Abstract
Purpose of Review
This review evaluates the current literature on robotic surgery for rectal cancer and discusses ongoing controversies related to outcomes, training, adoption, and cost-effectiveness.
Recent Findings
Robotic rectal surgery is associated with some benefits in short-term outcomes such as lower conversions compared to laparoscopy but also substantially higher costs. Data on long-term oncologic outcomes are still limited. Studies are inconsistent regarding benefits in margin positivity and other metrics. There is significant variability in training practices and adoption rates globally.
Summary
Robotic rectal surgery is increasingly utilized, especially for low rectal cancers where laparoscopy is more challenging. It allows more patients to undergo minimally invasive total mesorectal excision. However, benefit over laparoscopy is unclear for less complex cases. Ongoing controversies exist related to true outcomes, optimal training, cost-effectiveness, and credentialing of surgeons. Further data from high-quality trials are needed to better determine the definitive role of robotic platforms.
Similar content being viewed by others
Introduction
Minimally invasive surgery has become the standard of care for many colorectal procedures due to improved short-term outcomes compared to open techniques, including shorter hospital stays, decreased pain, faster return of bowel function, and improved cosmesis [1,2,3,4]. Laparoscopic colectomy is now widely utilized for colon cancer, with numerous studies demonstrating equivalent oncologic outcomes to open colectomy [5, 6]. However, adoption of laparoscopic techniques for rectal cancer has been slower due to the technical demands of operating in the confined pelvis and achieving an adequate total mesorectal excision (TME) [7, 8].
Robotic-assisted platforms have the potential to overcome some of the limitations of straight stick laparoscopy for pelvic dissection. Theoretical benefits include three-dimensional stereoscopic visualization, wristed instruments with a greater range of motion, reduced tremor, and improved surgeon ergonomics [9, 10]. These factors may facilitate maneuvers like splenic flexure mobilization, rectal transection, and precise TME in the deep pelvis. Early studies suggest robotic rectal surgery is associated with improvements in conversion rates, circumferential resection margin positivity, and other short-term outcomes compared to laparoscopy [10, 11, 12••, 13, 14••, 15]. However, robotic surgery also entails longer operating room times and substantially higher costs [11, 14••, 16, 17].
The current literature contains discrepancies regarding the benefits of robotic rectal surgery, especially when it comes to oncologic outcomes. There are also ongoing debates around the learning curve, training requirements, cost-effectiveness, and adoption rates. The aim of this review is to examine the evidence on robotic surgery for rectal cancer and provide an overview of the current controversies regarding its utilization. Determining the appropriate role of robotic platforms in treatment of rectal cancer remains an important question. With training programs transitioning towards minimally invasive approaches and increased availability of robotic systems, evaluating the benefits and limitations of this technology will have implications for practice.
Short-Term Outcomes
Multiple studies have compared short-term perioperative outcomes between robotic and laparoscopic rectal cancer surgery.
In terms of operative duration, robotic surgery is generally associated with significantly longer operating times. The randomized controlled trial by Kim et al. with 139 patients found median operating time was longer for robotic proctectomy compared to laparoscopy (190 min vs 155 min, p < 0.001) [18]. This aligns with multiple meta-analyses showing robotic rectal surgery having 28 to 93 min longer operating room times on average than laparoscopic surgery [19••, 20,21,22].
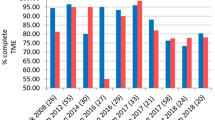
However, robotic surgery demonstrates lower rates of conversion to open surgery. Kim et al. reported only 1 conversion (1.2%) in the robotic group compared to 5 (7.6%) in the laparoscopic group [18]. Several meta-analyses found a significantly lower conversion rate with robotic surgery, ranging from 1.9 to 11% for robotic cases compared to 6.3% to 29% for laparoscopic cases [13, 14••, 21, 22].
When examining intraoperative complications, meta-analyses have found no significant differences between robotic and laparoscopic rectal surgery [21, 22]. In terms of postoperative morbidity, results are inconsistent. Some studies show lower rates of overall complications for robotic surgery [13, 17, 23], while other analyses demonstrate similar postoperative complication profiles between robotic and laparoscopic approaches [19••, 21].
Most studies report comparable lengths of hospital stay between robotic and laparoscopic rectal surgery, averaging 5–12 days [15, 18]. Both minimally invasive methods result in significantly shorter hospitalization compared to open surgery [21, 22].
In summary, while robotic rectal surgery requires longer operating room times, it demonstrates lower conversion rates back to open surgery compared to laparoscopy. Further data are required to determine if robotic techniques provide an advantage in reduced postoperative complications or quicker recovery times.
Oncologic Outcomes
Several studies have evaluated and compared oncologic outcomes between robotic and laparoscopic rectal cancer surgery.
The randomized trial by Kim et al. found no significant difference in circumferential resection margin (CRM) positivity rates between robotic and laparoscopic proctectomy (7.7% vs 13.2%, p = 0.513) [18]. Similar CRM positivity was reported in other studies, including the meta-analysis by Li et al. which found no difference between robotic and laparoscopic surgery after pooling 7 RCTs (RR 1.47, 95% CI 0.53–4.05, p = 0.46) [15, 16].
Regarding lymph node harvest, Kim et al. reported similar median lymph node yields between robotic and laparoscopic techniques (14 vs 12, p = 0.513) [18]. This aligns with the meta-analysis by Li et al. showing no difference in lymph node counts after pooling 7 RCTs (WMD − 0.56, 95% CI − 1.81 to 0.69, p = 0.38) [22]. However, Sun et al. reported a lower lymph node harvest with robotic surgery in their meta-analysis of 6 studies (WMD − 2.23, 95% CI − 3.51 to − 0.95, p = 0.0007) [22].
For low rectal cancers specifically, Lee et al. found comparable distal resection margins and CRM positivity rates when comparing robotic to laparoscopic intersphincteric resection in a meta-analysis of 510 patients [23]. This indicates the effectiveness of robotic surgery for low tumors requiring sphincter preservation.
Longer-term oncologic outcomes remain limited in studies given the relatively recent adoption of robotic surgery. However, the 2-year local recurrence rate was similar between robotic and laparoscopic surgery groups in the trial by Kim et al. (2.7% vs 4.8%, p = 0.999) [18].
In summary, current evidence suggests robotic rectal surgery provides equivalent oncologic outcomes to laparoscopy in terms of margin status, lymph node yields, and early recurrence rates. However, high-quality data on long-term survival are still lacking.
Learning Curve
Several studies have aimed to quantify the learning curve for robotic rectal surgery by analyzing how outcomes change with surgical experience.
The randomized trial by Kim et al. found operating time decreased from a median of 230 min for the first 10 robotic cases to 175 min for cases 11–20, and 150 min after 20 cases. Conversion rates also declined after the first 10 cases [18].
Olthof et al. reported improvements in operative duration, conversions, complications, and length of stay during a surgeon's first 15–25 robotic rectal resections before plateauing [16].
Additional studies found that major gains in operative time, EBL, lymph node harvest, and other outcomes occurred after the first 20 robotic cases [11, 24].
However, the exact number of cases needed to achieve proficiency remains controversial. Some studies suggest comprehensive training should include a minimum of 20–30 mentored cases before independence [13, 25]. But others note the learning curve is highly variable based on factors like prior experience [14••, 15, 16, 26].
Regardless, studies agree outcomes are inferior during the initial learning curve [11, 16, 18, 24]. This has led some experts to recommend restricting robotic rectal surgery to high-volume centers until surgeons achieve proficiency [19••, 20]. However, formal case volume requirements for competency are not standardized.
In summary, accumulating experience leads to improvements in outcomes for robotic rectal surgery. But the precise number of cases needed to reach mastery remains unclear. Careful monitoring of individual learning curves can help ensure optimal results.
Training and Adoption
Adoption of robotic rectal surgery has increased substantially but remains variable globally. Studies show utilization rates ranging from 1.5% in Germany to 54% in Korea [11, 17].
Optimal training methods remain controversial. Some experts advocate comprehensive training including modules, simulation, observation, bedside assisting, and graded progression to independence under proctoring [12••, 13, 14••, 21].
The Robotic Training Network recommends at least 20 mentored cases before independent practice [25]. Others have called for standardized virtual curricula to demonstrate competency prior to autonomous surgery [26].
However, most surgeons receive no formal credentialing or robotic training beyond their laparoscopic colorectal experience [15, 22]. One study found only 18% of robotic rectal surgeons had formal credentialing protocols [15].
This variability has led some to advocate restricting robotic surgery until stronger evidence on outcomes and credentialing emerges [19••, 20, 23]. Proposed requirements include minimum case volumes, proctoring, and maintenance of competence. However, formal guidelines are lacking in most countries.
In summary, adoption of robotic rectal surgery is increasing but optimal training and credentialing remain controversial. Further data will help establish standardized curricula to ensure responsible adoption.
In summary, adoption of robotic rectal surgery is increasing but remains inconsistent globally. Optimal training methods and credentialing requirements remain controversial. Further data will help establish optimal paradigms to ensure safe, responsible adoption of robotic techniques.
Cost Effectiveness
Robotic rectal surgery is consistently associated with substantially higher procedural costs compared to laparoscopic approaches. A major contributor is the use of proprietary robotic instruments which must be replaced after a certain number of uses.
The randomized trial by Kim et al. found the total cost was $9538 USD for robotic low anterior resection compared to $6966 USD for laparoscopic surgery. The main factors increasing robotic costs were longer operating room times and instrumentation expenses [18].
Other studies echo these findings. The meta-analysis by Sun et al. reported the median additional cost of robotic proctectomy ranged from $1300 to $8000 more than laparoscopy, with a pooled incremental cost of $3200 [16]. The meta-analyses by Martins et al. and Li et al. also found higher robotic surgery costs driven by instrumentation and longer operative durations [19••, 21].
Additional factors that contribute to higher costs for robotic surgery include longer operative times, the need for repairs/replacements of robotic arms and instruments, purchase and maintenance of the robotic system itself, and increased utilization of operating room resources [15, 24]. In our experience we found that after the first 5 cases, robotic proctectomy had similar overall costs compared to open surgery when a cost-conscious approach was used, despite longer operative times [27••]. Yet this approach requires proactive thinking towards minimizing unnecessary expenses during the robotic procedures such as not excessive instruments, vessel sealers.
However, there is debate whether the added expense of robotic technology is justified by potential clinical benefits such as lower conversions to open surgery, shorter hospital stays postoperatively, or improved oncologic outcomes. Some argue the technology provides good value given the reductions in conversion rates and complications [20]. Others argue the marginal benefits observed thus far do not warrant the substantially increased costs of robotic platforms [21, 28••].
Overall, the literature consistently demonstrates robotic rectal surgery carries a substantial price premium compared to laparoscopic surgery. But further data are required to determine if advantages in outcomes justify these additional expenditures.
Conclusions
The use of robotic platforms for rectal cancer resection has gained increasing adoption over the past decade, with utilization rates up to 50% reported in some centers. The technology offers theoretical benefits for performing total mesorectal excision in the confined pelvis.
Several studies suggest improvements in short-term outcomes compared to laparoscopy, including lower rates of conversion to open surgery. Robotic surgery also facilitates higher quality tissue and mesorectal specimen retrieval compared to laparoscopy in some reports, particularly for low rectal tumors requiring intersphincteric resection.
However, operating room times are longer for robotic surgery, and equipment costs per case are substantially higher amounting to several thousand dollars of incremental expense. Data on long-term oncologic outcomes are lacking given the relatively recent adoption of robotic techniques. And high-quality randomized trials have failed to demonstrate a clear advantage over laparoscopic surgery in terms of margin status, lymph node yields, quality of resection, and early recurrence rates.
Major controversies still exist regarding the true benefits of robotic rectal surgery and its optimal role. The literature contains discrepancies related to improvements in conversion rates, specimen quality, complications, and other metrics. And the higher costs associated with robotic platforms remain a major limitation inhibiting universal adoption.
To better determine the appropriate use of robotic assistance for rectal resections, future research should focus on high-quality randomized controlled trials with standardized protocols, surgeons, and equipment. Long-term follow-up for 5-year and 10-year oncologic outcomes will be essential to evaluate the technology's impact on survival. Detailed cost-effectiveness data are needed incorporating factors like operating room expenses, instrumentation costs, and hospitalization. And further evaluation of learning curves and optimal training methods can help ensure responsible adoption by credentialed surgeons.
Currently, robotic rectal surgery appears most beneficial for selected patients with mid to low rectal cancers, where the technical demands of laparoscopy are amplified. In the hands of experienced, high-volume surgeons at specialized centers, it allows an increased proportion of complex cases to be completed minimally invasively with low conversions. However, it remains unclear if advantages over laparoscopy exist for higher, less complicated rectal lesions within reach of standard multiport techniques. Moving forward, appropriate patient selection and limiting robotic surgery to trained proctors until mastery is demonstrated will be key to optimizing outcomes and justifying costs. While a valuable tool in the treatment arsenal for rectal cancer, numerous questions remain regarding the definitive role of robotic platforms.
Study | Year | Patients | Interventions | Outcomes | Key Findings |
|---|---|---|---|---|---|
Wang Y et al. | 2020 | 17 studies, 3193 patients | Robotic vs laparoscopic rectal resection | Postop complications (Clavien-Dindo classification) | Lower rates of overall (OR 0.69) and severe complications (grade III-V, OR 0.69) with robotic approach |
Wang X et al. | 2020 | 20 studies, 5496 patients | Robotic vs laparoscopic rectal resection | OR time, conversions, LOS, complications | Longer OR time but fewer conversions and faster recovery with robotic surgery |
Emile et al. | 2022 | 470 patients after propensity matching | Robotic vs laparoscopic resection for T4 rectal cancer | Conversions, margins, survival | Lower conversion rate (8.9% vs 17.9%) and better 5-year survival (56.2% vs 43.4%) with robotic |
Crippa et al. | 2020 | 600 patients | Robotic vs laparoscopic rectal resection | Complications, LOS | Lower complications (37.2% vs 51.2%) and faster recovery with robotic surgery |
Safiejko et al. | 2021 | 19,731 patients from 42 studies | Robotic vs laparoscopic rectal resection | 30-day mortality, LOS | Lower 30-day mortality (0.4% vs 1.2%) and LOS with robotic surgery |
Tong et al. | 2021 | 1436 patients from 9 studies | Robotic vs laparoscopic rectal resection | OR time, LOS, oncologic outcomes | Longer OR time but similar LOS and oncologic outcomes between techniques |
Olthof et al. | 2020 | 100 robotic, 220 laparoscopic rectal resections | Robotic vs laparoscopic rectal resection | OR time, complications, LOS | Faster OR time, fewer complications, and shorter LOS with robotic approach |
Martins et al. | 2023 | 869 patients from 7 studies | Robotic vs laparoscopic rectal resection | Quality of life | Similar overall QoL. Better physical functioning but worse nausea/vomiting with robotic surgery |
Flynn et al. | 2023 | 50 studies, n not reported | Robotic vs laparoscopic rectal resection | OR time, conversions, oncologic outcomes | Longer OR time but lower conversions and better oncologic outcomes with robotic |
Yung et al. | 2023 | 1678 patients from 11 studies | Robotic vs laparoscopic rectal resection | Urinary and sexual function | Better urinary (IPSS) and sexual (IIEF, FSFI) function scores with robotic approach |
Katsuno et al. | 2020 | Review article | Robotic rectal resection techniques and outcomes | Short-term outcomes, oncologic outcomes, costs | Robotic surgery may benefit obese patients and those needing sphincter preservation, but evidence still limited. Higher costs than laparoscopy |
Guo et al. | 2021 | 2711 patients from 14 studies | Robotic vs open rectal resection | Circumferential margin, lymph nodes, TME | Equivalent oncologic outcomes between robotic and open surgery |
Bao et al. | 2021 | Review article | Robotic vs laparoscopic ventral mesh rectopexy | Complications, costs, outcomes | Shorter LOS and lower complications but higher costs with robotic surgery |
Kim et al. | 2018 | 139 patients | Robotic vs laparoscopic rectal resection | TME quality, margins, complications | Similar TME quality, margins, complications. Better sexual function at 12 months with robotic |
Li et al. | 2019 | 1022 patients from 7 RCTs | Robotic vs laparoscopic rectal resection | Conversions, complications, margins | Lower conversion rate with robotic. Similar complications and margins |
Sun et al. | 2019 | 6 studies, n not reported | Robotic vs laparoscopic rectal resection | Complications, conversions, LOS | Lower complications and conversions but longer OR time with robotic |
Lee et al. | 2018 | 510 patients | Robotic vs laparoscopic intersphincteric resection | Conversions, complications, margins | Lower conversions and blood loss but longer OR time with robotic |
Cengiz et al. | 2021 | 32 robotic, 68 open proctectomies | Robotic vs open proctectomy | OR time, costs, complications, LOS | Longer OR time but fewer complications and similar costs after initial 5 cases with robotic approach |
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance, ••Of major importance
Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061–8. https://doi.org/10.1200/JCO.2006.09.7758.
Klaver CEL, Kappen TM, Borstlap WAA, Bemelman WA, Tanis PJ. Laparoscopic surgery for T4 colon cancer: a systematic review and meta-analysis. Surg Endosc. 2017;31(12):4902–12. https://doi.org/10.1007/s00464-017-5544-7.
Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363(9416):1187–92. https://doi.org/10.1016/S0140-6736(04)15947-3.
Samalavicius NE, Janusonis V, Siaulys R, et al. Robotic surgery using Senhance® robotic platform: single center experience with first 100 cases. J Robot Surg. 2020;14(2):371–6. https://doi.org/10.1007/s11701-019-01000-6.
Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34(6):498–504. https://doi.org/10.1016/j.ctrv.2008.03.011.
da Silva GM, Börjesson L, Wexner SD. Laparoscopy for colorectal cancer. Semin Laparosc Surg. 2004;11(1):3–12. https://doi.org/10.1177/107155170401100102.
Fernández-Hevia M, Delgado S, Castells A, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261(2):221–7. https://doi.org/10.1097/SLA.0000000000000865.
Wu WX, Sun YM, Hua YB, Shen LZ. Laparoscopic versus conventional open resection of rectal carcinoma: a clinical comparative study. World J Gastroenterol. 2004;10(8):1167–70. https://doi.org/10.3748/wjg.v10.i8.1167.
Tschann P, Weigl MP, Lechner D, et al. Is robotic assisted colorectal cancer surgery equivalent compared to laparoscopic procedures during the introduction of a robotic program? A propensity-score matched analysis. Cancers (Basel). 2022;14(13):3208. https://doi.org/10.3390/cancers14133208.
Gómez Ruiz M, Lainez Escribano M, Cagigas Fernández C, Cristobal Poch L, Santarrufina MS. Robotic surgery for colorectal cancer. Ann Gastroenterol Surg. 2020;4(6):646–51. https://doi.org/10.1002/ags3.12401.
Wang X, Cao G, Mao W, Lao W, He C. Robot-assisted versus laparoscopic surgery for rectal cancer: a systematic review and meta-analysis. J Cancer Res Ther. 2020;16(5):979–89. https://doi.org/10.4103/jcrt.JCRT_533_18.
••Emile SH, Horesh N, Freund MR, et al. Outcomes of laparoscopic versus robotic-assisted resection of T4 rectal cancer: propensity score-matched analysis of a national cancer database. Br J Surg. 2023;110(2):242–250. https://doi.org/10.1093/bjs/znac396. This 2023 study analyzed outcomes of robotic vs laparoscopic surgery specifically for advanced T4 rectal cancers using a large national database. It provides important evidence on robotic resection of high-risk rectal tumors
Crippa J, Grass F, Dozois EJ, et al. Robotic surgery for rectal cancer provides advantageous outcomes over laparoscopic approach: results from a large retrospective cohort. Ann Surg. 2021;274(6):e1218–22. https://doi.org/10.1097/SLA.0000000000003805.
••Safiejko K, Tarkowski R, Koselak M, et al. Robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection: a systematic review and meta-analysis of 19,731 patients. Cancers (Basel). 2021;14(1):180. https://doi.org/10.3390/cancers14010180. This 2021 meta-analysis included nearly 20,000 patients comparing robotic to laparoscopic rectal cancer surgery. It provides a comprehensive analysis of perioperative and oncologic outcomes between the techniques.
Tong G, Zhang G, Zheng Z. Robotic and robotic-assisted vs Laparoscopic rectal cancer surgery: a meta-analysis of short-term and long-term results. Asian J Surg. 2021;44(12):1549. https://doi.org/10.1016/j.asjsur.2021.08.053.
Olthof PB, Giesen LJX, Vijfvinkel TS, Roos D, Dekker JWT. Transition from laparoscopic to robotic rectal resection: outcomes and learning curve of the initial 100 cases. Surg Endosc. 2021;35(6):2921–7. https://doi.org/10.1007/s00464-020-07731-0.
Wang Y, Liu Y, Han G, Yi B, Zhu S. The severity of postoperative complications after robotic versus laparoscopic surgery for rectal cancer: a systematic review, meta-analysis and meta-regression. PLoS ONE. 2020;15(10): e0239909. https://doi.org/10.1371/journal.pone.0239909.
Kim MJ, Park SC, Park JW, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg. 2018;267(2):243–51. https://doi.org/10.1097/SLA.0000000000002321.
••Martins RS, Fatimi AS, Mahmud O, et al. Multidimensional quality of life after robotic versus laparoscopic surgery for rectal cancer: a systematic review and meta-analysis. World J Surg. 2023;47(5):1310–1319. https://doi.org/10.1007/s00268-023-06936-3. This 2023 systematic review specifically analyzed quality of life after robotic vs laparoscopic rectal resection, providing important data on patient-reported outcomes.
Flynn J, Larach JT, Kong JCH, et al. Operative and oncological outcomes after robotic rectal resection compared with laparoscopy: a systematic review and meta-analysis. ANZ J Surg. 2023;93(3):510–21. https://doi.org/10.1111/ans.18075.
Li L, Zhang W, Guo Y, et al. Robotic versus laparoscopic rectal surgery for rectal cancer: a meta-analysis of 7 randomized controlled trials. Surg Innov. 2019;26(4):497–504. https://doi.org/10.1177/1553350619839853.
Sun XY, Xu L, Lu JY, Zhang GN. Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2019;28(3):135–42. https://doi.org/10.1080/13645706.2018.1498358.
Lee SH, Kim DH, Lim SW. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2018;33(12):1741–53. https://doi.org/10.1007/s00384-018-3145-0.
Katsuno H, Hanai T, Masumori K, et al. Robotic surgery for rectal cancer: operative technique and review of the literature. J Anus Rectum Colon. 2020;4(1):14–24. https://doi.org/10.23922/jarc.2019-037.
Guo Y, Guo Y, Luo Y, Song X, Zhao H, Li L. Comparison of pathologic outcomes of robotic and open resections for rectal cancer: a systematic review and meta-analysis. PLoS ONE. 2021;16(1): e0245154. https://doi.org/10.1371/journal.pone.0245154.
Bao X, Wang H, Song W, Chen Y, Luo Y. Meta-analysis on current status, efficacy, and safety of laparoscopic and robotic ventral mesh rectopexy for rectal prolapse treatment: can robotic surgery become the gold standard? Int J Colorectal Dis. 2021;36(8):1685–94. https://doi.org/10.1007/s00384-021-03885-y.
••Cengiz TB, Benlice C, Ozgur I, et al. Cost-conscious robotic restorative proctectomy has similar economic and oncologic outcomes to open restorative proctectomy: Results of a long-term follow-up study. Int J Med Robot. 2021;17(6):e2331. https://doi.org/10.1002/rcs.2331. This 2021 study provided important long-term cost and oncologic outcome data on robotic vs open proctectomy to analyze value and cancer control.
••Yang H, Zhou L. The urinary and sexual outcomes of robot-assisted versus laparoscopic rectal cancer surgery: a systematic review and meta-analysis [published online ahead of print, 2023 Mar 21]. Surg Today. 2023; https://doi.org/10.1007/s00595-023-02671-3. This recent 2023 meta-analysis focused on urinary and sexual function outcomes comparing robotic to laparoscopic rectal surgery.
Funding
None.
Author information
Authors and Affiliations
Contributions
A.A and E.G wrote the main manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts to disclose.
Human and Animal Rights and Informed Consent
As this review article is based on previously published research and does not involve original studies with human or animal subjects, the section regarding Human and Animal Rights and Informed Consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alipouriani, A., Gorgun, E. Robotic Rectal Cancer Surgery: Current Controversies. Curr Surg Rep (2024). https://doi.org/10.1007/s40137-024-00397-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s40137-024-00397-w