Abstract
To analyze changes in cardiac output and hemodynamic volumes using transpulmonary ultrasound dilution (TPUD) in a neonatal animal model under different hemodynamic conditions. 7 lambs (3.5–8.3 kg) under general anesthesia received arterial and central venous catheters. A Gore-Tex® shunt was surgically inserted between the descending aorta and the left pulmonary artery to mimic a patent ductus arteriosus. After shunt opening and closure, induced hemorrhagic hypotension (by repetitive blood withdrawals) and repetitive volume challenges, the following parameters were assessed using TPUD: cardiac output, active circulating volume index (ACVI), central blood volume index (CBVI) and total end-diastolic volume index (TEDVI). 27 measurement sessions were analyzed. After shunt opening, there was a significant increase in TEDVI and a significant decrease in cardiac output with minimal change in CBVI and ACVI. With shunt closure, these results reversed. After progressive hemorrhage, cardiac output and all volumes decreased significantly, except for ACVI. Following repetitive volume resuscitation, cardiac output increased and all hemodynamic volumes increased significantly. Correlations between changes in COufp and changes in hemodynamic volumes (ACVI 0.83; CBVI 0.84 and TEDVI 0.78 respectively) were (slightly) better than between changes in COufp and changes in heart rate (0.44) and central venous pressure (0.7). Changes in hemodynamic volumes using TPUD were as expected under different conditions. Hemodynamic volumetry using TPUD might be a promising technique that has the potential to improve the assessment and interpretation of the hemodynamic status in critically ill newborns and children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hemodynamic monitoring is an integral part of adult critical care and used with increasing interest in critically ill children [1–4]. In the last decades a range of new cardiac monitoring devices (e.g. methods based on Fick principle, indicator dilution, Doppler ultrasound, arterial pulse contour or thoracic electrical impedance) have been introduced to provide information about the patient’s hemodynamic state [1]. Most of these techniques are not applicable in neonates due to technical and/or vascular access restraints or potential toxicity of the indicator. Some of them have not (yet) been validated or are not accurate, especially in the presence of intra- or extracardiac shunts [5]. Transpulmonary thermodilution (TPTD) is regularly used in pediatric intensive care patients, but due to the necessity of a dedicated thermistor tipped catheter not applicable in infants <3.5 kg. Therefore, the estimation of cardiac output and intravascular volume status in neonates is still mainly based on clinical parameters, despite their proven unreliability [6]. Functional echocardiography provides a lot of hemodynamic information and allows the monitoring of response to treatment, but is operator-dependent, requires training and is not always available at the bedside at any given time [7].
Transpulmonary ultrasound dilution (TPUD) is a relatively new method for estimation of cardiac output, which has been validated in adults [8, 9] and recently in pediatric patients [10]. The main advantage of this method is that it can be used with any (peripheral or umbilical) arterial and central venous catheter. The TPUD has proven to be easily applicable, reproducible and safe with acceptable accuracy and precision for measuring cardiac output, even in the presence of a significant left-to-right shunt in a juvenile animal model [11–13]. After injection of isotonic saline the ultrasound velocity in the blood will decrease and a dilution curve is obtained. Analysis of this dilution curve enables the calculation of cardiac output and several hemodynamic volumes, such as total end-diastolic volume (TEDV), central blood volume (CBV) and active circulation volume (ACV) [14]. TEDV is a surrogate for the end-diastolic heart volume (comparable to global end diastolic volume (GEDV) used by TPTD). CBV estimates the blood volume in the heart, lungs and large vessels (volume between the central venous and arterial catheter), resembling intrathoracic blood volume (ITBV). ACV—a new variable—differs from total blood volume, as it does not include the blood volume in the small peripheral vessels/microcirculation (‘high resistance vessels’). Several studies evaluated hemodynamic volumetry in pediatric animal models or critically ill children—all using TPTD technology—and found in agreement with studies in adult patients, that changes in cardiac output or stroke volume index correlated better with changes in GEDVI than with changes in CVP [15–18]. Although absolute values of GEDVI are not reliable, TPTD derived GEDVI can be used as trend monitoring [19]. In analogy with older children we assume it could be beneficial to use hemodynamic monitoring—including changes in hemodynamic volumes—in neonates to diagnose underlying conditions and improve or adapt treatment modalities.
The purpose of this study was to describe the effect of hemorrhagic hypotension and subsequent volume resuscitation on cardiac output, TEDVI, CBVI and ACVI measured by TPUD in an experimental animal model with an intermittently opened and closed aortopulmonary shunt.
2 Methods
2.1 Study design
The initial study protocol was designed to validate cardiac output measurement using TPUD in an animal model with a left-to-right shunt [13]. The data obtained for the current study were collected during the initial study. The study protocol was performed in accordance with Dutch national legislation concerning guidelines for the care and use of laboratory animals, approved by the Ethical Committee on Animal Research of the Radboud University Nijmegen (RU-DEC #2008-117). Seven random-bred lambs (3.5–8.3 kg, age 5–21 days) were premedicated with an intramuscular injection of ketamine (10 mg/kg), atropine (0.03 mg/kg) and midazolam (0.2 mg/kg), followed by intravenous administration of propofol (2 mg/kg). After orotracheal intubation (cuffed endotracheal tube (ID 4–5 mm; Kruse, Marslev, Denmark) they were mechanically ventilated in a pressure control mode using a Datex Ohmeda Excel 210 SE anesthesia machine (GE Healtcare, Waukesha, Wisconsin, USA) under general anesthesia (isoflurane inhalation (0.5–2.0 vol.%), intravenous administration of fentanyl (15–20 μg/kg/h), midazolam (0.2 mg/kg/h) and pancuronium (0.02 mg/kg/h) after a loading dose of 0.05 mg/kg). The ventilator settings were adjusted during and after thoracotomy in order to maintain normoxaemia (Sao2 90–95%) and normocapnia [Paco2 30–45 torr (4.0–6.0 kPa); end-tidal CO2 30–45 torr (4.0–6.0 kPa)]. The depth of anesthesia was repeatedly assessed by clinical parameters (heart rate, spontaneous ventilation or elevated arterial pressure) and pain stimuli and was adjusted when necessary.
Intravascular catheters were inserted by surgical cutdown via the femoral vessels. The tip of the arterial catheter (16 G/13 cm/1.7 mm, 681002, Secalon T™, Becton, Dickinson and Company, Oxford, United Kingdom) was positioned in the abdominal aorta and connected with the arterial limb of the extracorporeal circuit for TPUD measurement. A double-lumen central venous catheter (16 G/16 cm/1.7 cm, Arrow, Arrow International, Reading, USA) was positioned with the tip in the inferior vena cava. One lumen was used for connection with the venous limb of the AV-loop for TPUD measurement. The other lumen was used for administration of fluids and medication. Another arterial catheter was inserted in the opposite femoral artery to measure blood pressure continuously. A non-stretch, thin-walled vascular graft (ID 4–6 mm, Gore-Tex®, W.L. Gore & Associates Inc., Arizona, USA) was then inserted between the descending aorta and the left pulmonary artery through a left-sided thoracotomy. After a loading dose of heparin (100–120 IU/kg), continuous infusion of heparin (50–100 IU/kg/h) was used to prevent shunt thrombosis. In order to measure the cardiac output and shunt fraction, we placed perivascular ultrasonic flow probes (PAX series, Transonic Systems Inc, Ithaca, USA) around the main pulmonary artery (COufp) and proximal (QAOpre) and distal (QAOpost) to the aortopulmonary shunt on the descending aorta.
2.2 Study protocol (Fig. 1)
After a stabilization period of 15 min cardiac output and hemodynamic volumes were measured under different hemodynamic conditions: (1) phase 1 = normovolemia with open and closed shunt (by (un)clamping the aortopulmonary shunt), (2) phase 2 = hypovolemia, which was effected by stepwise withdrawal of blood from the central venous catheter until a decrease in mean arterial blood pressure of 10 mmHg within approximately 5 min was established (total of 3 times) and (3) phase 3 = volume expansion, which was achieved by stepwise administration of packed red blood cells erythrocyte concentrate (3 times 10 mL/kg). Sessions of TPUD measurements—consisting of three consecutive injections of 1.0 mL/kg isotonic saline at body temperature—were performed after a stabilization period of 15 min following each intervention. At the end of the experiment the animals were euthanized using a lethal dose of pentobarbital (150 mg/kg).
2.3 Transpulmonary ultrasound dilution cardiac output (TPUD)
The dilution method is based on the physiological principle that ultrasound velocity is different in both blood (1570–1585 m/s) and indicator (1533 m/s). It uses a disposable extracorporeal arteriovenous loop (priming volume 5 mL), which is connected to the inserted catheters. A peristaltic pump (6–12 mL/min) prevents stasis of blood and provides stable blood flow during the 6 min measurement sessions. Isotonic saline at body temperature is injected into the venous limb of the AV-loop. A venous sensor—situated nearby the injection site—calculates the exact volume of injected saline. The saline passes the venous catheter, mixes with blood while entering the inferior vena cava and the right heart, passes through the lungs and the left heart and finally arrives in the descending aorta where the arterial catheter is situated. In the presence of a left-to-right shunt, part of the indicator recirculates and makes an extra passage through the lungs before reaching the arterial catheter. The arterial sensor—positioned at the arterial side of the AV-loop—measures the decrease in ultrasound velocity of blood due to the concentration of the injected indicator and a dilution curve is obtained. After three measurements the loop is flushed and the pump stopped.
Cardiac output and hemodynamic volume values (indexed by weight) are displayed on the CO-status™ monitor screen (Transonic Systems Inc, Ithaca, USA). Calculation of the afore mentioned parameters is based on the ultrasound dilution curve(s) and generated by the CO-status software (Transonic Systems Inc, Ithaca, USA), first described by Krivitski et al [14] (Fig. 2). An extensive description and calculation of all parameters is shown in the “Appendix” section.
a Schematic overview of hemodynamic volumes and b dilution curves. MTTa, time during which the indicator travels from the injection site (venous sensor) to the arterial sensor; FWHMart and FWHMven, full width at half maximum of arterial and venous curves in minutes, respectively; H, the concentration of injected saline becomes largely stable within 60 s from the time of injection. An explanation of the calculation of the volumes can be found in the appendix session
2.4 Other measurements
We used biomedical data acquisition software (Poly, Inspektor Research Systems BV, Amsterdam, The Netherlands) to store COufp, QAOpre and QAOpost with a 200 Hz sampling rate. The exact span of the TPUD measurements was marked in the registration. The shunt flow was calculated as the difference between QAOpre and QAOpost, the shunt fraction as shunt flow/systemic blood flow [=(QAOpre − QAOpost)/COufp]. Before every measurement the adequacy of signal strength of the flow probes was checked. Additional acoustic gel was applied in case of decreased signal strength (quality <0.7 on analog meter in “TEST” mode). Continuous monitoring of heart rate, blood pressure and central venous pressure (CVP) was performed.
2.5 Data analysis
In phase 1, the data after shunt opening and closure were compared. In phase 2 and 3, only the data after total hemorrhage and total volume resuscitation with closed shunt were analyzed. Statistical calculations were performed with SPSS version 18.0, using a Wilcoxon signed rank test for paired comparisons of the afore mentioned variables during the different phases (normovolemia (open vs. closed shunt), hemorrhage versus normovolemia and hemorrhage vs. volume resuscitation). Pearson’s correlation was used to compare changes in cardiac output measured by ultrasonic flow probe (COufp) between phase 1 (closed shunt) and phase 2, and between phase 2 and phase 3 respectively with changes in hemodynamic volumes, heart rate and central venous pressure. Significance was set at a p value of less than 0.05. Values were indexed for body surface area using the BSA-formula for lambs (BW2/3 × 0.121) with BW = body weight [20].
3 Results
Table 1 shows the characteristics of the study population. The mean (±SD) weight was 6.4 (±1.4) kg. Mean age was 12.3 (±6) days. The native ductus arteriosus was closed in all animals. Lamb 1 did not receive volume expansion.
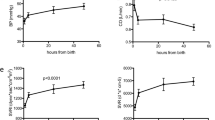
Twenty seven measurement sessions were analyzed. Cardiac output (COtpud) ranged from 104 to 310 mL/kg/min; cardiac output (COufp) ranged from 104 to 284 mL/kg/min. Mean (±SD) normovolemic values (with closed shunt) for cardiac output, ACVI, CBVI and TEDVI were 221 (±67) mL/kg/min, 50 (±8) mL/kg, 18 (±3) mL/kg and 14 (±3) mL/kg respectively. Qp/Qs ratio during open shunt varied between 1.3 and 2.2. Mean total blood withdrawal per lamb during the experiment was 19 (±3.5) mL/kg and mean total volume resuscitation 28 (±4) mL/kg. Results are displayed in Table 2 and Fig. 3.
Absolute changes of cardiac output measured by transpulmonary ultrasound dilution COtpud (a), active circulating volume index ACVI (b), central blood volume index CBVI (c) and total end-diastolic blood volume index TEDVI (d) during different hemodynamic phases for all subjects. Normo_O, normovolemic phase with open shunt; normo_C, normovolemic phase with closed shunt; volume resuscit, volume resuscitation
3.1 Response to shunt closure
There was a significant increase in TEDVI and a significant decrease in COtpud. CBVI and ACVI hardly changed. After shunt closure the opposite effects were observed.
3.2 Response to hemorrhage
COtpud and all measured hemodynamic volumes decreased significantly, except for ACVI.
3.3 Response to volume resuscitation
COtpud and all measured hemodynamic volumes increased significantly.
Figure 4 shows correlations between changes in COufp and changes in hemodynamic volumes (ACVI, CBVI and TEDVI) and hemodynamic parameters (heart rate and CVP) respectively. Correlations were better for the hemodynamic volumes as for heart rate and CVP.
4 Discussion
This is the first study describing the effect of different interventions on CO and hemodynamic volume changes in a neonatal (animal) population using TPUD. The main findings were: (1) COtpud and hemodynamic volumes all decreased after hemorrhage (except for ACVI) and increased after volume resuscitation, (2) opening of the left-to-right shunt resulted in a significant decrease in cardiac output and a significant increase in TEDVI, while CBVI and ACVI hardly changed. These changes were as physiologically expected.
The changes in cardiac output and hemodynamic volumes (except ACVI) during hemorrhage and volume resuscitation in populations without a shunt were also observed by others using TPTD [15, 17, 21]. In our study, CBVI and TEDVI both adequately reflect hemorrhage and volume loading. The decrease in ACVI during hemorrhage was less explicit, which might be due to a redistribution of blood from the peripheral vessels in an attempt to protect the vital organs. A study of hemodynamic volumes measured by TPUD in foals showed a decrease in TEDVI and ACVI during extensive hemorrhage (30 mL/kg) [22]. However, these authors found a high variability in CBVI measurements, probably due to the peripheral position of the arterial line which lead to an excessively prolonged mean transit time. The position of the arterial catheter is taken into account in the algorithm to calculate CBVI in order to correct for a prolonged mean transit time.
In the presence of a (significant) left-to-right shunt left ventricle end-diastolic volume and left ventricle output increases. Consequently, systemic blood flow distal to the origin of the shunt (i.e measured cardiac output) decreases. We indeed noticed a significant increase in TEDVI, although this increase might—except for the enlargement of the left atrium—also be partially due to an increase in full width at half maximum of the arterial curve as a result of recirculation through the left to right shunt. CBVI did not change during shunt opening: we hypothesized that as the blood volume in the lungs and left ventricle increases, the blood volume in the aorta is slightly diminished due to lower perfusion distal to the shunt insertion. ACVI did not significantly change in the presence of this shunt. However, when left-to-right shunting exists for a longer time, fluid retention may occur due to decreased urine output resulting in a consequently higher ACVI. In piglets with right-to-left shunts (after septostomy and partial pulmonary artery occlusion) Shih found a significant decrease in cardiac output and CBVI, but not in TEDVI and ACVI using TPUD [23].
We compared changes in cardiac output with changes in the hemodynamic volumes and central venous pressure and heart rate during phase 2 and 3. To rule out any mathematic coupling between the cardiac output measurement by TPUD (COtpud) and the hemodynamic volumes—both COtpud and the volumes are calculated by the same dilution curve—we chose to use COufp for these analyses. Changes in cardiac output correlated better with changes in hemodynamic volumes than changes in central venous pressure and heart rate. We did not calculate blood pressure changes in our analyses as this parameter was used to determine the amount of blood withdrawal during phase 2. We expected—in analogy with TPTD—that TEDVI would better reflect preload conditions than CBVI or ACVI, but the correlation between TEDVI and COufp was slightly less when compared to the other mentioned volumes. This might be due to (1) the relative small number of measurements and (2) the presence of 1 outlier (lamb 5, phase 3) for which we have no clear explanation (no system warning, no inotropic use). When recalculating correlations without this aberrant measurement TEDVI seems a better predictor for preload (Pearson’s correlation for ACVI = 0.85, CBVI = 0.89 respectively TEDVI = 0.89). In a study of 100 ventilated children the predictive value for fluid responsiveness (>15% change in stroke volume after a 10 mL/kg fluid bolus) was poor, although slightly better for hemodynamic volumes measured by TPUD than for CVP [24].
The changes in cardiac output and hemodynamic volumes measured by TPUD are in accordance with the expected physiological changes in this experimental setting. In our opinion hemodynamic volumetry could play—in analogy with the use of TPTD in adults and children—an important part in advanced hemodynamic monitoring of critically ill neonates as different clinical conditions may be associated with specific changes in cardiac output and hemodynamic volumes. We are aware that this current experimental model rather reflects acute hemodynamic changes, similar with clinical (neonatal) situations as acute (perinatal) blood loss, repetitive volume resuscitation during sepsis and ductal ligation. This model might therefore not be suitable to interpret changes in hemodynamic blood volumes in more chronic conditions as compensating mechanisms may alter blood volumes. However, we believe that combining repetitive measurements of CO and hemodynamic volumes and their dynamic interactions in response to time and treatment might be helpful in diagnosing underlying conditions and improving or adapting treatment modalities.
It was not our intention in this study to validate the accuracy of the hemodynamic indices in terms of absolute values, so we do not know whether the measured hemodynamic volumes are comparable with normal values for infants and neonates. To our knowledge no studies have been published that define normal values for hemodynamic volumes (especially CBV and ACV) in pediatric patients. To compare our observed values with those found in TPTD-studies in small children and animals we recalculated and indexed them by body surface area [20]. Comparison of absolute values of hemodynamic volumes measured by TPTD and TPUD in juvenile animals and children reveals that GEDVI and ITBVI—although proportionally smaller than in adults [15, 18]—are still 1.5–2 times higher than TEDVI and CBVI [25]. Our results confirm this finding. This discrepancy is caused by (1) the difference in the indicator used (TPTD uses a diffusible thermal indicator), (2) the mathematical models used for the calculation of hemodynamic values in TPUD and TPTD [14, 26] (see also “Appendix”) and (3) the algorithm used by TPTD for calculating GEDVI which is not appropriate for small children [16]. Therefore, volumes measured by TPUD might better reflect absolute volumes.
In addition to the relatively scarce data, the main limitation of our study is the lack of concomitant right and left ventricular end-diastolic volume measurement by using for example 3D-echocardiography or cardiac MRI, as these latter might estimate the actual TEDVI. However, as mentioned before, this was not the aim of our study. Secondly, TPUD is an invasive monitoring system requiring central venous and arterial lines. However, in contrast to TPTD, TPUD can be used with any indwelling catheter inserted in term and preterm infants. Additionally, the use of triple injections of 1.0 mL/kg per session could lead to fluid overload in vulnerable neonates when used repetitively. Previously, de Boode showed that for cardiac output measurements (1) using 0.5 mL/kg instead of 1.0 mL/kg of isotonic saline per injection and (2) performing 2 consecutive measurements (instead of 3), unless the difference between the two measurements exceeds 10%, is as precise and accurate as the recommended sessions [11]. We confirmed those findings in another study [13].
We conclude that TPUD can be used in an experimental neonatal population with or without a left-to-right shunt to adequately monitor changes in hemodynamic volumes under different hemodynamic conditions. Although its clinical utility in neonates is not yet proven, and absolute values of the hemodynamic volumes must be interpreted with caution, TPUD seems a promising technique that might improve the assessment and interpretation of the hemodynamic status in critically ill newborns and children, especially when used as a trend monitoring. Validation studies of TPUD in neonates and children in comparison with (3D) echocardiography or cardiac MRI are warranted to evaluate the accuracy of these blood volume measurements.
Abbreviations
- ACV(I):
-
Active circulating volume (index)
- AV-loop:
-
Arteriovenous loop
- CBV(I):
-
Central blood volume (index)
- COtpud:
-
Cardiac output measured by transpulmonary ultrasound dilution
- COufp:
-
Systemic blood flow/cardiac output measured by ultrasonic transit-time flow probe
- CVP:
-
Central venous pressure
- GEDV(I):
-
Global end-diastolic volume (index)
- ITBV(I):
-
Intrathoracic blood volume (index)
- MAP:
-
Mean arterial blood pressure
- TEDV(I):
-
Total end-diastolic volume (index)
- TPUD:
-
Transpulmonary ultrasound dilution
- TPTD:
-
Transpulmonary thermodilution
- QAOpre :
-
Blood flow proximal to the insertion of aortopulmonary shunt
- QAOpost :
-
Blood flow distal to the insertion of aortopulmonary shunt
References
Vincent JL, Rhodes A, Perel A, et al. Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care. 2011;15:229.
de Waal EE, Wappler F, Buhre WF. Cardiac output monitoring. Curr Opin Anaesthesiol. 2009;22:71–7.
Lemson J, Nusmeier A, van der Hoeven JG. Advanced hemodynamic monitoring in critically ill children. Pediatrics. 2011;128:560–71.
Nusmeier A, van der Hoeven JG, Lemson J. Cardiac output monitoring in pediatric patients. Expert Rev Med Devices. 2010;7:503–17.
de Boode WP. Cardiac output monitoring in newborns. Early Hum Dev. 2010;86:143–8.
Tibby SM, Hatherill M, Marsh MJ, Morrison G, Anderson D, Murdoch IA. Clinical validation of cardiac output measurements using femoral artery thermodilution with direct Fick in ventilated children and infants. Intensive Care Med. 1997;23:987–91.
de Waal K, Kluckow M. Functional echocardiography; from physiology to treatment. Early Hum Dev. 2010;86:149–54.
Eremenko AA, Safarov PN. Flow-regulated extracorporeal arteriovenous tubing loop for cardiac output measurements by ultrasound velocity dilution: validation in post-cardiac surgery intensive care unit patients. ASAIO J. 2010;56:522–6.
Tsutsui M, Matsuoka N, Ikeda T, Sanjo Y, Kazama T. Comparison of a new cardiac output ultrasound dilution method with thermodilution technique in adult patients under general anesthesia. J Cardiothorac Vasc Anesth. 2009;23:835–40.
Crittendon I III, Dreyer WJ, Decker JA, Kim JJ. Ultrasound dilution: an accurate means of determining cardiac output in children. Pediatr Crit Care Med. 2012;13(1):42–6.
de Boode WP, van Heijst AF, Hopman JC, Tanke RB, van der Hoeven HG, Liem KD. Cardiac output measurement using an ultrasound dilution method: a validation study in ventilated piglets. Pediatr Crit Care Med. 2010;11:103–8.
de Boode WP, van Heijst AF, Hopman JC, Tanke RB, van der Hoeven HG, Liem KD. Application of ultrasound dilution technology for cardiac output measurement: cerebral and systemic hemodynamic consequences in a juvenile animal model. Pediatr Crit Care Med. 2010;11:616–23.
Vrancken SL, de Boode WP, Hopman JC, Singh SK, Liem KD, van Heijst AF. Cardiac output measurement with transpulmonary ultrasound dilution is feasible in the presence of a left-to-right shunt: a validation study in lambs. Br J Anaesth. 2012;108:409–16.
Krivitski NM, Kislukhin VV, Thuramalla NV. Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive care unit patients. Pediatr Crit Care Med. 2008;9(4):423–8.
Schiffmann H, Erdlenbruch B, Singer D, Singer S, Herting E, Hoeft A, Buhre W. Assessment of cardiac output, intravascular volume status, and extravascular lung water by transpulmonary indicator dilution in critically ill neonates and infants. J Cardiothorac Vasc Anesth. 2002;16:592–7.
Lemson J, Merkus P, van der Hoeven JG. Extravascular lung water index and global end-diastolic volume index should be corrected in children. J Crit Care. 2011;26:432.e7–12.
Lopez-Herce J, Ruperez M, Sanchez C, Garcia C, Garcia E. Haemodynamic response to acute hypovolaemia, rapid blood volume expansion and adrenaline administration in an infant animal model. Resuscitation. 2006;68:259–65.
Lopez-Herce J, Bustinza A, Sancho L, Mencia S, Carrillo A, Moral R, Bellon JM. Cardiac output and blood volume parameters using femoral arterial thermodilution. Pediatr Int. 2009;51:59–65.
Proulx F, Lemson J, Choker G, Tibby SM. Hemodynamic monitoring by transpulmonary thermodilution and pulse contour analysis in critically ill children. Pediatr Crit Care Med. 2011;12:459–66.
Stowe CM, Good AL. Estimation of cardiac output in calves and sheep by the dye and Fick oxygen techniques. Am J Physiol. 1960;198:987–90.
Cecchetti C, Stoppa F, Vanacore N, Barbieri MA, Raucci U, Pasotti E, Tomasello C, Marano M, Pirozzi N. Monitoring of intrathoracic volemia and cardiac output in critically ill children. Minerva Anestesiol. 2003;69:907–18.
Vigani A, Shih A, Queiroz P, Pariaut R, Gabrielli A, Thuramalla N, Bandt C. Quantitative response of volumetric variables measured by a new ultrasound dilution method in a juvenile model of hemorrhagic shock and resuscitation. Resuscitation. 2012;83:1031–7.
Shih A, Maisenbacher H III, Vigani A, Estrada A, Pogue B, Berry C, Buckley G, Schrank H, Thuramalla N, Bandt C. Ultrasound dilution technique as a minimally invasive way to detect intracardiac shunt. Crit Care Med. 2011;39 (12 suppl):61.
Saxena R, Durward A, Murdoch I, Tibby S. Prediction of stroke volume response to fluid bolus in 100 children. Crit Care. 2013;17(2):207.
Boehne M, Schmidt F, Witt L, Koditz H, Sasse M, Sumpelmann R, Bertram H, Wessel A, Osthaus WA. Comparison of transpulmonary thermodilution and ultrasound dilution technique: novel insights into volumetric parameters from an animal model. Pediatr Cardiol. 2012;33:625–32.
Pulsion PiCCOplus operator’s manual. Pulsion Medical System. Munich, Germany; 2002.
Sakka SG, Rühl CC, Pfeiffer UJ, et al. Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med. 2000;26:180–7.
Michard F. Bedside assessment of extravascular lung water by dilution methods: temptations and pitfalls. Crit Care Med. 2007;35:1186–92.
Fracasso T, Vennemann M, Pfeiffer H, et al. Organ weights in cases of sudden infant death syndrome: a German study. Am J Forensic Med Pathol. 2009;30:231–4.
Acknowledgments
We would like to thank Mr. J.J.M. Menssen from the Department of Radiology, Medical Ultrasound Imaging Centre, Radboud University Nijmegen Medical Centre, Prof. P. Schoof, pediatric cardiothoracic surgeon, and Mr. A.E.J. Hanssen from the Animal Laboratory of the Radboud University Nijmegen for their outstanding support. Our research group received financial support for the technical realization of this experiment from Transonic Systems Inc. Ithaca, USA and Pulsion Medical Systems, Munich, Germany.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standard
This study protocol was performed in accordance with Dutch national legislation concerning guidelines for the care and use of laboratory animals, approved by the Ethical Committee on Animal Research of the Radboud University Nijmegen Medical Centre.
Author information
Authors and Affiliations
Corresponding author
Appendix: Calculation of hemodynamic volumes
Appendix: Calculation of hemodynamic volumes
1.1 TPUD [14]
Cardiac output is calculated using the Stewart–Hamilton equation: CO = Vinj/∫Ca(t), with Vinj = the volume of injected saline (mL) and ∫Ca(t) = the area under the dilution curve [saline concentration in arterial blood (mLsaline/mLblood × minute)].
Active circulating volume (ACV) is defined as the volume of blood in which the indicator mixes in a 1 min time period from the time of injection. It is calculated using the following formula: ACV = Vinj/H, where Vinj = volume of injected isotonic saline (mL); H = level of isotonic saline concentration in the blood (mLsaline/mLblood) at the end of the first minute after venous injection as recorded by the arterial sensor.
Central blood volume (CBV) is calculated as CBV = CO × (MTTa − MTTv − MTTt) with CO cardiac output and MTTa the mean transit time, which is the time span between injection of the indicator (measured by the venous sensor) and the time point when half of the indicator has passed the detection point (arterial sensor). As the mean transit time actually refers to the transit time of the indicator between the venous and arterial catheter a correction must be made for the time the indicator travels from the injection site to the end of the venous catheter (MTTv) and the mean transit time during which the indicator travels in the loop before reaching the arterial sensor (MTTt). CBV might vary slightly depending on the position of the tip of the arterial and central venous catheter.
Total end-diastolic volume’s (TEDV) calculation is based on the assumption that the change in spread of the ultrasound dilution curve from the initial venous shape results largely from mixing of the indicator in the heart chambers. This spread of the curve is defined as the full width at half maximum (FWMH) (1). The venous injection curve is small and high (no mixing of indicator yet) resulting in a small FWHMven, while the arterial dilution curve is broader and less high due to mixing of indicator (larger FWHMart). The higher the end-diastolic volume, the broader the arterial curve will be. As the spread of the curve will also be influenced by the heart rate the formula for TEDV calculation is corrected for this: TEDV = CO × (1.62/HR + 0.77 × FWHMc) where CO = cardiac output (mL min−1); FWHMc = (FWHMart2 − FWHMven2)1/2; HR = heart rate (bpm); FWHMart and FWHMven = the full width at half maximum of the arterial and venous curves in minutes, respectively. All the volumes are indexed by bodyweight.
1.2 Hemodynamic volumetry by TPTD [26]
The intrathoracic blood volume (ITBV) is defined as the sum of GEDV and pulmonary blood volume. It is calculated based on the assumption that the blood volume in the lungs and the intrathoracic vessels (with the exception of heart volume) constitutes 25 % of GEDV:
This assumed factor of 1.25 is based on one study in adult subjects [27]. However, there exists a rather large intra- and interindividual variability [28, 29]. Global end-diastolic (GEDV) is defined as the sum of all end-diastolic volumes of atria and ventricles. It is calculated by subtracting the pulmonary thermal volume (PTV) from the intrathoracic thermal volume (ITTV):
where ITTV is the distribution of the thermal volume in the thorax and calculated as cardiac output multiplied by the mean transit time of the thermal indicator:
Rights and permissions
About this article
Cite this article
Vrancken, S.L., van Heijst, A.F., Hopman, J.C. et al. Hemodynamic volumetry using transpulmonary ultrasound dilution (TPUD) technology in a neonatal animal model. J Clin Monit Comput 29, 643–652 (2015). https://doi.org/10.1007/s10877-014-9647-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9647-6