Abstract
To identify the microstructural factors effecting the electro gas welding (EGW) weld metal properties, this study investigated the influence of different prototype welding consumables and shielding gases on the microstructural composition and mechanical-technological properties. The aim was to adjust the weld metal properties as a trade-off between strength, ductility, and impact toughness to fulfill typical weld metal material specifications in cruise vessel shipbuilding under consideration of the manufacturing conditions at European shipyards. The microstructure is analyzed by quantitative metallography of the ferritic matrix, martensite-retained austenite (M/A) constituents, and non-metallic inclusions (NMI). The influence of the Ni content, the deoxidation concept by variation of Si and Ti contents, and different shielding gas activity by variation of the Ar proportions is discussed. The interaction of ferritic matrix with high acicular ferrite content of about 70 ± 10%, the existence of larger grain boundary ferrite formations, and the M/A morphology plus distribution are considered as the determining factors for the material properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electro gas welding (EGW) is a highly efficient arc-based process used for welding upwards in vertical position of long straight butts in steel ship structures with thicknesses of about t = 12–40 mm by single-layer welding using one electrode (see Fig. 1) and up to t = 80 mm by using two electrodes. The process is characterized by relatively high inputs, i.e., arc energy of about E = 6–25 kJ/mm and up to > 80 kJ/mm when using two electrodes, which significantly influence the weld metal metallurgy and thus the microstructure, cf. [1,2,3,4,5,6,7]. Filler alloy concepts ensuring specified ferritic weld metal properties are widely validated for arc-based processes like shielded metal arc welding (SMAW) and gas metal arc welding (GMAW) with lower heat inputs, i.e., E = 0.5–2 kJ/mm. However, to achieve sufficient material properties in low-alloyed steel, weld metal microstructures formed by relatively low cooling rates different approaches were taken. The activity of former austenite (γ) grain boundaries is attempted to be reduced to prevent from high-temperature ferrite phase formation, i.e., intergranular allotriomorphic ferrite and Widmanstätten ferrite with coarse structure. Dispersed NMI (non-metallic inclusions) are aimed to act as nuclei for desirable acicular ferrite (AF) formation facilitating sufficient strength and appropriate toughness [8, 9]. Commonly, high AF contents of about 70% are considered to ensure preferably low ductile-to-brittle temperatures of C-Mn weld metals [3, 10]. Normally, the strength and impact toughness properties of EGW welds can be reached by using commercial consumables. For specific applications in hull manufacturing of cruise vessels, the technical rules of classification societies require enhanced ductility in the form of relatively high elongation at fracture of the weld metal. However, higher ductility is usually promoted by larger ferrite morphologies like primary ferrite causing lower toughness and strength.
Electro gas welding of vertical butt joints: a schematic single-sided EGW process using a copper shoe and b hull section in production at the shipyard of NEPTUN WERFT, cf. [11]
Since the development of EGW process in the 1950s [1, 12], there have been specially designed welding electrodes. The Western European in-house developments are based on basic cored wires. The latest development has its origins in the 1970s and is essentially a non-microalloyed CSiMnNiMo type. The widespread use in Asian shipbuilding is often based on a similar type of alloy, which has, however, been continuously further developed. Today, filler metal for EGW is alloyed with small amounts of Ti and B as important drivers for the crystallization of acicular ferrite during ferrite formation [4, 13]. Due to the constant demand for cruise ships, the construction strategy of MEYER WERFT shipyard has been optimized. The so-called floating engine room units (FERU) are built and supplied as floating parts. These engine room modules (approx. 140 m long) have a relatively large number of straight butt joints in vertical position (often t = 14–20 mm). This application is realized by EGW. In order to ensure optimum weld metal properties, extensive tests were carried out, which led to a new flux-cored wire electrode, which was especially composed to the conditions of European cruise shipbuilding.
In this study, the influence of different prototype welding consumables and shielding gases on the microstructural composition and mechanical-technological properties of the weld metal is investigated. The aim was to adjust the weld metal properties as a trade-off between strength, ductility, and impact toughness to fulfill typical weld metal material specifications in cruise vessel shipbuilding, e.g., classification rules of DNV, RINA, ABS, and BV, under consideration of manufacturing conditions at European shipyards.
2 Classification and influencing of the microstructure
A classification of ferritic components is possible according to their formation, morphology [10, 14], or effect on the macro-mechanical-technological behavior [8, 15, 16]. Since correlations to mechanical-technological properties could be proven, a simplified rough categorization is possible [10, 16]. For the analysis of the ferritic weld metal by means of light optical microscopy (LOM), the IIW guideline for the classification of microstructure [17, 18] applies, which was extended by Thewlis [14]. A scheme for phase classification regarding morphological character is defined, which is generally applicable for low-alloyed steels, HAZ, and weld metal. An optically magnified, metallographically prepared microsection is analyzed for quantitative microstructure description. A simplified evaluation methodology, especially for single-layer welds in as welded state, which is used to estimate the resulting material properties, was presented in [16]. This approach has already been used for the evaluation of weld metal microstructures of different processes, such as submerged arc, electro gas, electro slag, and stud welding [19, 20].
The ferrite forms can be read off as an example on the basis of their formation temperatures in the schematic continuous cooling transformation diagram for the weld metal (see Fig. 2), following [16, 21]. The main microstructural constituents of ferritic weld metal and a corresponding categorization are listed in Table 1.
Schematic continuous cooling transformation diagram for non- or low-alloyed ferritic weld metal and transformations from the austenite region, cf. [20]
In primary metallurgy, delta ferrite (δ) is present as a cast structure during solidification. On further cooling, however, the δ-γ-transformation takes place, which nucleates at the δ grain boundaries and reproduces its columnar structure. Therefore, only phase transformation from γ below Ar3 is discussed. Accordingly, a homogeneous austenite (γ) is assumed in the solidified weld metal. At high crystallization temperatures, proeutectoid ferrite PF is initially formed, which usually shows relatively coarse grains. Although this exhibits high ductility, it causes reduced toughness. Due to the simple diffusion paths, allotriomorphic ferrite initially forms along the γ boundaries. On further cooling, growth takes place in Widmanstätten form since diffusion of substitutional elements is no longer involved and growth does not come to a standstill at lower temperatures. Furthermore, idiomorphic ferrite can form intragranular at potent inclusions, called “active.” The group of ferrites with secondary phases FS comprises ferrites with aligned martensite-austenite-carbide phases MAC as Widmanstätten ferrite WF and forms of parallel ferrite plates with MAC on the grain boundaries (upper bainite UB), as well as ferrite with undirected secondary phases. The group of FS favors unstable crack growth and should therefore be avoided in the weld metal of low-alloyed steels. At lower temperatures, the formation of pearlite as ferrite with lamellar cementite plates takes place, which is assigned to the group ferrite-carbide aggregates FCA. Furthermore, acicular ferrite AF is formed between the crystallization temperatures of WF and UB, providing that sufficiently large former γ grains are present (longer C-diffusion paths) [21]. This fine-grained ferrite structure crystallizes intragranular at heterogeneous nucleation sites, especially on NMIs of Mn- and Ti–rich oxides and nitrides [5, 10, 14] as well as complex oxides containing Mn and Si [9]. Furthermore, AF forms on primary plates by sympathetic nucleation [21]. Due to the composition of ferrite lancets with carbon-enriched islands of austenite or martensite, this microstructure is also called granular bainite. The chaotic arrangement and the relatively small grain size of the ferrites cause many large-angle grain boundaries and a high dislocation density, so that good strength and toughness properties are achieved [22]. AF is therefore considered to be the desired morphology for weld metal in the moderate strength range [8, 10, 22]. At high cooling rates, phase transformation occurs via a cooperative shear mechanism or displacive transformation. Lower bainite LB or lath martensite M(L) results. Due to the precipitation characteristics caused by the auto-tempering effect, these phases appear very similar and prevent a clear differentiation by LOM [18] so that they are combined in the group LB/M.
In addition to the components of the ferritic matrix, even in low-alloyed steels with C: < 0.25 wt.%, there may occur areas between transformed α grains that are locally highly C-enriched due to microsegregation, i.e., C: 0.5–1.3 wt.% [23, 24]. In these areas, depending on the concentration, either the critical cooling rate is exceeded, so that non-annealed martensite or retained austenite results at room temperature. These particles are referred to as martensite-austenite M/A constituents [23, 24] or generally microphases [14, 21] due to their comparatively small size. The M/A particles mostly show heterogeneous C distribution with a C-rich center and a lower-C shell [25] (see Fig. 3a). Depending on the hardness and morphology of these particles relative to the surrounding matrix as well as their distribution, the toughness is strongly influenced. These M/A constituents represent local brittle zones [26], provided they exceed a minimum size [27] or fraction [28], or exhibit a directional, quasi-intergranular arrangement [15, 29]. Relatively large blocky or massive particles, as well as particles with a slender shape, e.g., elongated or necklace-type [23, 27], are considered to promote embrittlement and further favor crack initiation. In contrast, a finely dispersed distribution of M/A particles is assumed to have a strength and toughness-increasing effect [16, 27].
The ferritic matrix and the M/A particles determine the mechanical-technological properties of the weld metal. Both components can be assigned intragranular and intergranular morphologies (see Fig. 3). Approaches are listed in [19] that the former γ grain boundary with coarse ferrite types, viz., PF(GB), FS(GB), summarized to αGB, and large M/A5 particles with an individual size ≥ 5 µm2 is particularly related to the impact properties. These intragranular representatives, which tend to have negative connotations, are contrasted with AF as an intragranular type and finely dispersed M/A particles.
The γ-α transformation and M/A formation is influenced by the chemical conditions in the molten pool or weld metal, in addition to the thermal undercooling, which in the case of EGW is associated with the plate thickness to be welded [2]. These in turn depend on the chemical composition of the filler material and the base material due to relatively strong dilution, as well as on the composition of the shielding gas. Alloying elements such as Mn, Ni, and Mo impede C diffusion and thus shift the transformation temperature to lower temperatures and suppress the high-temperature types of ferrite, such as grain boundary ferrite. Ti and B as a micro-alloy concept have become indispensable in modern EGW welds. B reduces the free enthalpy at the γ grain boundary through segregation, thus preventing the ferrite nucleus from stabilizing and growing. Due to its high affinity (low enthalpy of formation) for N, Ti protects B from binding with N. Furthermore, complex oxides with titanium oxide surfaces are essential as efficient nucleation sites for the desired AF [3, 4, 8, 30].
The O content in the EGW weld metal is supplied via the shielding gas. In Asia, EGW is usually performed with 100% CO2 [7, 30], whereas in Europe, Ar-rich gas mixtures are currently predominantly used. In any case, the oxygen interacts with the wire electrode alloy, resulting in a loss of alloying elements (burn-off), which must be considered for the target analysis of the weld metal. The amount of deoxidation elements used, such as Si, Mn, and Ti, depends on the composition of the shielding gas. It determines the O content in weld metal and thus the number, size, and distribution of the oxides being decisive for intragranular nucleation.
In addition to the O content, which is expected to reach an optimum at approx. 250 ppm [10], the chemical composition of the NMIs is also determined by the type of deoxidizing agent, e.g., B. The epitaxial effect of the NMIs for the AF is reduced by excessive amounts of Al on the oxide surfaces [31, 32]; the M/A shares also increase with increasing Al content. Although Si can be used as a deoxidation element to adjust the O content, it is known to significantly increase the M/A ratio in steels. In bainitic steels, this is explained by the fact that Si is not soluble in cementite, accumulates in the surrounding Fe-mixed crystal, and thus represents a diffusion barrier for carbon. This then diffuses into segregation areas of Mn and Ni (γ stabilization), and there is increased M/A formation [33].
The relationships described mean that there cannot be a single filler metal/welding gas combination for EGW to cover the entire plate thickness range, and thus the arc energy, for single-wire variants from t = 12–40 mm. For special applications, either the electrode alloy or the shielding gas must be varied to achieve a balance between the required properties by the rules. In the field of European cruise ship construction, the plate metal thicknesses of t = 12–25 mm (shell plates) are relatively moderate applications. [11]
3 Experimental methodology
3.1 Materials and sample welding
Weld samples were produced with an older commercial EGW welding machine (ESAB, Mini Vertomatic NC/W) and a one-side water cooled copper shoe plus ceramic backing. One electrode without oscillation was used. This is sufficient for electrode development. Modern welding technology (Hyundai, Mini EG) was used at the NEPTUN WERFT shipyard for procedure qualification, based on the findings presented here (see Fig. 4).
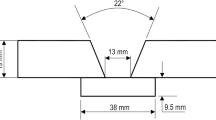
Butt joints (t × 1000 × 500 mm3 each plate) of shipbuilding steel grade A36 with single-V groove preparation (40 ± 2°, and 6 ± 1 mm root gap) and a thickness of t = 15 mm were welded with an arc energy of about E = 8 kJ/mm without preheating. The “ESAB Vertomax 2MG” tubular cored electrode was used for the first welding test and serves as a reference (Ref.). Six different flux cored wire prototypes with a basic-based slag were then used for the welding tests. The aim was to create microstructures that were as different as possible to enable a correlation between weld metal properties and microstructure. In addition, metallurgical processes in the areas of deoxidation, oxygen supply by means of shielding gas, and variation of the Ni content were examined with chemical conditions kept as constant as possible using the same electrode “E4”; see test program (Table 2).
The parameters used for sample welding are listed in Table 3. The welds were performed with shielding gases acc. to ISO 14175 as a mixture of Ar with different percentages of active components, viz., CO2 or O2, respectively, or pure CO2. All welds were welded with approx. 35 V and 380 A. Small changes in the welding speed due to varying seam preparation resulted in minimal differences in the arc energy. Only samples #6 (M20) and #7 (M13) resulted in noticeably lower arc energies. The reason for this is a reduction in voltage (M20: 34,5 V vs. M13: 28,5 V) so that the arc cannot migrate to the welding torch due to the increased Ar content. This leads to process instabilities and associated imperfections, i.e., pores.
The base materials used were selected based on their composition. The dilution (here approximately 10–25%, see Fig. 5b)) of the weld pool with the base material should therefore only have a very small influence on the weld metal chemistry. Chemically, the two base materials reflect a common shipbuilding steel in Europe of grade A36 (see Table 4). The composition of the weld metal, set by variation in the electrode alloy and shielding gas, meets the requirements. The Nb and Al content in weld metal is very low, at well below 100 ppm. These elements are more likely to result from dilution than from the shell of the cored wire electrodes. The P and S content, on the other hand, can come from the flux filling of the tubular electrodes. Ni in particular can be contaminated due to its high affinity for these S and P and can explain the higher values compared to the base materials. In the case of S, it can also be due to Mo content in the flux filling.
To determine the cooling conditions, temperature measurements were carried out in the weld metal for single-sided EGW of a steel grade A36 with t = 13 mm. The used shielding gas was M21 (82/18), and the arc energy was about E = 7.1 kJ/mm. The results of the measurement using a type S thermocouple placed in the weld metal from the root side resulted in a cooling time of t8/5 = 104 s, describing the time interval for the cooling span from 800 to 500 °C of the weld metal; see Fig. 5a), cf. [34].
3.2 Material testing
The samples were all tested in the as welded condition without any further heat treatment. To characterize the material behavior regarding toughness Charpy V-notch (CVN) impact tests were performed acc. to ISO 148-1. A 450-J-pendulum testing machine (WPM, PSd 450 + Zwick/Roell) with a 2-mm striker was used. Standard test pieces (10 × 10 × 55 mm3) were extracted from the center of weld (VWT 0/2.5 position acc. to ISO 9016). A set of three specimens were tested each at different temperatures in the range of − 60 to + 20 °C. The ductile–brittle transition temperature (DBBT) curves were estimated by individual regression curves of the averaged value for specific temperatures to determine the T100J transition temperatures regarding 100 J impact energy. The strength and ductility properties represented by the characteristic stress values upper yield strength ReH or proof strength, plastic extension Rp0.2, and ultimate tensile strength Rm, as well as the strain values elongation at break A and percentage reduction of area Z, were determined by tensile tests acc. to ISO 6892-1. Two longitudinal test pieces with a round cross-section (Ø8 mm, L0 = 40 mm) were taken from the center of each weld sample. To examine the weld metal composition, optical emission spectrometry using a spark analyzer (SPECTRO, Spectromaxx) as well as inert gas fusion analysis using a carrier gas melt-extraction system (Bruker, G8 Galileo) for O/N determination was performed (see Table 4).
3.3 Metallography preparation and analysis
The metallographic investigation is based on a complementary microstructure analysis of the ferritic matrix structure, M/A microphase constituent, and NMI in the center of weld metal. For LOM-based investigations, image acquisition at 500X and 1000X was performed using a microscope (Leica, DM6000B) in bright field mode. The welded samples were cross-sectioned and prepared by grinding/polishing before contrasting the microstructure. In this condition, the NMIs were analyzed by digital image processing based on five images at 1000X with 73,000 µm2 each. The NMI’s total area fraction “NMI”, the average diameter “NMIØ”, and the areal distribution “D(NMI)”, equivalent to the dispersity of the inclusions, were evaluated.
On the same sample, grain etching by Nital—3% nitric acid in ethyl alcohol—was applied to reveal the ferritic matrix. Additionally, LePera’s tint etching—1 wt.% aqueous solution of sodium metabisulfite and 4% picric acid in ethyl alcohol in a ratio of 1:1 [35]—was applied to reveal the microphases consisting of C-enriched martensite or retained austenite (M/A) that result by microsegregation during phase transformation. Three images with a total area of 165,000 µm2 were analyzed each.
Quantitative metallography was performed by manual point counting for matrix analysis. A square grid of equidistant horizontal and vertical lines was overlayed on the microscopic photos (see Fig. 6a). The present phase at the 99 intersections was determined according to a classification scheme based on mechanical-technological properties [14, 16]. The value αGB, representing PF(GB) and FS(GB) in total, is also given here. Due to the distinguished contrast after tint etching, the quantitative M/A analysis was performed by digital image processing (see Fig. 6b). The segmentation and classification were based on binary images created by thresholding, cf. [36]. The M/A analysis includes distribution, i.e., number density D(M/A), and morphologic characteristics of the particles. Proportions of larger particles with an individual area ≥ 5 µm2 are given as M/A5 representing the share of the entire M/A area.
Exemplary complementary quantitative microstructure analysis in weld metal region: a point counting method for ferritic matrix and b image processing for M/A constituent, cf. [20]
In addition to LOM analysis, selected samples were examined by scanning electron microscopy (SEM) and energy-dispersive micro-area X-ray spectrometry (EDX) using an electron microscope system (JOEL, JSM-IT100). The EDX detector (JOEL, Dry SD25) operating at a working distance of 10 mm enables the analysis of elements in the spectrum from Be to U. The energy resolution is less than 129 eV. The same Nital etched microsections as used for LOM investigations regarding quantification of the matrix microstructure were analyzed. In addition to M/A particles, NMIs were analyzed in the matrix regarding their chemical composition. Only active inclusions (10 per specimen) regarding AF nucleation were analyzed. The maximum possible area of the NMIs was analyzed by EDX and 150 screenings of the areas evaluated.
4 Results and discussion
For plate thicknesses in the range of t = 12–25 mm, which is relevant for cruise ship construction, cooling times of about t8/5 = 100–250 s are present [2]. Even for a relatively thin joint of t = 13 mm, this means that the weld metal is already exposed to a temperature > 1000 °C for more than 30 s at the root side (see Fig. 5), cf. [34]. The very low cooling rate leads to different microstructures compared to conventional GMAW processes with lower arc energy, i.e., 0.5–2 kJ/mm. The microstructure and the influence of Ni contents, as well as different oxygen supplies, varied via active component shares in shielding gases, and different deoxidation strategies by variation of Si and Ti contents are discussed.
4.1 Microstructure and mechanical properties
A wide variance of microstructural composition in EGW weld metal was achieved (see Table 5). Both the ferritic matrix and the M/A particles show clear differences in their morphology; see exemplarily Fig. 7 based on a comparison of the samples “Ref.” and #9. The strong differences regarding the AF content and the dispersity of M/A particles are very striking. Despite the significant differences, it can be stated that the Ref. sample has an AF content of about 50%. Accordingly, there are also potent inclusions for the formation of AF.
By SEM and EDX analysis, the inclusions were identified to contain Ti and to be active regarding AF nucleation (see Fig. 8b and c), cf. [5]. By using increased primary voltage of 20 kV, contrasting the M/A particles is possible in the SEM image (see Fig. 8a). The large size of the M/A particles in the “Ref.” sample allows to analyze the C-gradients between the ferritic matrix and the M/A particles. The total area fraction M/A and the relative share M/A5 are very pronounced in the “Ref.” sample.
The mechanical material properties of the weld metals are listed in Table 6 and compared to the requirements in shipbuilding [37]. The yield strengths and tensile strengths vary remarkably. The ductility also shows considerable variation, i.e., the elongation in a range of A = 21.6–31.5%. In addition to the tensile test results, the impact energy also proves that the microstructure has a significant influence on the mechanical properties. From the DBBT curves, T100J was found to be in a wide range of + 25 to − 49 °C. The impact energies ranged from KV2 = 50–140 J at − 20 °C.
The strict requirements regarding the weld metal material properties could only be met by sample #1. However, the authors consider sample #8 as potentially suitable for further development due to its high ductility. Sample #9 is said to have great potential for large sheet thicknesses and possibly high-strength steels, e.g., grade E40/E46.
4.2 Variation of Ni content
In the series of tests presented here, the Ni content was varied while the overall chemistry remained relatively constant (see Table 4). The area fraction of AF gradually increases from 41 to 69% with increasing Ni content, viz., from 0.02 to 0.72 wt.%. At the same time, the fraction of coarse ferrite types on the prior γ grain boundary αGB decreases (see Fig. 9). For M/A particles, the area fraction increases slightly, just like the M/A5 share, with increasing Ni content, whereas the dispersity of the particles increases significantly. Overall, however, the M/A constituent in the weld metal is quite low.
In addition to the moderate increase in strength, i.e., roughly + 50 MPa for yield and ultimate tensile strength, the ductility reserve drops very slightly, i.e., roughly − 1%, with increasing Ni content from 0.01 up to 0.72 wt.%, or is comparable to the Ni-free alloy type CMnSiMoTiB (#3). However, the impact property is dramatically increased by + 78 J at − 20 °C for the higher Ni content.
The AF and αGB content in correlation with the T100J transition temperature is shown in Fig. 10 for different Ni contents. It seems that the greatest influence of Ni on the microstructure of the alloy type is more likely to be found in the ferritic matrix. However, due to the finely dispersed M/A particles with increasing Ni content, this effect could be promoted, but the M/A fractions are rather low. In order not to reduce the ductility too much, a Ni content of 0.7–0.8 wt.% is considered appropriate in weld metal (Mo: approx. 0.15 wt.%). The potential for further increase of mechanical properties by adding more Ni to the weld metal alloy is therefore not seen, at least not for the examined plate thickness.
4.3 Oxygen supply and deoxidation
Furthermore, attempts were made to adapt the nucleation conditions for AF and M/A particles through adapted deoxidation (samples: Ref; #1; #5; #8; #9) and oxygen supply (samples: #4; #5; #6; #7) in the shielding gas. These two series are explained in detail below and presented in context. First, the series with different Ar percentage in the shielding gas using the same consumable “E4” are discussed. In the course of the gradual increase of Ar percentage, viz., 0, 82, 90, and 97 vol.%, the active component used was CO2 for samples #4 to #6 and O2 for sample #7. The weld metal composition gradually changed up to the state shown in Table 7.
The C, Si, Mn, Ni, Mo, P, S, Ti, and B contents increase with a higher percentage of Ar or a decreasing active gas share in the gas mixture. This can be explained by the reduced reaction between oxygen and these elements. Conversely, however, this means that weld metal produced with pure CO2 (C1) will have a higher purity regarding S, C, and P than those welded with Ar-rich gases when using the same electrode. Similar results are achieved in steel production by refining, which is state of the art to reduce undesired C, S, and P contents. On the other hand, the elemental burn-off caused by the reactions with the active gas must be compensated by increasing the alloy content in the wire to achieve the target weld metal composition. The positive effect of welding with pure CO2 in terms of purity is opposed to other properties of the gas mixtures. The use of Ar‐rich gases means less alloying of the filler material and the possibility of reducing the O content in weld metal. However, identical O contents are possible by EGW with pure CO2 as with M21 shielding gas. Advantages only arise from approx. 90 vol.% Ar with acceptable process stability. Due to process instabilities, Ar ≥ 90 vol.% is not recommended. It is also noticeable that samples with low O content, viz., samples #6 and #7, have a significantly increased strength. For sample #7, this can be explained by the high AF content (Hall-Patch relationship), which is not exceptionally high in sample #6. The size of the inclusions may play a role here with respect to particle hardening, too.
Table 8 lists the results of the LOM investigations on NMIs. The size, the area fraction, and the dispersity were evaluated. It can be stated that with increasing O content in the weld metal, the mean diameter, the total proportion, and the dispersity of the inclusions increase (see Fig. 11). The change in shielding gas leads to a significant change in the weld metal composition. Therefore, the microstructure is enormously influenced.
The AF content increases and, at the same time, the content of αGB decreases with increasing Ar percentage in the shielding gas (see Fig. 12).
Since NMIs were identified as sites for AF crystallization, further investigations were made using EDX (see Table 9). In addition to the shielding gas variation (samples #4 to #7), samples with different Si and Ti contents were selected for this investigation, viz., “Ref.,” #1, #8, and #9. Regarding the NMI composition, there is a small anomaly. Within sample #9, which by far has the highest Ti content of 590 ppm, N could be detected in the area of an active inclusion, cf. [5].
From Fig. 13a, it can be stated that the Si and Mn content in the active NMI decreases significantly with increasing Ar percentage in the shielding gas (Ti content increases, too), while the content of M/A particles increases. It is possible that instead of being bound in NMI, Si and Mn are now present in the solid solution. This could intensify the processes explained (see 2), which could lead to an increased formation of M/A. This effect could be reinforced by the overall increase in Mn and Si content in weld metal due to an increased Ar percentage.
Figure 13b shows samples with low and high Si content and different Ti contents in weld metal. Samples with high Si content have higher M/A fractions. With increasing Ti content, the Si and Mn content in the active NMI decreases significantly, similar to the increase in the Ar percentage in the shielding gas. However, this does not change the M/A area fraction significantly for weld metals with little Si or only slightly for those with higher Si content. The essential factor in M/A formation is seen in the general weld metal composition, i.e., C, Si, and Mn.
With increasing Ti content in the active NMIs, the concentration of Si and Mn in the active NMIs of the welds from the gas variation series decreases (see Fig. 14a). At the same time, the AF content increases > 80%, with less than 8 wt.% of Ti being detected in the active NMI. When plotting the AF content from the welds of the deoxidation tests against the Ti content of the active inclusions, it can be seen that only approx. 5 wt.% is required to produce 70% AF in the weld metal. The Si content in the weld metal is irrelevant here (see Fig. 14b). For maximum toughness and ductility with moderate strength in the EGW weld metal (#1, #8), only 70% AF is required while maintaining the lowest possible M/A, M/A5, and αGB (see Table 5 and Table 6).
5 Conclusions
EGW welding tests were carried out with different wire electrode alloys and shielding gases to examine the microstructure and effect on the mechanical properties of the weld metal. The following main conclusions are drawn:
-
a)
CMnNiMoTiB filler can provide excellent mechanical properties despite the very low cooling rates.
-
b)
The balance of strength, ductility, and toughness with regard to applicable regulations (classification rules) is not easy to find. A suitable electrode/shielding gas combination for cruise ship construction (sample #1) was found, and two promising combinations (#8, #9) in addition.
-
c)
It is assumed that approx. 70% AF is sufficient for a balanced weld metal if there are as few coarse ferrite types (αGB) and as few coarse M/A particles (M/A5) on the prior γ grain boundaries.
-
d)
Si plays a crucial role in the M/A formation. Low Si levels produce lower M/A content.
-
e)
Ni has a decisive influence on the ferritic matrix within the investigated alloy. With an increasing Ni content (up to 0.72 wt.%), the AF content increases, and the proportion of αGB decreases. The proportion of M/A was only slightly affected, but the dispersity was greatly increased. Increasing Ni content leads to a moderate increase in strength and a slight reduction in ductility, while toughness has increased significantly.
-
f)
Like pure CO2, Ar-rich gas mixtures are well suited for EGW. Significantly more than 90 vol.% Ar in the shielding gas is not recommended due to the risk of process instabilities.
-
g)
EGW with pure CO2 produces very pure weld metal, i.e., low C, S, and P content, and can produce the same O content as for welding with M21 through sufficient deoxidation.
-
h)
With increasing Ti contents, the proportion of Si and Mn in the NMIs, which are active for AF nucleation, decreases.
-
i)
From about 5 wt.% Ti in the NMI, 70% AF can be generated in the weld metal.
An interesting experiment would be to make an alloy with as low Si content as possible to reduce M/A. The deoxidation should then be compensated with Ti, so that at least 5 wt.% are present in NMI. The B content must be adjusted regarding the Ti content. The remaining composition could be oriented as follows: Ni: 0.7–0.8 wt.%, Mn: 1.6 wt.%, and Mo: 0.15 wt.%.
Data Availability
The data that support the findings of this study are available from the corresponding author, O.B., upon reasonable request.
References
Fu J, Tao Q, Yang X, Nenchev B, Li M, Tao B, Dong H (2022) The effect of heat source path on thermal evolution during electro-gas welding of thick steel plates. Mater 15(6):2215. https://doi.org/10.3390/ma15062215
Seo K, Ryoo H, Kim HJ, Yoon JG, Lee C (2021) Characterization of the local brittle layer formed in electro-gas weld metals. Weld World 65:513–524. https://doi.org/10.1007/s40194-020-01032-9
Al Z, Pan C, Tian Z (2015) Influence of Ti on weld microstructure and mechanical properties in large heat input welding of high strength low alloy steels. J Iron Steel Res Int 22(5):431–437. https://doi.org/10.1016/S1006-706X(15)30023-6
Fengyu S, Lingzhen Y, Yanmei L (2020) In-situ observation on the influences of Ti on phase transformation of weld metal processed by high-heat input welding. Adv Mech Eng 12(9):1–9. https://doi.org/10.1177/1687814020962289
Seo K, Ryoo H, Kim HJ, Yoon JG, Lee C (2022) Nature of nonmetallic inclusions in electrogas weld metal. Weld World 66:379–390. https://doi.org/10.1007/s40194-021-01246-5
Xie X, Zhao T, Zhao H, Wang C (2021) Heterogeneous microstructure-induced mechanical responses in various sub-zones of eh420 shipbuilding steel welded joint under high heat input electro-gas welding. Acta Metall Sin (Engl. Lett.) 34:1427–1433. https://doi.org/10.1007/s40195-021-01245-x
Seo K, Ryoo H, Kim HJ, Park C, Lee C (2020) Local variation of impact toughness in tandem electro-gas welded joint. Weld World 64:457–465. https://doi.org/10.1007/s40194-019-00844-8
Jorge JCF, De Souza LFG, Mendes MC, Bott IS, Araújo LS, Dos Santos VR, Rebello JMA, Evans GM (2021) Microstructure characterization and its relationship with impact toughness of C-Mn and high strength low alloy steel weld metals – a review. J Mater Res Technol 10:471–501. https://doi.org/10.1016/j.jmrt.2020.12.006
Oh YJ, Lee S-Y, Byun J-S, Shim J-H, Cho YW (2000) Non-metallic inclusions and acicular ferrite in low carbon steel. Mater Trans JIM 41(12):1663–1669. https://doi.org/10.2320/matertrans1989.41.1663
Evans GM, Bailey N (1997) Metallurgy of basic weld metal. Woodhead Publishing Limited, Abington
Banaschik R, Otto A, Gericke A (2022) Wirtschaftlich und prozesssicher schweißen: Elektrogasschweißen in der schiffbaulichen Praxis der Neptun Werft Rostock. Der Praktiker 4:146–151
Krieweth W (1960) Das elektrische Vertikal-CO2-Schweißen mit zwangsweiser Schweißnahtbegrenzung. Dissertation. TH Aachen, Institut für Schweißtechnische Fertigungsverfahren
Zhang P, Zhang J, Li B (2022) Mechanical properties and microstructure transformation behavior for welded joints in ship plate steel with high-heat input welding. J Mater Eng Perf 31:944–952. https://doi.org/10.1007/s11665-021-06224-y
Thewlis G (2004) Classification and quantification of microstructures in steels. J Mater Sci Technol 20(2):143–160. https://doi.org/10.1179/026708304225010325
Bonneviea E, Ferrièrea G, Ikhlefa A, Kaplanb D, Oraina JM (2004) Morphological aspects of martensite–austenite constituents in intercritical and coarse grain heat affected zones of structural steels. J Mater Sci Eng A 385(1–2):352–358. https://doi.org/10.1016/j.msea.2004.06.033
Banaschik R, Brätz O, Henkel K-M (2017) Systematic expansion of the microstructural characterization of ferritic weld metals. Pract Metallogr 54(10):669–684. https://doi.org/10.3139/147.110466
Pargeter RJ, Dolby RE (1986) Guidelines for classification of ferritic steel weld metal microstructures in the light microscope. Weld World 24(7/8):144–149
Abson DJ, Duncan A, Pargeter RJ (1988) Guide to the light microscope examination of ferritic steel weld metals. IIW Doc IX-1533–88
Banaschik R, Brätz O, Henkel K-M (2019) Schweißmetallurgische Untersuchung des Elektroschlacke-Kanalschweißens zur Qualifizierung für stahlbauliche Anwendungen im Dickblechbereich. DVS Ber 355:318–326
Brätz O, Henkel K-M (2023) Investigations on microstructure of drawn arc stud welds on structural steels by quantitative metallography. Weld World 67:195–208. https://doi.org/10.1007/s40194-022-01417-y
Loder D, Sk M, Mayerhofer A, Bernhard C (2017) On the capability of nonmetallic inclusions to act as nuclei for acicular ferrite in different steel grades. Metall Mater Trans B 48:1992–2006. https://doi.org/10.1007/S11663-017-0984-Y
Bhadeshia HKDH, Honeycombe R (2017) STEELS – microstructure and properties, 4th edn. Butterworth-Heinemann, Oxford/Cambridge
Matsuda F, Ikeuchi K, Fukada Y, Horii Y, Okada H, Shiwaku T, Shiga C, Suzuki S (1995) review of mechanical and metallurgical investigations of M-A constituent in welded joint in Japan. Trans JWRl 24(1):1–24
Lan L, Qui C, Zhao D, Gao X, Du L (2012) Analysis of martensite austenite constituent and its effect on toughness in submerged arc welded joint of low carbon bainitic steel. Mater Sci 47:4732–4742. https://doi.org/10.1007/s10853-012-6346-x
Lee S, Kim BC, Kwon D (1993) fracture toughness analysis of heat-affected zones in high-strength low-alloy steel welds. Metallurg Trans A 24:1133–1141. https://doi.org/10.1007/BF02657244
Kim BC, Lee S, Kim NJ, Lee DY (1991) microstructure and local brittle zone phenomena in high-strength low-alloy steel welds. Metall Trans A 22(2):139–149. https://doi.org/10.1007/BF03350956
Lou X, Chen X, Wang T, Pan S, Wang Z (2018) Effect of morphologies of martensite–austenite constituents on impact toughness in intercritically reheated coarse-grained heat-affected zone of HSLA steel. Mater Sci Eng A 710:192–199. https://doi.org/10.1016/j.msea.2017.10.079
Lu F, Gheng G, Chai F, Pan T, Shi Z, Su H, Yang C (2016) Effect of heat input on cleavage crack initiation of simulated coarse grain heat-affected zone in microalloyed offshore platform steel. J Iron Steel Res Int 23(10):1086–1095. https://doi.org/10.1016/S1006-706X(16)30161-3
Li X, Fan Y, Ma X, Subramanian S, Shang C (2015) Influence of martensite-austenite constituents formed at different intercritical temperatures on toughness. Mater Des 67:457–463. https://doi.org/10.1016/j.matdes.2014.10.028
Xie X, Zhong M, Zhao T, Wang C (2023) Probing microstructural evolution in weld metals subjected to varied CaF2–TiO2 flux cored wires under high heat input electro-gas welding. J Iron Steel Res Int 30:150–157. https://doi.org/10.1007/s42243-022-00814-3
Bhadeshia HKDH (2001) Bainite in steels: transformations, microstructure and properties 2nd edn. IOM Communications Ltd., London
Babu SS, Fox AG, Evans GM (2012) A comparative study of the non-metallic inclusions in C-Mn steel weld metals containing titanium or aluminium. 9th Int. Conf. on Trends in Welding Research. Chicago
Bayraktar E, Kaplan D (2004) Mechanical and metallurgical investigation of martensite–austenite constituents in simulated welding conditions. J Mater Proces Technol 153–154:87–92. https://doi.org/10.1016/j.jmatprotec.2004.04.021
Banaschik R (2021) Mikrostrukturelle Charakterisierung langsam abkühlender ferritischer Schweißgüter am Beispiel des Elektrogasschweißens. Dissertation. University of Rostock. https://doi.org/10.18453/rosdok_id00003552
LePera FS (1980) Improved etching technique to emphasize martensite and bainite in high-strength dual-phase steel. JOM 32(38):29. https://doi.org/10.1007/BF03354553
Brätz O (2021) Methodik zur Qualifizierung des Lichtbogenbolzenschweißens mit Hubzündung für den Einsatz im Unterwasserbereich. Dissertation. University of Rostock. https://doi.org/10.18453/rosdok_id00003723
DNV-CP-0069 (2022) Class programme: type approval – welding consumables. DNV AS, Høvik
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection was performed by R.B. The analysis was performed by R.B., with help from O.B. The first draft of the manuscript was written by R.B. and O.B. Figures were designed by O.B. A.G. commented on previous versions of the manuscript. R.B. and K.M.H conceived the original idea. K.M.H. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended for publication by Commission II - Arc Welding and Filler Metals
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banaschik, R., Brätz, O., Gericke, A. et al. Modification of electro gas weld metal microstructure reflecting mechanical property specifications in cruise vessel shipbuilding. Weld World 68, 1625–1639 (2024). https://doi.org/10.1007/s40194-024-01779-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-024-01779-5