hornsby PR, Sharma HSS, Lyons G. Preparation and characterisation of cellulose nanofibres. J Mater Sci. 2011;46:6029–45.
Brinchi L, Cotana F, Fortunati E, Kenny JM. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr Polym. 2013;94:154–69.
Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls. Bioresour Technol. 2008;99:1664–71.
Hassan ML, Mathew AP, Hassan EA, El-Wakil NA, Oksman K. Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol. 2012;46:193–205.
Ferrer A, Filpponen I, Rodríguez A, Laine J, Rojas OJ. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour Technol. 2012;125:249–55.
Chen W, Yu H, Liu Y, Hai Y, Zhang M, Chen P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose. 2011;18:433–42.
Wang S, Cheng Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J Appl Polym Sci. 2009;113:1270–5.
Fatah IYA, Khalil HPSA, Hossain MS, Aziz AA, Davoudpour Y, Dungani R, et al. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers Multidisciplinary Digital Publishing Institute. 2014;6:2611–24.
Ha TLB, Quan TM, Vu DN, Si DM. Naturally Derived Biomaterials: Preparation and Application. Regen Med Tissue Eng [Internet]. IntechOpen; 2013 [cited 2021 Apr 6]; Available from: https://www.intechopen.com/books/regenerative-medicine-and-tissue-engineering/naturally-derived-biomaterials-preparation-and-application
Fabian C, Ju Y-H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit Rev Food Sci Nutr. 2011;51:816–27 (Taylor & Francis).
Sharma K, Mujawar MA, Kaushik A. State-of-Art Functional Biomaterials for Tissue Engineering. Front Mater [Internet]. Frontiers; 2019 [cited 2021 Apr 6];6. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fmats.2019.00172/full
Ramesh N, Moratti SC, Dias GJ. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J Biomed Mater Res B Appl Biomater. 2018;106:2046–57.
Ho M, Wang H, Lee J-H, Ho C, Lau K, Leng J, et al. Critical factors on manufacturing processes of natural fibre composites. Compos Part B Eng. 2012;43:3549–62.
Baino F, Novajra G, Vitale-Brovarone C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front Bioeng Biotechnol [Internet]. Frontiers; 2015 [cited 2021 Apr 6];3. Available from: https://www.frontiersin.org/articles/https://doi.org/10.3389/fbioe.2015.00202/full
Pina S, Oliveira JM, Reis RL. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv Mater. 2015;27:1143–69.
Edwards A, Jarvis D, Hopkins T, Pixley S, Bhattarai N. Poly(ε-caprolactone)/keratin-based composite nanofibers for biomedical applications. J Biomed Mater Res B Appl Biomater. 2015;103:21–30.
Bulota M, Jääskeläinen AS, Paltakari J, Hughes M. Properties of biocomposites: influence of preparation method, testing environment and a comparison with theoretical models. J Mater Sci. 2011;46:3387–98.
Fu W, Liu Z, Feng B, Hu R, He X, Wang H, et al. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int J Nanomedicine. 2014;9:2335–44.
Kijeńska E, Prabhakaran MP, Swieszkowski W, Kurzydlowski KJ, Ramakrishna S. Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J Biomed Mater Res B Appl Biomater. 2012;100B:1093–102.
Arun Kumar R, Sivashanmugam A, Deepthi S, Iseki S, Chennazhi KP, Nair SV, et al. Injectable Chitin-Poly(ε-caprolactone)/Nanohydroxyapatite Composite Microgels Prepared by Simple Regeneration Technique for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:9399–409 (American Chemical Society).
Lu T, Li Q, Chen W, Yu H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos Sci Technol. 2014;94:132–8.
Wu S, Applewhite AJ, Niezgoda J, Snyder R, Shah J, Cullen B, et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv Skin Wound Care. 2017;30:S1-18.
Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules American Chemical Society. 2014;15:635–43.
Nasri-Nasrabadi B, Mehrasa M, Rafienia M, Bonakdar S, Behzad T, Gavanji S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr Polym. 2014;108:232–8.
Salgado AJ, Sousa RA, Fraga JS, Pego JM, Silva BA, Malva JO, et al. Effects of Starch/ Polycaprolactone-based Blends for Spinal Cord Injury Regeneration in Neurons/Glial Cells Viability and Proliferation. J Bioact Compat Polym. 2009;24:235–48 (SAGE Publications Ltd STM).
Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8:3714–22.
Zhang C, Salick MR, Cordie TM, Ellingham T, Dan Y, Turng L-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater Sci Eng C. 2015;49:463–71.
Wei G, Li C, Fu Q, Xu Y, Li H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int Urol Nephrol. 2015;47:95–9.
Semnani D, Naghashzargar E, Hadjianfar M, Manshadi FD, Mohammadi S, Karbasi S, et al. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int J Polym Mater Polym Biomater. 2017;66:149–57 (Taylor & Francis).
Nazeer MA, Yilgör E, Yilgör I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr Polym. 2017;175:38–46.
Sambudi NS, Sathyamurthy M, Lee GM, Park SB. Electrospun chitosan/poly(vinyl alcohol) reinforced with CaCO3 nanoparticles with enhanced mechanical properties and biocompatibility for cartilage tissue engineering. Compos Sci Technol. 2015;106:76–84.
Sambudi NS, Park SB, Cho K. Enhancing the mechanical properties of electrospun chitosan/poly(vinyl alcohol) fibers by mineralization with calcium carbonate. J Mater Sci. 2016;51:7742–53.
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3:278–314.
Ude AU, Eshkoor RA, Zulkifili R, Ariffin AK, Dzuraidah AW, Azhari CH. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater Des. 2014;57:298–305.
Teimouri A, Ghorbanian L, Najafi Chermahini A, Emadi R. Fabrication and characterization of silk/forsterite composites for tissue engineering applications. Ceram Int. 2014;40:6405–11.
Tajbakhsh S, Hajiali F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater Sci Eng C. 2017;70:897–912.
Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials. 2010;3:1863–87 (Molecular Diversity Preservation International).
Gurunathan T, Mohanty S, Nayak SK. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos Part Appl Sci Manuf. 2015;77:1–25.
Mukhopadhyay S, Fangueiro R. Physical Modification of Natural Fibers and Thermoplastic Films for Composites — A Review. J Thermoplast Compos Mater. 2009;22:135–62 (SAGE Publications Ltd STM).
Macocinschi D, Filip D, Vlad S, Butnaru M, Knieling L. Evaluation of polyurethane based on cellulose derivative-ketoprofen biosystem for implant biomedical devices. Int J Biol Macromol. 2013;52:32–7.
Rani MU, Rastogi NK, Anu Appaiah KA. Statistical Optimization of Medium Composition for Bacterial Cellulose Production by Gluconacetobacter hansenii UAC09 Using Coffee Cherry Husk Extract - an Agro-Industry Waste. J Microbiol Biotechnol. 2011;21:739–45 (The Korean Society for Microbiology and Biotechnology).
Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res. 2012;30:217–24 (SAGE Publications Ltd STM).
Archana G, Sabina K, Babuskin S, Radhakrishnan K, Fayidh MA, Babu PAS, et al. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr Polym. 2013;98:89–94.
Athinarayanan J, Periasamy VS, Alhazmi M, Alatiah KA, Alshatwi AA. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram Int. 2015;41:275–81.
Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773–81.
Cheirmadurai K, Thanikaivelan P, Murali R. Highly biocompatible collagen–Delonix regia seed polysaccharide hybrid scaffolds for antimicrobial wound dressing. Carbohydr Polym. 2016;137:584–93.
Chinga-Carrasco G, Ehman NV, Pettersson J, Vallejos ME, Brodin MW, Felissia FE, et al. Pulping and Pretreatment Affect the Characteristics of Bagasse Inks for Three-dimensional Printing. ACS Sustain Chem Eng. 2018;6:4068–75 (American Chemical Society).
Li Q, Lu F, Zhou G, Yu K, Lu B, Xiao Y, et al. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules. 2017;18:3766–75 (American Chemical Society).
Mandal BB, Grinberg A, Gil ES, Panilaitis B, Kaplan DL. High-strength silk protein scaffolds for bone repair. Proc Natl Acad Sci. 2012;109:7699–704 (National Academy of Sciences).
Tovar-Carrillo KL, Sueyoshi SS, Tagaya M, Kobayashi T. Fibroblast Compatibility on Scaffold Hydrogels Prepared from Agave Tequilana Weber Bagasse for Tissue Regeneration. Ind Eng Chem Res. 2013;52:11607–13 (American Chemical Society).
Kumar A, Negi YS, Choudhary V, Bhardwaj NK. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J Mater Phys Chem. 2014;2:1–8 (Science and Education Publishing).
Cherian BM, Leão AL, de Souza SF, Costa LMM, de Olyveira GM, Kottaisamy M, et al. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr Polym. 2011;86:1790–8.
Czaja W, Krystynowicz A, Bielecki S, Brown RM. Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145–51.
Hoyer B, Bernhardt A, Heinemann S, Stachel I, Meyer M, Gelinsky M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules American Chemical Society. 2012;13:1059–66.
Jithendra P, Rajam AM, Kalaivani T, Mandal AB, Rose C. Preparation and Characterization of Aloe Vera Blended Collagen-Chitosan Composite Scaffold for Tissue Engineering Applications. ACS Appl Mater Interfaces. 2013;5:7291–8 (American Chemical Society).
Nakasone K, Ikematsu S, Kobayashi T. Biocompatibility Evaluation of Cellulose Hydrogel Film Regenerated from Sugar Cane Bagasse Waste and Its in Vivo Behavior in Mice. Ind Eng Chem Res. 2016;55:30–7 (American Chemical Society).
Silvério HA, Flauzino Neto WP, Pasquini D. Effect of Incorporating Cellulose Nanocrystals from Corncob on the Tensile, Thermal and Barrier Properties of Poly(Vinyl Alcohol) Nanocomposites. J Nanomater. 2013;2013:e289641 (Hindawi).
dos Santos RM, Flauzino Neto WP, Silvério HA, Martins DF, Dantas NO, Pasquini D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind Crops Prod. 2013;50:707–14.
Gade R, Tulasi MS, Bhai VA. Seaweeds: a novel biomaterial. Int J Pharm Pharm Sci. 2013;5:975–1491.
Akhavan O, Bijanzad K, Mirsepah A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv Royal Society of Chemistry. 2014;4:20441–8.
Chen D, Lawton D, Thompson MR, Liu Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr Polym. 2012;90:709–16.
Salgado AJ, Coutinho OP, Reis Rl. Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue eng. 2004;10(3-4):465–474.
Chowdhury A, Anthony P. A review on biodegradable polymeric materials derived from vegetable oils for diverse applications. Int J Sci Res. 2016;5:1786–1791.
Lönnberg H, Larsson K, Lindström T, Hult A, Malmström E. Synthesis of Polycaprolactone-Grafted Microfibrillated Cellulose for Use in Novel Bionanocomposites-Influence of the Graft Length on the Mechanical Properties. ACS Appl Mater Interfaces. 2011;3:1426–33 (American Chemical Society).
Venkatesan J, Kim S-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar Drugs. 2010;8:2252–66 (Molecular Diversity Preservation International).
Venkatesan J, Qian Z-J, Ryu B, Ashok Kumar N, Kim S-K. Preparation and characterization of carbon nanotube-grafted-chitosan – Natural hydroxyapatite composite for bone tissue engineering. Carbohydr Polym. 2011;83:569–77.
Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, et al. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:27226 (Nature Publishing Group).
Prasadh S, Suresh S, Wong R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials. 2018;11:1430 (Multidisciplinary Digital Publishing Institute).
Luef KP, Stelzer F, Wiesbrock F. Poly(hydroxy alkanoate)s in Medical Applications. Chem Biochem Eng Q. 2015;29:287–97 (Hrvatsko društvo kemijskih inženjera i tehnologa).
Soler-Botija C, Bagó JR, Llucià-Valldeperas A, Vallés-Lluch A, Castells-Sala C, Martínez-Ramos C, et al. Engineered 3D bioimplants using elastomeric scaffold, self-assembling peptide hydrogel, and adipose tissue-derived progenitor cells for cardiac regeneration. Am J Transl Res. 2014;6:291–301.
Yahyavi-Firouz-Abadi N, Menias CO, Bhalla S, Siegel C, Gayer G, Katz DS. Imaging of Cosmetic Plastic Procedures and Implants in the Body and Their Potential Complications. Am J Roentgenol. 2015;204:707–15 (American Roentgen Ray Society).
Williams C. Granugel: hydrocolloid gel. Br J Nurs Mark Allen Group. 1996;5:188–90.
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015;65:252–67.
Francesko A, Tzanov T. Chitin, Chitosan and Derivatives for Wound Healing and Tissue Engineering. In: Nyanhongo GS, Steiner W, Gübitz G, editors. Biofunctionalization Polym Their Appl [Internet]. Berlin, Heidelberg: Springer; 2011 [cited 2021 Apr 6]. p. 1–27. Available from: https://doi.org/10.1007/10_2010_93
Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano PA, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–64.
Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Synergistic Combination of Chitosan Acetate with Nanoparticle Silver as a Topical Antimicrobial: Efficacy against Bacterial Burn Infections. Antimicrob Agents Chemother. 2011;55:3432–8 (American Society for Microbiology Journals).
Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ. 2006;332:962–5 (British Medical Journal Publishing Group).
Lawin PB, Silverstein P, Still JM. E-Z DERM™ A Porcine Heterograft Material. Am J Clin Dermatol. 2002;3:507–507.
Pajardi G, Rapisarda V, Somalvico F, Scotti A, Russo GL, Ciancio F, et al. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: a retrospective observational study. Int Wound J. 2016;13:44–52.
Varkey M, Ding J, Tredget EE. Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J Funct Biomater. 2015;6:547–63 (Multidisciplinary Digital Publishing Institute).
Dvir T, Kohane DS, Langer RS, Timko B. Nanowired three dimensional tissue scaffolds [Internet]. 2015 [cited 2021 Apr 6]. Available from: https://patents.google.com/patent/US9114009B2/en
Lelkes PI, Senel HG, Brookstein D, Govindaraj M. Textile-templated electrospun anisotropic scaffolds for tissue engineering and regenerative medicine [Internet]. 2017 [cited 2021 Apr 6]. Available from: https://patents.google.com/patent/US9668846B2/en
Silva AKA, Juenet M, Meddahi-Pellé A, Letourneur D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr Polym. 2015;116:267–77.
Note, I. Important. Hyaluronic Acid Derivatives: Durolane®, Gel-One®, GelSyn-3™, GenVisc 850®, Hyalgan™, Hymovis®, Monovisc®, Orthovisc™, Supartz/Supartz FX™, Synojoynt™, TriVisc™, VISCO-3™, Triluron™, sodium hyaluronate 1%. 2020;9.
Binder Ronald K., Rodés-Cabau Josep, Wood David A., Mok Michael, Leipsic Jonathon, De Larochellière Robert, et al. Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc Interv. American College of Cardiology Foundation; 2013;6:293–300.
Shih M, Chang J, Rhee R. Chapter 6 - Complications of the Medtronic Endurant Stent Graft. In: Dryjski ML, Harris LM, editors. Complicat Endovasc Surg [Internet]. Philadelphia: Elsevier; 2022 [cited 2021 Apr 6]. p. 39–44. Available from: https://www.sciencedirect.com/science/article/pii/B9780323554480000061
Du J, Chen H, Qing L, Yang X, Jia X. Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater Sci. 2018;6:1299–311 (The Royal Society of Chemistry).
Acknowledgements
Authors are thankful to MHRD (Govt. of India) for motivating to contribute towards Clean India and Wealth from Waste initiatives. Also, authors express their gratitude towards continuous support from the Institutes (Indian Institute of Technology, Hyderabad and Dr. B. R. Ambedkar National Institute of Technology Jalandhar, Punjab, India).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest in any manner regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sah, M.K., Mukherjee, S., Flora, B. et al. Advancement in “Garbage In Biomaterials Out (GIBO)” concept to develop biomaterials from agricultural waste for tissue engineering and biomedical applications. J Environ Health Sci Engineer 20, 1015–1033 (2022). https://doi.org/10.1007/s40201-022-00815-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-022-00815-0