Abstract
Purpose of Review
The purpose of this review was to provide an overview of the recent concepts regarding cardiac output measurement devices that utilize non-pulse contour methods, especially in an intraoperative setting. The techniques include inert gas rebreathing method, partial CO2 rebreathing method, impedance cardiography, and its derivative technologies such as electrical velocimetry and bioreactance, transesophageal echocardiography/Doppler, and transthoracic echocardiography/Doppler. We focused on the invasiveness of the devices and their underlying technology.
Recent Findings
Although various types of cardiac output monitoring devices are available, none of them may be considered as an ideal device in terms of accuracy, trending ability of cardiac output changes, and reproducibility of measurements. There are increasing types of devices applicable for intraoperative use, yet only few data are available regarding the trending ability of cardiac output changes and reproducibility of the measurements. Therefore, the empirical application of these devices for various surgical patients may be done under the consideration of their invasiveness and their underlying technology, and it may provide us with more data over time.
Summary
The non-pulse contour-derived cardiac output measurement devices are classified according to their underlying principles, which closely reflect their advantages and disadvantages in the perioperative setting.
Similar content being viewed by others
Introduction
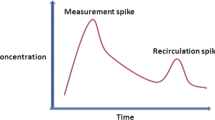
Cardiac output (CO) monitoring devices are used for detecting hemodynamic changes and guiding further therapy in both perioperative and postoperative periods. Perioperative CO monitoring has the potential benefits of reducing morbidity and mortality by optimizing perioperative fluid management. Less invasive CO monitoring devices are emerging as substitutes to the more invasive pulmonary artery catheterization (PAC). PAC is increasingly being avoided owing to its invasiveness [1]. The potential benefits of PAC are offset by its complications, which may also be responsible for the failure of PAC-based CO optimization in improving outcomes [2]. As the decision-making process in medical practice involves balancing the benefits and risks, the disadvantages of the various monitoring methods should be considered when adopting devices into clinical practice. The possible risks of CO monitoring devices are invasiveness, cost, and inaccuracy. Inaccuracy may result in incorrect decision-making owing to an incorrect interpretation of a situation (Fig. 1) Different monitoring devices have their own advantages and limitations related to their underlying technology. In this monograph, we classified and reviewed intraoperative non-pulse contour CO monitoring devices according to (1) their invasiveness and (2) their underlying technology.
The pulse contour method is defined as a technique for measuring and monitoring stroke volume (SV) on a beat-to-beat basis based on the morphological information obtained on an arterial pulse pressure waveform [3•]. The arterial pressure waveform may be obtained invasively (by using an arterial catheter) or non-invasively (without the use of an arterial line: volume clamp method, applanation tonometry, and photoelectric plethysmography). Pulse contour analysis-derived CO measurements may be used in an uncalibrated form (not calibrated to biometric and physiological data) or calibrated to an external CO measurement (transpulmonary thermodilution [TPTD] and lithium dilution.) The commercially available devices that are based on pulse contour methods include the FloTrac System, PiCCO-Technology, LiDCO System, VolumeView System, CNAP Monitor, PRAM System, and T-Line System. There are several comprehensive reviews available concerning pulse contour methods [4].
Classification of CO Monitoring Devices by Their Invasiveness
The devices can be stratified into several levels based on their invasiveness (Table 1). Except for the direct aortic flow probe insertion and other invasive perivascular flow probe placements that require surgical procedures, PAC (thermodilution) may be considered as the most invasive of all CO monitoring techniques. Numerous complications of PAC have been identified such as dysrhythmia, infection, pulmonary artery rupture, injury to adjacent arteries, embolization, pulmonary infarction, cardiac valvular damage, pericardial effusion, and intracardiac catheter knotting [5,6,7,8] (Table 1). The complication rate of PAC is about 5–10% [8] and is higher if access-related complications are included. The group with the second level of invasiveness includes TPTD, lithium dilution technique, and ultrasound dilution technique, which require the placements of both arterial pressure catheters and central venous catheters (CVC). The complications related to CVC insertion are pneumothorax, hemothorax, thrombus, bleeding, hematoma, arterial catheterization, vascular injury, extravenous wire malposition, stroke or cerebral ischemia, infection (local or systemic), arrhythmia, and right atrial perforation or other heart-related complications [9]. The complication rate with these techniques is in the range of 1–20% [9,10,11]. Although ultrasound-guided insertion may reduce the complication rate, the risk of complications is still substantial [9, 12, 13]. The transpulmonary lithium dilution technique involves an additional risk of lithium toxicity. The third level of invasiveness includes insertion of arterial pressure lines or transesophageal ultrasonic probes. This group of devices includes pulse contour analyses and transesophageal echocardiography (TEE)/Doppler (TED). Arterial cannulation has generally been found to be a safe procedure, and the incidence of serious complications is less than 1% [14, 15]. Similarly, the major complication rate associated with TEE is less than 1% [16], while no complications have been reported for TED [17]. Finally, the least invasive techniques, also known as non-invasive or minimally invasive methods [18,19,20], include the bioimpedance/bioreactance method, partial CO2 rebreathing method, inert gas rebreathing (IGR) method, transthoracic echocardiography (TTE)/Doppler (TTD) method, and non-invasive pulse contour method.
Classification of Non-pulse Contour CO Monitoring Devices by Their Underlying Technology
The determination of CO has been the subject of extensive developments over the last decade. The monitoring devices may be classified according to their underlying principles (Appendix).
CO Monitoring Devices: Principles and Practices
Fick’s Principle
This is an invasive and time-consuming method that is not suitable for continuous measurement as it requires drawing of arterial/mixed venous blood samples from the arterial line and the PAC, in addition to the measurement of exhaled oxygen volume. Although this method is considered the gold standard for CO measurement, it is rarely used for intraoperative monitoring.
Partial Gas Rebreathing Method
The NICO system (Novametrix Medical Systems, Wallingford, CT, USA) measures CO via a disposable rebreathing circuit that is added to the ventilator tubing and automatically provides a non-invasive CO measurement at 3-min intervals. Its easy application and the availability of automatic and continuous measurement make it suitable for perioperative use. This technique was found to have good agreement with PAC thermodilution in animal models [21], surgical/ICU (intensive care unit) patients [22, 23], pediatric patients [24], and cardiac surgery patients [25]; moderate agreement in thoracic surgery patients [26] and cardiac surgery patients [27]; and poor agreement in post-cardiac surgery patients [28, 29] and acute lung injury patients [30]. The device appears to have a high repeatability (4%) [28, 31]. There is no study adequately analyzing the trending ability of CO on this device.
The drawbacks of this technique are the need for stable CO2 elimination, which prevents its use in awake, spontaneously breathing subjects, and its unreliability in patients with chest trauma or pulmonary pathology, [28, 30, 32, 33]. Because CO2 detection is crucial in this technique, ventilator setting is an important factor in ensuring the accuracy of measurements. Tidal volumes (TV) of 10 mL/kg result in good agreement between this method and PAC thermodilution, whereas lower TV (6 mL/kg) may result in underestimation of CO [33]. Similarly, measurement in children with low TV may also be less accurate [24]. The accuracy of this technique also depends on shunt fraction [34]. As patients with severe lung injury have increased shunt fractions, it is difficult to estimate CO using this technique. Further, arterial blood gas analysis is required for shunt estimation [35]. The device seems to be more accurate in low-to-moderate CO than in high CO [22, 23, 36].
Although the device is considered harmless to patients, there is a potential risk of elevated arterial CO2 levels, which excludes its use in patients with critically increased intracranial pressure or pulmonary hypertension (PH). Arterial CO2 partial pressures can rise by about 10% of their initial values owing to the intermittent addition of extra dead space. It is also unclear whether this technique may be used during laparoscopy and CO2 insufflation. Despite these limitations, this device has the potential for intraoperative use in selected patients, provided its trending ability is validated.
Inert Gas Rebreathing Method
Innocor (Innovision, Glamsbjerg, Denmark) is the commercially available product that applies the IGR method for CO monitoring. This system utilizes two types of physiologically inert gases: a blood-soluble gas (0.5% nitrous oxide, N2O) and an inert insoluble gas (0.1% sulfur hexafluoride, SF6). The rebreathing bag is prefilled with an O2-enriched mixture of these two gases. A photoacoustic infrared gas analyzer is used for the continuous and simultaneous measurement of the levels of the inert gases and of CO2. Photoacoustic analyzers measure gas concentrations over a 5- to 6-breath or 10- to 20-s interval of rebreathing into the closed system. The concentration of the blood-insoluble SF6 following equilibration aids in the calculation of the total system volume (lungs, valve, and rebreathing bag). Subsequently, the rate of disappearance of N2O reflects its uptake by pulmonary blood flow (PBF). These data (total system volume and rate of N2O disappearance) are used for calculating the total PBF that participates in gas exchange. This system was developed for spontaneously breathing patients and has been extensively validated in various types of spontaneously breathing patient populations including patients with heart failure [37, 38], PH [39, 40], atrial fibrillation [41, 42], and pulmonary disease [43, 44], as well as in pediatric patients [45]. Overall, IGR demonstrated good agreement with gold standard methods including PAC thermodilution, the Fick method, and cardiac magnetic resonance-derived CO in these patient populations. However, only few studies [46, 47••] validated its accuracy in ventilated patients, who are the main subjects of this review. Reutershan indicated good interchangeability with PAC thermodilution in mechanically ventilated patients with acute respiratory distress syndrome by using an older generation IGR device [46]. Similarly, Perak showed good agreement between CO determined by IGR and the Fick equation in pediatric patients [47••]. The authors indicated the need for a valve adapter that allowed for rebreathing. Data regarding the repeatability and trending ability of this device in mechanically ventilated patients are not available as of now. Peyton et al. reported the repeatability of this device as being less than 20% in spontaneously breathing cardiac patients and healthy volunteers [48].
The drawbacks of the IGR device are its lower accuracy in high CO conditions [41, 49] and its inability to provide continuous measurements. However, one study suggested that an IGR device could provide measurements once in every 5 min [48]. This is a potential intraoperative CO monitoring device as it is completely non-invasive, safe, easy-to-use, and accurate in various patient groups, especially patients with cardiac rhythm failure and pulmonary disease, who are unsuitable for monitoring with other techniques. Further validation of this technique is required in ventilated patients and in the perioperative setting.
Indicator Dilution Technique
The DDG pulsed dye densitometry series (Nihon Kohden, Tokyo, Japan) is one of the commercially available product using the indicator dilution technique. In this technique, signal detection in the arterial blood is performed in a manner similar to that of pulse oximetry. Transcutaneous measurements of two wavelengths (805 and 940 nm) are used to calculate the ratio between hemoglobin and the injected indicator, indocyanine green (pulse dye densitometry; PDD). Indocyanine green distributes exclusively in the intravascular space following a bolus injection and is cleared via hepatic elimination at a half-life of 4.1 min. Hemoglobin concentration is required for the calculation of the absolute indocyanine green concentration. CO is calculated from the indocyanine green dye-dilution curve (dye densitogram) according to the Stewart-Hamilton equation. Similar to pulse oximetry, factors that compromise signal detection, such as vasoconstriction, interstitial edema, movement, or ambient light artifacts, may limit the reliability of CO assessment by using this method [50, 51]. There are conflicting reports regarding the accuracy of PDD. The indocyanine green dye dilution CO measurement was found to have acceptable agreement with PAC thermodilution in various surgery patients [52], cardiac surgery patients with limited performance [51, 53], post-cardiac surgery patients [54], and critically ill pediatric animal models (acceptable bias and precision) [55]. Bremer et al. found the technique to be reliable only at CO values > 5.0 L/min [56]. A similar trend of increased overestimation in the low CO range was also reported by other studies [52]. Kroon et al. reported concordance rate of 81% for the trending ability of PDD, when compared with PAC thermodilution CO [54]. They also demonstrated that the device had good reproducibility. However, the intraoperative use of this technique may be limited owing to the following reasons: limited performance on trending ability and lack of continuous measurement of CO as subsequent measurements cannot be performed until indocyanine green is excreted. Theoretically, the total elimination of indocyanine green takes about three half-lives, and the residual plasma indocyanine green may contribute to errors.
Transpulmonary ultrasound dilution (TPUD) is another method that utilizes the indicator dilution technique. This is used in the commercially available product called COstatus (Transonic Systems Inc., Ithaca, NY, USA). This device consists of an extracorporeal arteriovenous loop set that is connected to an arterial catheter on one side and a CVC on the other side. Two reusable sensors for blood ultrasound velocity and flow are clamped on to the loop, and a roller pump circulates blood through the arteriovenous loop for each measurement session. Isotonic saline, which is used as the indicator, is injected into the venous side of the arteriovenous loop, randomly during a respiratory cycle, at the rate of 0.5–1.0 mL/kg, to a maximum volume of 30 mL for each measurement. The venous sensor detects the indicator and determines the time and volume of injection. Following its passage through the cardiopulmonary system, the arterial sensor records the travel time of the indicator and the changes in blood ultrasound velocity. At the end of three consecutive measurements, blood is returned to the patient by flushing the arteriovenous loop with heparinized saline, thus avoiding any blood loss. The device was found to have good agreement with PAC thermodilution in critically ill post-cardiac surgery patients [57], although this study did not provide any evidence of its use in the intraoperative setting. The reproducibility of TPUD is indicated by its coefficient of variance of 6% [57]. The device was found to have good agreement and trending ability in children undergoing heart surgery [58] and in neonatal animal models [59,60,61, 62•].
The limitations of this device are the following: arterial pressures are not available during the time that the CO is measured (5–6 min when the pump is operated); the volume of isotonic saline injections may be an issue in patients who are extremely hypervolemic; and an arterial catheter and CVC are required for monitoring. The device may have the potential for intraoperative use, especially in small children with arterial catheters and CVC.
Thermodilution Technique
A detailed description of PAC thermodilution and TPTD is beyond the scope of this review and is attempted elsewhere [1, 6, 63].
Impedance Cardiography
The conventional impedance cardiography (ICG) devices involve placing electrodes on the patient’s body surface, which may interfere with the performance of surgical procedures. Several products incorporate the classic thoracic ICG, such as the Niccomo monitor (Medis GmbH, Ilmenau, Germany) and the BioZ monitor (Cardio Dynamics, San Diego, CA, USA). Niccomo is reported as lacking adequate accuracy [64, 65], although there is no evidence comparing it with PAC thermodilution. There was inadequate agreement between BioZ and PAC continuous thermodilution in patients undergoing off-pump coronary artery bypass graft, with a tendency to overestimate and underestimate CO in low CO and high CO conditions, respectively [66].
Another device, ECOM (ConMed, Irvine, USA), is based on a modification of the thoracic electrical bioimpedance method and involves electrodes attached to a specially designed endotracheal tube. There was inadequate agreement between ECOM and PAC thermodilution in patients undergoing cardiac surgery [67,68,69,70], along with poor trending ability [68, 70]. The repeatability of this device appeared to be good (10% of precision) [70]. ECOM is a simple system to employ in the perioperative setting, although it lacks the required accuracy. However, the direct evidence of improved postoperative outcomes following the use of ECOM encourages its perioperative use [71••].
NICaS (NIMedical, Petah Tikva, Israel) utilizes whole-body impedance, which is detected peripherally as impedance signals, for determining CO. Although it is not validated in the intraoperative setting, studies have reported poor agreement in PH patients [72] and good agreement in cardiac disease patients [73]. Although there are recent case series on its perioperative use in patients with pheochromocytoma [74] and those undergoing Cesarean sections [75], there is inadequate evidence to support its intraoperative use.
NICOM (Cheetah medical, Portland, USA) is a device that uses the bioreactance method and has been validated in various intraoperative settings. Studies have reported poor agreement with Doppler echo-derived CO in patients undergoing abdominal surgery [76,77,78] and with PAC thermodilution for living donor liver transplantation [79, 80]. This device seems to be unreliable in abdominal surgery, possibly owing to the massive fluid shifts and tissue retraction in these procedures. It has been demonstrated as having poor accuracy in pediatric population, although the studies involved inconsistent physical conditions and settings [78, 81,82,83,84]. The repeatability of the device is reported to be good [31], while its trending ability is relatively reliable [83, 85,86,87, 88•]. Although the device has inadequate accuracy, it may be useful in some perioperative settings, as it provides continuous CO measurement and SV variation, which are proven parameters to guide fluid therapy [84, 89,90,91,92].
There is no commercially available transbrachial electrical velocimetry device. A prototype of the device has been validated in volunteers with favorable results [93, 94••]. Although no study has been reported under perioperative setting, the precision of the device as well as its accuracy against magnetic resonance image-derived CO have been confirmed [94••]. Considering its potential for the perioperative use, its empirical perioperative use may be allowed and further analysis is expected in this field.
Aesculon and its portable version, Icon (Osypka Medical, Berlin, Germany), employ electrical velocimetry (EV) methods to determine CO. The device has been validated in intraoperative settings and found to have good agreement with TEE for coronary artery surgery [95]. However, other studies have identified it as having poor agreement with various reference methods in cardiac disease patients [96] and critically ill patients [97]. In contrast, the device may be useful as it has acceptable accuracy in pediatric patients with various conditions [98••, 99,100,101,102,103,104,105,106,107]. The device was found to have high reproducibility [106, 108], although data on its trending ability in humans are lacking. The device has the potential for intraoperative use in pediatric patients.
Continuous Doppler and Morphological Echocardiography
TTE has limited applicability in the perioperative setting owing to the difficulty in obtaining continuous and consistent measurement of CO with it. TEE is a currently used monitoring technique in the perioperative setting as it provides information concerning cardiac and aortic structures, cardiac contractility, and volume/fluid assessment. Similar to TTE, TEE has inaccuracy or inconsistency issues that are related to operator skill. To overcome the drawback of it being an operator-dependent measure, a TTD-based monitoring method called USCOM (Uscom, Sydney, Australia) and a TED-based monitoring method called CardioQ (Deltex Medical, Chichester, UK) have been developed. USCOM utilizes continuous wave Doppler to obtain velocity-time integrals of either transaortic blood flow at the left ventricular outflow tract or pulmonary valve blood flow, by applying a non-imaging probe in the suprasternal notch (aortic valve) or left parasternal site (pulmonary valve). The cross-sectional area of the aortic root may be a source of error as it is derived from nomograms based on patient height [109], without the use of 2-dimensional (2D) echo. Chong and Peyton conducted a meta-analysis of six studies that analyzed USCOM and identified a mean weighted bias of − 0.39 L/min, precision of 1.27 L/min, and percentage error of 42.7% [110]. Recently available data have confirmed it as having acceptable agreement with 3D-TTE in pregnant women [111•]. Further, USCOM was successful in detecting blood loss in healthy volunteers [112]. Another study demonstrated that the tandem use of USCOM and CardioQ provided reliable accuracy as well as trending ability of CO [113•]. The limitation of this device is its reliance on operator skill. Adequate training to achieve the appropriate angle of insonation for optimizing flow signal may improve the measurement accuracy [114, 115].
CardioQ utilizes a disposable probe that is inserted in the esophagus to measure blood flow in the descending aorta for the continuous measurement of CO [116]. It only measures flow in the descending thoracic aorta, which is 70% of the total flow. Therefore, an additional conversion factor is needed to compensate aortic arch flow. Similar to USCOM, the diameter of the descending aorta is not directly measured but estimated from nomograms based on patient characteristics. As the thoracic aorta elongates and unfolds with advancing age, the reliability of the technique reduces owing to probe displacement [117]. CardioQ is endorsed by the British guidelines for use in patients undergoing major or high-risk surgery [118]. Moller-Sorensen et al. found that this technique had poor agreement with PAC thermodilution in patients undergoing coronary artery bypass graft. However, they reported the device as having good precision (12.8%) and an acceptable trending ability [119•]. The device-derived SV respiratory variation accurately predicted fluid responsiveness under general anesthesia [120, 121].
Modified Pulse Wave Transient Time
The esCCO (Nihon Kohden, Tokyo, Japan) technique estimates CO by utilizing modified pulse wave transient time (mPWTT). This system uses an existing electrocardiogram and a specific type of pulse oximeter. Recently available data demonstrate its poor agreement and insufficient trending ability with TPTD in off-pump coronary artery surgery patients [122]. Similar results have been reported in critically ill patients [123,124,125,126, 127•, 128, 129•], cardiac surgery patients [130], and liver transplantation patients [131]. In contrast, it was found to have good agreement with acceptable trending ability in renal transplant patients [132] and poor agreement with acceptable trending ability in liver surgery patients [133]. The inaccuracy with this technique may be related to low systemic vascular resistance [126, 129, 131]. This device appears to have lower accuracy in low CO conditions [122]. It was found to have acceptable reproducibility [127, 128, 134]. Intraoperative interventions such as administration of vasoactive drugs and anesthetics, intrathoracic pressure changes, systemic vascular resistance changes, cardiac displacement, and arrhythmias are likely to affect the quality of the ECG and pulse oximetry signals. Further, its relatively low trending ability may also limit its intraoperative use.
Conclusions
Although various types of less invasive methods for CO measurement have been developed, none of them are entirely ideal for perioperative use. Each device has its own advantages and drawbacks related to its underlying technology. The current evidence for their perioperative use is limited. The empirical application of these devices for various surgical patients may provide us with more data over time.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993-2004. JAMA. 2007;298(4):423–9. https://doi.org/10.1001/jama.298.4.423.
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14. https://doi.org/10.1056/NEJMoa021108.
• Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42(9):1350–9. https://doi.org/10.1007/s00134-016-4375-7. A comprehensive review of monitoring devices.
Schloglhofer T, Gilly H, Schima H. Semi-invasive measurement of cardiac output based on pulse contour: a review and analysis. Can J Anaesth = J Can Anesth. 2014;61(5):452–79. https://doi.org/10.1007/s12630-014-0135-8.
Smart FW, Husserl FE. Complications of flow-directed balloon-tipped catheters. Chest. 1990;97(1):227–8.
Evans DC, Doraiswamy VA, Prosciak MP, Silviera M, Seamon MJ, Rodriguez Funes V, et al. Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand J Surg. 2009;98(4):199–208. https://doi.org/10.1177/145749690909800402.
Practice guidelines for pulmonary artery catheterization: an updated report by the American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Anesthesiology. 2003;99(4):988–1014.
Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: impact data and complications. Crit Care. 2006;10(Suppl 3):S8. https://doi.org/10.1186/cc4834.
Lennon M, Zaw NN, Popping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234–40.
Malbezin S, Gauss T, Smith I, Bruneau B, Mangalsuren N, Diallo T, et al. A review of 5434 percutaneous pediatric central venous catheters inserted by anesthesiologists. Paediatr Anaesth. 2013;23(11):974–9. https://doi.org/10.1111/pan.12184.
Janik JE, Conlon SJ, Janik JS. Percutaneous central access in patients younger than 5 years: size does matter. J Pediatr Surg. 2004;39(8):1252–6.
Heidemann L, Nathani N, Sagana R, Chopra V, Hueng M. A contemporary assessment of mechanical complication rates and trainee perceptions of central venous catheter insertion. J Hosp Med. 2017;12(8):646–51. 10.12788/jhm.2784.
Perbet S, Pereira B, Grimaldi F, Duale C, Bazin JE, Constantin JM. Guidance and examination by ultrasound versus landmark and radiographic method for placement of subclavian central venous catheters: study protocol for a randomized controlled trial. Trials. 2014;15:175. https://doi.org/10.1186/1745-6215-15-175.
Scheer BV, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6(3):199. https://doi.org/10.1186/cc1489.
Belda FJ, Aguilar G, Teboul JL, Pestana D, Redondo FJ, Malbrain M, et al. Complications related to less-invasive haemodynamic monitoring. Br J Anaesth. 2011;106(4):482–6. https://doi.org/10.1093/bja/aeq377.
Daniel WG, Erbel R, Kasper W, Visser CA, Engberding R, Sutherland GR, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation. 1991;83(3):817–21. https://doi.org/10.1161/01.cir.83.3.817.
Schober P, Loer SA, Schwarte LA. Perioperative hemodynamic monitoring with transesophageal Doppler technology. Anesth Analg. 2009;109(2):340–53. https://doi.org/10.1213/ane.0b013e3181aa0af3.
Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010;113(5):1220–35. https://doi.org/10.1097/ALN.0b013e3181ee3130.
Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth. 2016;30(3):461–80. https://doi.org/10.1007/s00540-016-2154-9.
Hofer CK, Rex S, Ganter MT. Update on minimally invasive hemodynamic monitoring in thoracic anesthesia. Curr Opin Anaesthesiol. 2014;27(1):28–35. https://doi.org/10.1097/aco.0000000000000034.
Haryadi DG, Orr JA, Kuck K, McJames S, Westenskow DR. Partial CO2 rebreathing indirect Fick technique for non-invasive measurement of cardiac output. J Clin Monit Comput. 2000;16(5–6):361–74.
Murias GE, Villagra A, Vatua S, del Mar Fernandez M, Solar H, Ochagavia A, et al. Evaluation of a noninvasive method for cardiac output measurement in critical care patients. Intensive Care Med. 2002;28(10):1470–4. https://doi.org/10.1007/s00134-002-1477-1.
Odenstedt H, Stenqvist O, Lundin S. Clinical evaluation of a partial CO2 rebreathing technique for cardiac output monitoring in critically ill patients. Acta Anaesthesiol Scand. 2002;46(2):152–9.
Levy RJ, Chiavacci RM, Nicolson SC, Rome JJ, Lin RJ, Helfaer MA, et al. An evaluation of a noninvasive cardiac output measurement using partial carbon dioxide rebreathing in children. Anesth Analg. 2004;99(6):1642–1647, table of contents. https://doi.org/10.1213/01.ane.0000136952.85278.99.
Binder JC, Parkin WG. Non-invasive cardiac output determination: comparison of a new partial-rebreathing technique with thermodilution. Anaesth Intensive Care. 2001;29(1):19–23.
Ng JM, Chow MY, Ip-Yam PC, Goh MH, Agasthian T. Evaluation of partial carbon dioxide rebreathing cardiac output measurement during thoracic surgery. J Cardiothorac Vasc Anesth. 2007;21(5):655–8. https://doi.org/10.1053/j.jvca.2007.01.012.
Botero M, Kirby D, Lobato EB, Staples ED, Gravenstein N. Measurement of cardiac output before and after cardiopulmonary bypass: comparison among aortic transit-time ultrasound, thermodilution, and noninvasive partial CO2 rebreathing. J Cardiothorac Vasc Anesth. 2004;18(5):563–72.
Nilsson LB, Eldrup N, Berthelsen PG. Lack of agreement between thermodilution and carbon dioxide-rebreathing cardiac output. Acta Anaesthesiol Scand. 2001;45(6):680–5.
Mielck F, Buhre W, Hanekop G, Tirilomis T, Hilgers R, Sonntag H. Comparison of continuous cardiac output measurements in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17(2):211–6. https://doi.org/10.1053/jcan.2003.49.
Valiatti JL, Amaral JL. Comparison between cardiac output values measured by thermodilution and partial carbon dioxide rebreathing in patients with acute lung injury. Sao Paulo Medical Journal = Revista paulista de Medicina. 2004;122(6):233–8.
Rich JD, Archer SL, Rich S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J. 2013;42(1):125–33. https://doi.org/10.1183/09031936.00102212.
Maxwell RA, Gibson JB, Slade JB, Fabian TC, Proctor KG. Noninvasive cardiac output by partial CO2 rebreathing after severe chest trauma. J Trauma. 2001;51(5):849–53.
Tachibana K, Imanaka H, Takeuchi M, Takauchi Y, Miyano H, Nishimura M. Noninvasive cardiac output measurement using partial carbon dioxide rebreathing is less accurate at settings of reduced minute ventilation and when spontaneous breathing is present. Anesthesiology. 2003;98(4):830–7.
Rocco M, Spadetta G, Morelli A, Dell'Utri D, Porzi P, Conti G, et al. A comparative evaluation of thermodilution and partial CO2 rebreathing techniques for cardiac output assessment in critically ill patients during assisted ventilation. Intensive Care Med. 2004;30(1):82–7. https://doi.org/10.1007/s00134-003-2069-4.
Young BP, Low LL. Noninvasive monitoring cardiac output using partial CO(2) rebreathing. Crit Care Clin. 2010;26(2):383–392, table of contents. https://doi.org/10.1016/j.ccc.2009.12.002.
van Heerden PV, Baker S, Lim SI, Weidman C, Bulsara M. Clinical evaluation of the non-invasive cardiac output (NICO) monitor in the intensive care unit. Anaesth Intensive Care. 2000;28(4):427–30.
Sobanski P, Sinkiewicz W, Kubica J, Blazejewski J, Bujak R. The reliability of noninvasive cardiac output measurement using the inert gas rebreathing method in patients with advanced heart failure. Cardiol J. 2008;15(1):63–70.
Hassan M, Wagdy K, Kharabish A, Selwanos PP, Nabil A, Elguindy A, et al. Validation of noninvasive measurement of cardiac output using inert gas rebreathing in a cohort of patients with heart failure and reduced ejection fraction. Circ Heart fail. 2017;10(3):e003592. https://doi.org/10.1161/circheartfailure.116.003592.
McLure LE, Brown A, Lee WN, Church AC, Peacock AJ, Johnson MK. Non-invasive stroke volume measurement by cardiac magnetic resonance imaging and inert gas rebreathing in pulmonary hypertension. Clin Physiol Funct Imaging. 2011;31(3):221–6. https://doi.org/10.1111/j.1475-097X.2010.01004.x.
Farina S, Teruzzi G, Cattadori G, Ferrari C, De Martini S, Bussotti M, et al. Noninvasive cardiac output measurement by inert gas rebreathing in suspected pulmonary hypertension. Am J Cardiol. 2014;113(3):546–51. https://doi.org/10.1016/j.amjcard.2013.10.017.
Saur J, Trinkmann F, Doesch C, Weissmann J, Hamm K, Schoenberg SO, et al. Non-invasive measurement of cardiac output during atrial fibrillation: comparison between cardiac magnetic resonance imaging and inert gas rebreathing. Cardiology. 2010;115(3):212–6. https://doi.org/10.1159/000288712.
Osbak PS, Henriksen JH, Kofoed KF, Jensen GB. Non-invasive measurements of cardiac output in atrial fibrillation: inert gas rebreathing and impedance cardiography. Scand J Clin Lab Invest. 2011;71(4):304–13. https://doi.org/10.3109/00365513.2011.563790.
Saur J, Trinkmann F, Doesch C, Scherhag A, Brade J, Schoenberg SO, et al. The impact of pulmonary disease on noninvasive measurement of cardiac output by the inert gas rebreathing method. Lung. 2010;188(5):433–40. https://doi.org/10.1007/s00408-010-9257-0.
Perrault H, Richard R, Kapchinsky S, Baril J, Bourbeau J, Taivassalo T. Addressing assumptions for the use of non-invasive cardiac output measurement techniques during exercise in COPD. Copd. 2016;13(1):75–81. https://doi.org/10.3109/15412555.2015.1043985.
Marma AK, Opotowsky AR, Fromm BS, Ubeda-Tikkanen A, Porras D, Rhodes J. Noninvasive cardiac output estimation by inert gas rebreathing in pediatric and congenital heart disease. Am Heart J. 2016;174:80–8. https://doi.org/10.1016/j.ahj.2016.01.006.
Reutershan J, Schmitt A, Dietz K, Fretschner R. Non-invasive measurement of pulmonary blood flow during prone positioning in patients with early acute respiratory distress syndrome. Clin Sci (Lond). 2004;106(1):3–10. https://doi.org/10.1042/cs20030157.
•• Perak AM, Opotowsky AR, Walsh BK, Esch JJ, DiNardo JA, Kussman BD, et al. Noninvasive cardiac output estimation by inert gas rebreathing in mechanically ventilated pediatric patients. J Pediatr. 2016;177:184–90.e3. https://doi.org/10.1016/j.jpeds.2016.07.007. Accuracy and precision data of inert gas rebreathing methods in pediatric mechanically ventilated patients.
Peyton PJ, Bailey M, Thompson BR. Reproducibility of cardiac output measurement by the nitrous oxide rebreathing technique. J Clin Monit Comput. 2009;23(4):233–6. https://doi.org/10.1007/s10877-009-9187-7.
Saur J, Fluechter S, Trinkmann F, Papavassiliu T, Schoenberg S, Weissmann J, et al. Noninvasive determination of cardiac output by the inert-gas-rebreathing method—comparison with cardiovascular magnetic resonance imaging. Cardiology. 2009;114(4):247–54. https://doi.org/10.1159/000232407.
Haruna M, Kumon K, Yahagi N, Watanabe Y, Ishida Y, Kobayashi N, et al. Blood volume measurement at the bedside using ICG pulse spectrophotometry. Anesthesiology. 1998;89(6):1322–8.
Hofer CK, Buhlmann S, Klaghofer R, Genoni M, Zollinger A. Pulsed dye densitometry with two different sensor types for cardiac output measurement after cardiac surgery: a comparison with the thermodilution technique. Acta Anaesthesiol Scand. 2004;48(5):653–7. https://doi.org/10.1111/j.1399-6576.2004.00371.x.
Imai T, Takahashi K, Fukura H, Morishita Y. Measurement of cardiac output by pulse dye densitometry using indocyanine green: a comparison with the thermodilution method. Anesthesiology. 1997;87(4):816–22.
Baulig W, Bernhard EO, Bettex D, Schmidlin D, Schmid ER. Cardiac output measurement by pulse dye densitometry in cardiac surgery. Anaesthesia. 2005;60(10):968–73. https://doi.org/10.1111/j.1365-2044.2005.04296.x.
Kroon M, Groeneveld AB, Smulders YM. Cardiac output measurement by pulse dye densitometry: comparison with pulmonary artery thermodilution in post-cardiac surgery patients. J Clin Monit Comput. 2005;19(6):395–9. https://doi.org/10.1007/s10877-005-6865-y.
Taguchi N, Nakagawa S, Miyasaka K, Fuse M, Aoyagi T. Cardiac output measurement by pulse dye densitometry using three wavelengths. Pediatr Crit Care Med. 2004;5(4):343–50.
Bremer F, Schiele A, Tschaikowsky K. Cardiac output measurement by pulse dye densitometry: a comparison with the Fick’s principle and thermodilution method. Intensive Care Med. 2002;28(4):399–405. https://doi.org/10.1007/s00134-002-1252-3.
Eremenko AA, Safarov PN. Flow-regulated extracorporeal arteriovenous tubing loop for cardiac output measurements by ultrasound velocity dilution: validation in post-cardiac surgery intensive care unit patients. ASAIO J. 2010;56(6):522–6. https://doi.org/10.1097/MAT.0b013e3181effdf8.
Lindberg L, Johansson S, Perez-de-Sa V. Validation of an ultrasound dilution technology for cardiac output measurement and shunt detection in infants and children. Pediatr Crit Care Med. 2014;15(2):139–47. https://doi.org/10.1097/pcc.0000000000000053.
Krivitski NM, Kislukhin VV, Thuramalla NV. Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive care unit patients. Pediatr Crit Care Med. 2008;9(4):423–8. https://doi.org/10.1097/01.PCC.0b013e31816c71bc.
Vrancken SL, de Boode WP, Hopman JC, Singh SK, Liem KD, van Heijst AF. Cardiac output measurement with transpulmonary ultrasound dilution is feasible in the presence of a left-to-right shunt: a validation study in lambs. Br J Anaesth. 2012;108(3):409–16. https://doi.org/10.1093/bja/aer401.
Vrancken SL, de Boode WP, Hopman JC, Looijen-Salamon MG, Liem KD, van Heijst AF. Influence of lung injury on cardiac output measurement using transpulmonary ultrasound dilution: a validation study in neonatal lambs. Br J Anaesth. 2012;109(6):870–8. https://doi.org/10.1093/bja/aes297.
• Vrancken SL, van Heijst AF, Hopman JC, Liem KD, van der Hoeven JG, de Boode WP. Hemodynamic volumetry using transpulmonary ultrasound dilution (TPUD) technology in a neonatal animal model. J Clin Monit Comput. 2015;29(5):643–52. https://doi.org/10.1007/s10877-014-9647-6. Providing explanation of transpulmonary ultrasound dilution technique.
Gidwani UK, Mohanty B, Chatterjee K. The pulmonary artery catheter: a critical reappraisal. Cardiol Clin. 2013;31(4):545–565, viii. https://doi.org/10.1016/j.ccl.2013.07.008.
Lorne E, Mahjoub Y, Diouf M, Sleghem J, Buchalet C, Guinot PG, et al. Accuracy of impedance cardiography for evaluating trends in cardiac output: a comparison with oesophageal Doppler. Br J Anaesth. 2014;113(4):596–602. https://doi.org/10.1093/bja/aeu136.
Fellahi JL, Caille V, Charron C, Deschamps-Berger PH, Vieillard-Baron A. Noninvasive assessment of cardiac index in healthy volunteers: a comparison between thoracic impedance cardiography and Doppler echocardiography. Anesth Analg. 2009;108(5):1553–9. https://doi.org/10.1213/ane.0b013e31819cd97e.
Cheung H, Dong Q, Dong R, Yu B. Correlation of cardiac output measured by non-invasive continuous cardiac output monitoring (NICOM) and thermodilution in patients undergoing off-pump coronary artery bypass surgery. J Anesth. 2015;29(3):416–20. https://doi.org/10.1007/s00540-014-1938-z.
Ball TR, Culp BC, Patel V, Gloyna DF, Ciceri DP, Culp WC Jr. Comparison of the endotracheal cardiac output monitor to thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2010;24(5):762–6. https://doi.org/10.1053/j.jvca.2010.04.008.
Maus TM, Reber B, Banks DA, Berry A, Guerrero E, Manecke GR. Cardiac output determination from endotracheally measured impedance cardiography: clinical evaluation of endotracheal cardiac output monitor. J Cardiothorac Vasc Anesth. 2011;25(5):770–5. https://doi.org/10.1053/j.jvca.2010.12.017.
van der Kleij SC, Koolen BB, Newhall DA, Gerritse BM, Rosseel PM, Rijpstra TA, et al. Clinical evaluation of a new tracheal impedance cardiography method. Anaesthesia. 2012;67(7):729–33. https://doi.org/10.1111/j.1365-2044.2012.07089.x.
Moller-Sorensen H, Hansen KL, Ostergaard M, Andersen LW, Moller K. Lack of agreement and trending ability of the endotracheal cardiac output monitor compared with thermodilution. Acta Anaesthesiol Scand. 2012;56(4):433–40. https://doi.org/10.1111/j.1399-6576.2011.02615.x.
•• Leclercq T, Lilot M, Schulz T, Meyer A, Farhat F, Fellahi JL. Endotracheal bioimpedance cardiography improves immediate postoperative outcome: a case-control study in off-pump coronary surgery. J Clin Monit Comput. 2017; https://doi.org/10.1007/s10877-017-9996-z. The authors have revealed that utilization of endotracheal bioimpedance cardiography improved postoperative outcome.
Taniguchi Y, Emoto N, Miyagawa K, Nakayama K, Kinutani H, Tanaka H, et al. Noninvasive and simple assessment of cardiac output and pulmonary vascular resistance with whole-body impedance cardiography is useful for monitoring patients with pulmonary hypertension. Circ J. 2013;77(9):2383–9.
Paredes OL, Shite J, Shinke T, Watanabe S, Otake H, Matsumoto D, et al. Impedance cardiography for cardiac output estimation: reliability of wrist-to-ankle electrode configuration. Circ J. 2006;70(9):1164–8.
Matsuda Y, Kawate H, Shimada S, Matsuzaki C, Nagata H, Adachi M, et al. Perioperative sequential monitoring of hemodynamic parameters in patients with pheochromocytoma using the Non-Invasive Cardiac System (NICaS). Endocr J. 2014;61(6):571–5.
Ram M, Lavie A, Lev S, Blecher Y, Amikam U, Shulman Y, et al. Cardiac hemodynamics before, during and after elective cesarean section under spinal anesthesia in low-risk women. J Perinatol: official journal of the California Perinatal Association. 2017; https://doi.org/10.1038/jp.2017.53.
Huang L, Critchley LA, Zhang J. Major upper abdominal surgery alters the calibration of bioreactance cardiac output readings, the NICOM, when comparisons are made against suprasternal and esophageal Doppler intraoperatively. Anesth Analg. 2015;121(4):936–45. https://doi.org/10.1213/ane.0000000000000889.
Conway DH, Hussain OA, Gall I. A comparison of noninvasive bioreactance with oesophageal Doppler estimation of stroke volume during open abdominal surgery: an observational study. Eur J Anaesthesiol. 2013;30(8):501–8. https://doi.org/10.1097/EJA.0b013e3283603250.
Dubost C, Bougle A, Hallynck C, Le Dorze M, Roulleau P, Baujard C, et al. Comparison of monitoring performance of bioreactance versus esophageal Doppler in pediatric patients. Ind J Crit Care Med. 2015;19(1):3–8. https://doi.org/10.4103/0972-5229.148630.
Han S, Lee JH, Kim G, Ko JS, Choi SJ, Kwon JH, et al. Bioreactance is not interchangeable with thermodilution for measuring cardiac output during adult liver transplantation. PLoS One. 2015;10(5):e0127981. https://doi.org/10.1371/journal.pone.0127981.
Shih PY, Lin WY, Hung MH, Cheng YJ, Chan KC. Evaluation of cardiac output by bioreactance technique in patients undergoing liver transplantation. Acta Anaesthesiol Taiwanica : Off J Taiwan Soc Anesthesiol. 2016;54(2):57–61. https://doi.org/10.1016/j.aat.2016.06.001.
Weisz DE, Jain A, McNamara PJ, EL-Khuffash A. Non-invasive cardiac output monitoring in neonates using bioreactance: a comparison with echocardiography. Neonatology. 2012;102(1):61–7. https://doi.org/10.1159/000337295.
Ballestero Y, Urbano J, Lopez-Herce J, Solana MJ, Botran M, Vinciguerra D, et al. Pulmonary arterial thermodilution, femoral arterial thermodilution and bioreactance cardiac output monitoring in a pediatric hemorrhagic hypovolemic shock model. Resuscitation. 2012;83(1):125–9. https://doi.org/10.1016/j.resuscitation.2011.06.039.
Weisz DE, Jain A, Ting J, McNamara PJ, El-Khuffash A. Non-invasive cardiac output monitoring in preterm infants undergoing patent ductus arteriosus ligation: a comparison with echocardiography. Neonatology. 2014;106(4):330–6. https://doi.org/10.1159/000365278.
Vergnaud E, Vidal C, Verchere JM, Taright H, Meyer PG, Carli PA, et al. Noninvasive cardiac output measurement using bioreactance in postoperative pediatric patients. Paediatr Anaesth. 2015;25(2):160–6. https://doi.org/10.1111/pan.12412.
Raval NY, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;22(2):113–9. https://doi.org/10.1007/s10877-008-9112-5.
Marque S, Cariou A, Chiche JD, Squara P. Comparison between Flotrac-Vigileo and Bioreactance, a totally noninvasive method for cardiac output monitoring. Crit Care (London). 2009;13(3):R73. https://doi.org/10.1186/cc7884.
Squara P, Rotcajg D, Denjean D, Estagnasie P, Brusset A. Comparison of monitoring performance of bioreactance vs. pulse contour during lung recruitment maneuvers. Crit Care (London). 2009;13(4):R125. https://doi.org/10.1186/cc7981.
• Berlin DA, Peprah-Mensah H, Manoach S, Heerdt PM. Agreement of bioreactance cardiac output monitoring with thermodilution during hemorrhagic shock and resuscitation in adult swine. Crit Care Med. 2017;45(2):e195–201. https://doi.org/10.1097/ccm.0000000000002071. The report provides accuracy data under the various hemodynamic conditions.
Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875–81. https://doi.org/10.1007/s00134-010-1990-6.
Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364–70. https://doi.org/10.1378/chest.12-1274.
Lee JY, Kim JY, Choi CH, Kim HS, Lee KC, Kwak HJ. The ability of stroke volume variation measured by a noninvasive cardiac output monitor to predict fluid responsiveness in mechanically ventilated children. Pediatr Cardiol. 2014;35(2):289–94. https://doi.org/10.1007/s00246-013-0772-7.
Min JJ, Lee JH, Hong KY, Choi SJ. Utility of stroke volume variation measured using non-invasive bioreactance as a predictor of fluid responsiveness in the prone position. J Clin Monit Comput. 2017;31(2):397–405. https://doi.org/10.1007/s10877-016-9859-z.
Bernstein DP, Henry IC, Banet MJ, Dittrich T. Stroke volume obtained by electrical interrogation of the brachial artery: transbrachial electrical bioimpedance velocimetry. Physiol Meas. 2012;33(4):629–49. https://doi.org/10.1088/0967-3334/33/4/629.
•• Bernstein DP, Henry IC, Lemmens HJ, Chaltas JL, DeMaria AN, Moon JB, et al. Validation of stroke volume and cardiac output by electrical interrogation of the brachial artery in normals: assessment of strengths, limitations, and sources of error. J Clin Monit Comput. 2015;29(6):789–800. https://doi.org/10.1007/s10877-015-9668-9. The authors have investigated a brand new technology, the transbrachial electrical bioimpedance velocimetry method.
Schmidt C, Theilmeier G, Van Aken H, Korsmeier P, Wirtz SP, Berendes E, et al. Comparison of electrical velocimetry and transoesophageal Doppler echocardiography for measuring stroke volume and cardiac output. Br J Anaesth. 2005;95(5):603–10. https://doi.org/10.1093/bja/aei224.
Trinkmann F, Berger M, Doesch C, Papavassiliu T, Schoenberg SO, Borggrefe M, et al. Comparison of electrical velocimetry and cardiac magnetic resonance imaging for the non-invasive determination of cardiac output. J Clin Monit Comput. 2016;30(4):399–408. https://doi.org/10.1007/s10877-015-9731-6.
Raue W, Swierzy M, Koplin G, Schwenk W. Comparison of electrical velocimetry and transthoracic thermodilution technique for cardiac output assessment in critically ill patients. Eur J Anaesthesiol. 2009;26(12):1067–71. https://doi.org/10.1097/EJA.0b013e32832bfd94.
•• Suehiro K, Joosten A, Murphy LS, Desebbe O, Alexander B, Kim SH, et al. Accuracy and precision of minimally-invasive cardiac output monitoring in children: a systematic review and meta-analysis. J Clin Monit Comput. 2016;30(5):603–20. https://doi.org/10.1007/s10877-015-9757-9. The literature provides thorough information on agreement of cardiac output monitoring devices in pediatric patients.
Norozi K, Beck C, Osthaus WA, Wille I, Wessel A, Bertram H. Electrical velocimetry for measuring cardiac output in children with congenital heart disease. Br J Anaesth. 2008;100(1):88–94. https://doi.org/10.1093/bja/aem320.
Noori S, Drabu B, Soleymani S, Seri I. Continuous non-invasive cardiac output measurements in the neonate by electrical velocimetry: a comparison with echocardiography. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F340–3. https://doi.org/10.1136/fetalneonatal-2011-301090.
Grollmuss O, Demontoux S, Capderou A, Serraf A, Belli E. Electrical velocimetry as a tool for measuring cardiac output in small infants after heart surgery. Intensive Care Med. 2012;38(6):1032–9. https://doi.org/10.1007/s00134-012-2530-3.
Cote CJ, Sui J, Anderson TA, Bhattacharya ST, Shank ES, Tuason PM, et al. Continuous noninvasive cardiac output in children: is this the next generation of operating room monitors? Initial experience in 402 pediatric patients. Paediatr Anaesth. 2015;25(2):150–9. https://doi.org/10.1111/pan.12441.
Rauch R, Welisch E, Lansdell N, Burrill E, Jones J, Robinson T, et al. Non-invasive measurement of cardiac output in obese children and adolescents: comparison of electrical cardiometry and transthoracic Doppler echocardiography. J Clin Monit Comput. 2013;27(2):187–93. https://doi.org/10.1007/s10877-012-9412-7.
Grollmuss O, Gonzalez P. Non-invasive cardiac output measurement in low and very low birth weight infants: a method comparison. Front Pediatr. 2014;2:16. https://doi.org/10.3389/fped.2014.00016.
Blohm ME, Obrecht D, Hartwich J, Mueller GC, Kersten JF, Weil J, et al. Impedance cardiography (electrical velocimetry) and transthoracic echocardiography for non-invasive cardiac output monitoring in pediatric intensive care patients: a prospective single-center observational study. Critical Care (London). 2014;18(6):603. https://doi.org/10.1186/s13054-014-0603-0.
Narula J, Chauhan S, Ramakrishnan S, Gupta SK. Electrical cardiometry: a reliable solution to cardiac output estimation in children with structural heart disease. J Cardiothorac Vasc Anesth. 2016; https://doi.org/10.1053/j.jvca.2016.12.009.
Torigoe T, Sato S, Nagayama Y, Sato T, Yamazaki H. Influence of patent ductus arteriosus and ventilators on electrical velocimetry for measuring cardiac output in very-low/low birth weight infants. J Perinatol: official journal of the California Perinatal Association. 2015;35(7):485–9. https://doi.org/10.1038/jp.2014.245.
Trinkmann F, Berger M, Hoffmann U, Borggrefe M, Kaden JJ, Saur J. A comparative evaluation of electrical velocimetry and inert gas rebreathing for the non-invasive assessment of cardiac output. Clin Res Cardiol. 2011;100(10):935–43. https://doi.org/10.1007/s00392-011-0329-9.
Nidorf SM, Picard MH, Triulzi MO, Thomas JD, Newell J, King ME, et al. New perspectives in the assessment of cardiac chamber dimensions during development and adulthood. J Am Coll Cardiol. 1992;19(5):983–8.
Chong SW, Peyton PJ. A meta-analysis of the accuracy and precision of the ultrasonic cardiac output monitor (USCOM). Anaesthesia. 2012;67(11):1266–71. https://doi.org/10.1111/j.1365-2044.2012.07311.x.
• McNamara H, Barclay P, Sharma V. Accuracy and precision of the ultrasound cardiac output monitor (USCOM 1A) in pregnancy: comparison with three-dimensional transthoracic echocardiography. Br J Anaesth. 2014;113(4):669–76. https://doi.org/10.1093/bja/aeu162. The authors proved the adequate accuracy of USCOM in pregnant women.
O’Loughlin E, Ward M, Crossley A, Hughes R, Bremner AP, Corcoran T. Evaluation of the utility of the Vigileo FloTrac(), LiDCO(), USCOM and CardioQ() to detect hypovolaemia in conscious volunteers: a proof of concept study. Anaesthesia. 2015;70(2):142–9. https://doi.org/10.1111/anae.12949.
• Li H, Critchley LA, Zhang J. Does using two Doppler cardiac output monitors in tandem provide a reliable trend line of changes for validation studies? J Clin Monit Comput. 2016;30(5):559–67. https://doi.org/10.1007/s10877-015-9753-0. The authors provide a practical way of measurement to obtain accuracy.
Corley A, Barnett AG, Mullany D, Fraser JF. Nurse-determined assessment of cardiac output. Comparing a non-invasive cardiac output device and pulmonary artery catheter: a prospective observational study. Int J Nurs Stud. 2009;46(10):1291–7. https://doi.org/10.1016/j.ijnurstu.2009.03.013.
Dey I, Sprivulis P. Emergency physicians can reliably assess emergency department patient cardiac output using the USCOM continuous wave Doppler cardiac output monitor. Emerg Med Australas. 2005;17(3):193–9. https://doi.org/10.1111/j.1742-6723.2005.00722.x.
Huang L, Critchley LA. Study to determine the repeatability of supra-sternal Doppler (ultrasound cardiac output monitor) during general anaesthesia: effects of scan quality, flow volume, and increasing age. Br J Anaesth. 2013;111(6):907–15. https://doi.org/10.1093/bja/aet254.
Zhang J, Critchley LA, Huang L. The effect of aorta unfolding and remodelling on oesophageal Doppler readings as probe depth is varied. Br J Anaesth. 2015;115(5):708–15. https://doi.org/10.1093/bja/aev343.
The National Institute for Health and Care Excellence (NICE). 2011. https://www.nice.org.uk/guidance/mtg3. Accessed 1 Aug 2017.
• Moller-Sorensen H, Cordtz J, Ostergaard M, Nilsson JC, Hansen KL. Transesophageal Doppler reliably tracks changes in cardiac output in comparison with intermittent pulmonary artery thermodilution in cardiac surgery patients. J Clin Monit Comput. 2017;31(1):135–42. https://doi.org/10.1007/s10877-015-9806-4. The authors properly validate precision of the method.
Raux O, Spencer A, Fesseau R, Mercier G, Rochette A, Bringuier S, et al. Intraoperative use of transoesophageal Doppler to predict response to volume expansion in infants and neonates. Br J Anaesth. 2012;108(1):100–7. https://doi.org/10.1093/bja/aer336.
Guinot PG, de Broca B, Bernard E, Abou Arab O, Lorne E, Dupont H. Respiratory stroke volume variation assessed by oesophageal Doppler monitoring predicts fluid responsiveness during laparoscopy. Br J Anaesth. 2014;112(4):660–4. https://doi.org/10.1093/bja/aet430.
Smetkin AA, Hussain A, Fot EV, Zakharov VI, Izotova NN, Yudina AS, et al. Estimated continuous cardiac output based on pulse wave transit time in off-pump coronary artery bypass grafting: a comparison with transpulmonary thermodilution. J Clin Monit Comput. 2017;31(2):361–70. https://doi.org/10.1007/s10877-016-9853-5.
Bataille B, Bertuit M, Mora M, Mazerolles M, Cocquet P, Masson B, et al. Comparison of esCCO and transthoracic echocardiography for non-invasive measurement of cardiac output intensive care. Br J Anaesth. 2012;109(6):879–86. https://doi.org/10.1093/bja/aes298.
Fischer MO, Balaire X, Le Mauff de Kergal C, Boisselier C, Gerard JL, Hanouz JL, et al. The diagnostic accuracy of estimated continuous cardiac output compared with transthoracic echocardiography. Can J Anaesth. 2014;61(1):19–26. https://doi.org/10.1007/s12630-013-0055-z.
Sinha AC, Singh PM, Grewal N, Aman M, Dubowitz G. Comparison between continuous non-invasive estimated cardiac output by pulse wave transit time and thermodilution method. Ann Card Anaesth. 2014;17(4):273–7. https://doi.org/10.4103/0971-9784.142059.
Permpikul C, Leelayuthachai T. Non-invasive estimated continuous cardiac output (escCO) during severe sepsis and septic shock resuscitation. J Med Assoc Thailand = Chotmaihet thangphaet. 2014;97(Suppl 3):S184–8.
• Thonnerieux M, Alexander B, Binet C, Obadia JF, Bastien O, Desebbe O. The ability of esCCO and ECOM monitors to measure trends in cardiac output during alveolar recruitment maneuver after cardiac surgery: a comparison with the pulmonary thermodilution method. Anesth Analg. 2015;121(2):383–91. https://doi.org/10.1213/ane.0000000000000753. The authors properly handled the precision and trending ability.
Feissel M, Aho LS, Georgiev S, Tapponnier R, Badie J, Bruyere R, et al. Pulse wave transit time measurements of cardiac output in septic shock patients: a comparison of the estimated continuous cardiac output system with transthoracic echocardiography. PLoS One. 2015;10(6):e0130489. https://doi.org/10.1371/journal.pone.0130489.
• Biais M, Berthezene R, Petit L, Cottenceau V, Sztark F. Ability of esCCO to track changes in cardiac output. Br J Anaesth. 2015;115(3):403–10. https://doi.org/10.1093/bja/aev219. The trending ability of esCCO has been validated.
Ball TR, Tricinella AP, Kimbrough BA, Luna S, Gloyna DF, Villamaria FJ, et al. Accuracy of noninvasive estimated continuous cardiac output (esCCO) compared to thermodilution cardiac output: a pilot study in cardiac patients. J Cardiothorac Vasc Anesth. 2013;27(6):1128–32. https://doi.org/10.1053/j.jvca.2013.02.019.
Magliocca A, Rezoagli E, Anderson TA, Burns SM, Ichinose F, Chitilian HV. Cardiac output measurements based on the pulse wave transit time and thoracic impedance exhibit limited agreement with thermodilution method during orthotopic liver transplantation. Anesth Analg. 2017; https://doi.org/10.1213/ane.0000000000002171.
Terada T, Oiwa A, Maemura Y, Robert S, Kessoku S, Ochiai R. Comparison of the ability of two continuous cardiac output monitors to measure trends in cardiac output: estimated continuous cardiac output measured by modified pulse wave transit time and an arterial pulse contour-based cardiac output device. J Clin Monit Comput. 2016;30(5):621–7. https://doi.org/10.1007/s10877-015-9772-x.
Tsutsui M, Araki Y, Masui K, Kazama T, Sugo Y, Archer TL, et al. Pulse wave transit time measurements of cardiac output in patients undergoing partial hepatectomy: a comparison of the esCCO system with thermodilution. Anesth Analg. 2013;117(6):1307–12. https://doi.org/10.1213/ANE.0b013e3182a44c87.
Raissuni Z, Zores F, Henriet O, Dallest S, Roul G. Can we obtain a noninvasive and continuous estimation of cardiac output? Comparison between three noninvasive methods. Int Heart J. 2013;54(6):395–400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yohei Fujimoto, Koichi Suehiro, Akira Mukai, and Kiyonobu Nishikawa declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Advances in Monitoring for Anesthesia
Electronic Supplementary Material
ESM 1
(DOCX 30.1 kb).
Rights and permissions
About this article
Cite this article
Fujimoto, Y., Suehiro, K., Mukai, A. et al. Perioperative Cardiac Output Monitoring Utilizing Non-pulse Contour Methods. Curr Anesthesiol Rep 7, 399–409 (2017). https://doi.org/10.1007/s40140-017-0240-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-017-0240-7