Abstract
Background
Photobiomodulation (PBM) relies on the pathophysiological mechanism whereby red to near-infrared light can target mitochondrial activity and promote ATP synthesis. Preclinical and clinical studies have shown promising results in treating intermediate age-related macular degeneration (AMD), since PBM can produce photochemical reactions in endogenous retinal chromophores. Currently, PBM is approved by the Food and Drug Administration and by the European Medicines Agency for the treatment of intermediate AMD. This narrative review aimed to evaluate the available evidence on the effectiveness and safety of PBM in treating intermediate AMD.
Methods
A comprehensive search was conducted using the PubMed database, employing the keywords “photobiomodulation” and “age-related macular degeneration.” All English-language studies published up to June 2023 were reviewed, and the search was expanded to include relevant references from selected articles. The included publications were analyzed for this review.
Results
The available studies on PBM in AMD demonstrated promising but inconsistent results. PBM showed potential in improving best-corrected visual acuity (BCVA) and contrast sensitivity (CS) in patients with AMD. Some studies also suggested a reduction in AMD lesions, such as drusen volume. However, the long-term efficacy and optimal treatment parameters of PBM in AMD remained to be fully determined due to the limitations of the available studies. These included variations in irradiation techniques, wavelengths, exposure times, and treatment sessions, making it challenging to generalize the effectiveness of PBM. Furthermore, the lack of accurate classification of AMD phenotypes in the available studies hindered the understanding of which phenotypes could truly benefit from this treatment. Finally, the strength of evidence varied among studies, with limited sample sizes, unpublished results, and only three randomized sham-controlled trials.
Conclusions
Currently, the effectiveness of PBM in promoting drusen resorption or preventing progression to advanced forms of AMD, as observed in the cited studies, remains uncertain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intermediate age-related macular degeneration (AMD) represents the most crucial stage of age-related macular degeneration, since it is still possible to slow disease progression towards the most severe forms. |
Photobiomodulation (PBM) has recently gained attention as a potential treatment for intermediate AMD, diabetic macular edema, and pachychoroid diseases, mainly acting on mitochondrial metabolism. |
Preclinical and clinical studies have shown promising but inconsistent evidence in improving functional and anatomical parameters in intermediate AMD. Thus, their results should be interpreted with caution. |
Several limitations of existing studies make it difficult to ascertain the efficacy of PBM in slow progression of intermediate AMD. Further studies with larger sample size and longer follow-up are needed to define the real pathophysiological impact of PBM on AMD. |
Introduction
Age-related macular degeneration (AMD) is a chronic progressive macular disease causing an irreversible and profound vision loss in older people [1]. It is the most common cause of blindness in developed countries [2,3,4,5,6], affecting about 7–8% of the population worldwide [7]. The phenotype is classified into dry (not exudative) AMD and exudative AMD, with three described stages: early, intermediate, and late (neovascular, and geographic atrophy, GA) AMD. Intermediate AMD represents the most crucial stage, since it is still possible to slow disease progression to more severe forms [4]. This form is typically characterized by the presence of large drusen (> 125 μm) or medium drusen (> 63 μm) in addition to pigmentary abnormalities. The pathogenesis of AMD is complex and multifactorial. Intrinsic and extrinsic stress factors lead to a progressive accumulation of waste materials, including extracellular and intracellular deposits. The former is called drusen and consists of extracellular deposits of cellular debris, lipids, lipoproteins, and amyloid. Drusen contains a variety of pro-inflammatory factors which may stimulate inflammation through various pathways, such as complement cascade. Intracellular deposits are made of lipofuscin, a waste metabolite produced by the outer segment of photoreceptors that represents the main source of reactive oxygen species (ROS). With aging, there is an excess of ROS production that conditions an oxidative stress state, and thus further retinal damage and atrophy. Mitochondria are key regulators of inflammatory and oxidative signaling pathways, and the major intracellular source of ROS as well.
Current treatments are available for neovascular AMD (e.g., ranibizumab, bevacizumab, aflibercept, brolucizumab, and faricimab). However, no therapy for GA has yet been validated, except for pegcetacoplan. Unfortunately, therapeutical options for intermediate forms are limited to a healthy lifestyle and nutritional lutein/zeaxanthin supplements. The Age-Related Eye Disease Study (AREDS)2 revealed that patients with intermediate AMD who were taking these supplements experienced a decreased risk of developing advanced AMD as compared to the control group who did not receive these supplements [8].
Photobiomodulation (PBM), also known as low-level light therapy (LLLT), has recently gained attention as a potential treatment for intermediate AMD. The objective of this review was to analyze the evidence surrounding the efficacy of photobiomodulation as a treatment option for intermediate AMD.
Methods
A PubMed engine search was performed including the terms “photobiomodulation” and “age-related macular degeneration.” All studies published in English up to June 2023 were reviewed, and a bibliographic search was expanded to the bibliographies of selected articles. All pertinent publications were included. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Biochemical Basis of Retinal Photobiomodulation
The basic principle of photobiomodulation is that light can be absorbed by molecules. Irradiation of cells at specific wavelengths can activate native molecules, thus modulating biochemical reactions and cellular metabolism. Different wavelengths of light have different degrees of absorption, scattering, and reflection by biological tissues. For instance, water molecules significantly absorb light energy at wavelengths longer than 970 nm, whereas wavelengths shorter than 600 nm are absorbed by flavin, hemoglobin, and melanin. PBM therapy acts in an “optical window” ranging from the red to near-infrared (NIR) regions of the light spectrum (500–1000 nm spectrum) generated either by a laser or by non-coherent light sources. Light penetrates tissues depending on the wavelength and stimulates cellular function through the activation of specific pathways or molecules. After absorbing light, the molecule assumes an excited state, leading to a measurable biological effect [9]. The underlying molecular mechanisms of light–tissue interaction are very complex due to the intracellular chromophore components. A variety of interactions have been proposed to explain the therapeutic effects of PBM. One of the main targets of PBM is mitochondrial activity. Mitochondria are sensitive to irradiation with red–NIR light. In vitro experiments on murine mitochondria have shown that illumination increased adenosine triphosphate (ATP) synthesis and O2 consumption [10]. The main chromophore thought to be responsible for PBM-induced intracellular metabolic activity is cytochrome c oxidase (CCO) [11]. CCO synthesizes ATP in the mitochondrial electron transport chain, and acts as chromophore for the red–NIR light spectrum through a domain of iron and copper ions [9, 12]. PBM might increase the bioavailability of nitric oxide (NO), prompting its release from intracellular stores. It is proposed that PBM causes the photodissociation of NO from CCO [13]. Since NO is known to inhibit electron transport, PBM can increase the mitochondrial membrane potential, O2 consumption, proton gradient, and finally ATP production. NO can also diffuse outside, as a messenger for several pathways including vasodilation. PBM was shown to reduce oxidative stress [14,15,16,17] and inflammation [18,19,20,21] and to modulate cell signaling [9, 22,23,24,25] and gene expression [26,27,28].
Preclinical Animal Model Studies
Several preclinical animal models of ocular disorders support the aforementioned mechanisms of action of PBM. However, since most of these studies involved murine models, their results should be interpreted with caution. For instance, mice naturally tend to avoid light due to their lack of a central retina. Consequently, AMD must be artificially induced in these animals (e.g., by using light), which leads to an inflammatory retinal degeneration that simulates human AMD. The demonstrated effects of PBM include a reduction in aging-related damage [29], methanol toxicity [30], and inflammation [31]. Furthermore, PBM was demonstrated to be protective against bright light-induced retinal degeneration, even when NIR treatment was applied after exposure to light [32]. The efficacy of PBM was also demonstrated in mice after exposure five times to 670 nm light for 90 s. The treatment reduced the number of macrophages as well as inflammation biomarkers such as tumor necrosis factor alpha (TNF-α) and complement components [33]. Albarracin et al. demonstrated that PBM decreased vimentin and glial fibrillary acidic protein, which are Müller cell-specific markers for stress and inflammation [34]. PBM was shown to reduce inflammation in complement factor H knockout mice, which is another murine model of AMD [31]. These studies suggest that PBM may enhance recovery from retinal injury and other ocular diseases where mitochondria may play a role. Thus, PBM would slow the progression of AMD by inhibiting the complement system, reversing oxidative and inflammatory damage, and improving mitochondrial function.
Concluded and Ongoing Clinical Studies
The use of photobiomodulation in age-related macular degeneration has been evaluated in a small number of clinical studies (Table 1). The first evidence was reported in 2008 in all forms of AMD using a semiconductor laser diode emitting continuous light (Laser Components Germany GmbH, Germany; wavelength: 780 nm; dose: 5 mW/cm2; irradiation time: 40 s) [35]. A total of four treatments were given over 2 weeks. Best-corrected visual acuity (BCVA) improved significantly. A decrease in AMD signs (e.g., pigment accumulations, drusen, macular edema, retinal bleeding) and visual symptoms (e.g., metamorphopsia, scotoma, dyschromatopsia) were reported. No adverse systemic or local side effects were observed. The study highlighted the potential of PBM as a new treatment for both early and advanced forms of AMD, suggesting its role in preventing vision loss with no side effects. However, this study had some limitations, such as the inclusion of different stages of disease (no distinction between intermediate and late AMD) and the absence of any structural parameters.
The Toronto and Oak Ridge Photobiomodulation Study for Dry Age Related Macular Degeneration (TORPA) [36] investigated the impact of PBM in intermediate AMD with visual acuity between 20/20 and 20/200. Primary outcomes were change in BCVA, contrast sensitivity (CS), and microperimetry fixation stability. Two different light-emitting diode (LED) lasers were used for a direct-transpupillary irradiation: Quantum Warp 10 (Quantum Devices, Inc., USA; wavelength: 670 nm; dose: 50–80 mW/cm2; exposure time: 88 s) and GentleWaves (Light BioScience LLC, USA; wavelength: 590–790 nm; dose: 4–0.6 mW/cm2; irradiation time: 35 s). Eighteen eyes were treated sequentially by both devices three times per week for 6 weeks (total: 18 treatments). Statistically significant improvements in BCVA and CS were observed after 6 weeks and after 1 year from the treatment.
The TORPA II study [37] tested the reproducibility of the results of TORPA and explored the potential benefits of PBM in various stages of AMD. As inclusion criteria, AMD (AREDS 2–4) and BCVA letter score of 50 or better were considered. Forty-two eyes of 24 patients were treated sequentially by two LED devices with multiple wavelengths during nine sessions over a 3-week period. Primary efficacy endpoints were changes in BCVA and CS from baseline. Secondary efficacy endpoints included changes in optical coherence tomography (OCT) (e.g., drusen volume, DV, central drusen thickness, CDT, central retinal thickness, GA area) and fundus autofluorescence parameters at baseline, after the first 3-week treatment, and at 3 months. Significant improvement in BCVA was observed after PBM treatment (+5.9 letters) and at the 3-month follow-up. Among patients, 59.5% gained more than one line and 11.9% gained more than two lines after the 3-week treatment. Interestingly, visual improvement was related to baseline BCVA, since acuity of 70–82 letters was most commonly associated with a gain of five letters or 15 letters. Contrast sensitivity increased as well. DV and CDT were reduced at the initial 3-week follow-up and at 3 months. No significant variations in central retinal thickness or GA area were observed. Overall, this study suggested that PBM may be a therapeutic option for patients affected by dry AMD, with both functional and anatomical beneficial effects. Despite its valuable contributions, however, the TORPA II study showed certain limitations that should be considered. The sample size was relatively small, and a control group was not included. Furthermore, this study focused primarily on short-term outcomes, with no long-term follow-up data.
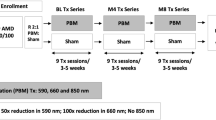
LIGHTSITE I [38] was the first single-center randomized sham-controlled study to evaluate PBM efficacy in AMD. This trial used the Valeda Light Delivery System (LumiThera, Inc., USA), which delivers three distinct wavelengths of light, including yellow (590 nm), red (660 nm), and near-infrared (850 nm). The treatment is divided into four phases lasting 250 s. In the first and third phases (35 s), the patient’s eyes are opened, and the yellow and near-infrared wavelengths are delivered in a pulsed mode. In the second and fourth phases (90 s), the patient’s eyes are closed, and the red wavelength is delivered continuously. Subjects received two PBM treatment series (at baseline and after 6 months), with nine treatment sessions per series spread over 3 or 4 weeks. Forty-six eyes of 30 individuals affected by dry AMD (AREDS grades 2–4) and visual acuity between 20/40 and 20/200 were enrolled. Subjects were assessed for BCVA, CS, quality of life, and retinal sensitivity by means of microperimetry, OCT (DV, CDT, GA area, retinal volume) and fundus autofluorescence. Treated subjects showed an increase in BCVA (from 73.8 ± 1.9 letters to 77.7 ± 2.5 letters) that declined around month 6 (76.1 ± 2.3 letters) just before the second series of treatments, which conferred a new visual gain (78 ± 2.4 letters) that decreased again after 6 months (74.2 ± 2.6 letters).
A greater response was observed in patients with drusen or GA with foveola sparing. CS improved significantly in PBM-treated eyes. No significant variations were observed in fixation stability at microperimetry between the two groups. Over time, most PBM-treated patients (70%) exhibited a decrease in DV that was statistically significant at month 12 in comparison to the control group. No significant differences in other anatomical parameters were observed between the two groups after 12 months of treatment. In summary, the key finding was the significant reduction in DV in the PBM-treated group as compared to the sham treatment, with no significant variations in other anatomical parameters.
The LIGHTSITE II [39] study further explored the effectiveness and safety of PBM therapy using the Valeda system (LumiThera, Inc., USA) in individuals with intermediate AMD (mainly AREDS 3) and BCVA between 20/32 and 20/100. A total of 44 subjects and 53 eyes were included in this randomized sham-controlled multicenter study. The primary outcome was the change in BCVA from baseline to month 9. PBM-treated patients showed a significant visual improvement (2.30 letters) as compared to baseline, although no significant differences between the groups were reported. Secondary outcomes related to visual function (CS, visual function questionnaire) and anatomical changes (DV, CDT, GA area) were also assessed. No significant changes in secondary outcome parameters were observed. Interestingly, GA lesions grew over time in both groups, with a slower growth rate in the PBM-treated group, suggesting a potential benefit of PBM treatment in reducing anatomical progression. However, this trial showed several limitations that should be taken into consideration. Because of its small sample size, its results may not be representative of the overall population. Moreover, the COVID-19 pandemic significant affected patient withdrawal from the study and missing data collection. Finally, four patients left the study due to adverse events unrelated to the treatment.
LIGHTSITE III (NCT04065490) partly overcame the limitations of the previous LIGHTSITE trials by enrolling a larger sample size with a longer follow-up and novel structural and functional parameters. This prospective randomized sham-controlled multicenter study included 148 eyes of 100 patients with foveal-sparing dry AMD, mainly at intermediate state, and vision between 20/32 and 20/100. Over a 24-month period, patients received six series of PBM or sham treatments using the Valeda system (LumiThera, Inc., USA), administered three times per week for 3–4 weeks. As clinical outcomes, BCVA, low-luminance BCVA, CS, reading speed, color vision, Visual Function Questionnaire-25 (VFQ-25), and perimetry were evaluated. Independent analysis of OCT, fundus autofluorescence, and color fundus imaging was performed by a reading center with masked evaluators at selected time intervals. Preliminary results at 13 months showed a significant improvement in BCVA (5.5 letters) in the group receiving PBM compared to the sham. Within the treated group, 55% exhibited a greater than 5-letter improvement (9.7 letters), and another 26% achieved an improvement of greater than 10 letters (12.8 letters). Among the control eyes, 9.1% progressed to new GA, in comparison to 1.1% in the photobiomodulation-treated group. Conversely, the conversion rate from non-exudative to exudative AMD was higher for the PBM group (5.4% vs. 1.8% for the control group). The results at 24 months are yet to be published.
The ELECTROLIGHT study (https://clinicaltrials.gov/ct2/show/NCT04522999) was another pilot study based on the Valeda Light Delivery System (LumiThera, Inc., USA). It aimed to evaluate the impact of PBM by measuring electroretinograms in 23 eyes of 15 patients with intermediate AMD and with BCVA between 20/32 and 20/100. As of now, the results of this study are still pending.
Grewal et al. [40] published the results of a pilot study based on a custom LED system (custom device; wavelength: red light ranging from 650 to 700 nm; dose: 40 mW/cm2; irradiation time: 120 s). Visual function was measured in terms of BCVA, low-luminance BCVA, scotopic thresholds, and rod intercept time. PBM had no significant impact on any of the parameters over a period of 12 months. This is the first study in discordance with the other published studies. However, the ELECTROLIGHT study displayed several differences from the previous literature, including the lack of a control arm, younger inclusion age, use of a single wavelength, longer exposure, and a higher dose.
Main Limitations of Previous and Ongoing Clinical Studies
The aforementioned studies showed several limitations. First, PBM was performed using different techniques with several wavelengths and exposure times. Additionally, the time interval between different treatment sessions and the number of sessions varied across studies. Therefore, the results of each study cannot be extrapolated to PBM in general, but only to the specific type of treatment used. Secondly, all studies lacked accurate classification of AMD phenotype, thus making it impossible to determine which AMD phenotypes could truly benefit from this treatment. Thirdly, the strength of the proposed evidence was not the same for all studies, since not all trials were randomized, placebo-controlled, and with a large sample size. Finally, although all studies investigated almost the same functional and structural parameters, their results varied due to different baseline conditions. Interestingly, the evidence of a decrease in DV could be regarded as “weak,” given that their resorption could potentially be attributed to the normal cycle of deposition and resorption of drusen material, as observed even in untreated patients. Thus, a larger sample and more precise measurements are needed to ascertain whether PBM promotes a significant reduction in DV. Moreover, even if the resorption of drusen is often seen as a positive outcome, it is important to note that there is no guarantee of its benefits over longer periods than trial follow-up. In fact, the process of drusen resorption can potentially give rise to the development of nascent GA and advanced macular degeneration. Further research is necessary to understand the mechanisms involved in drusen resorption and to determine whether it truly benefits retinal health in the long term.
Photobiomodulation in Other Macular Diseases
Photobiomodulation in Diabetic Macular Edema
Given that some preclinical [41, 42] and pilot studies [43] confirmed the impact of PBM on diabetic retinopathy and center-involved diabetic macular edema (DME), the Protocol AE trial [41] evaluated the efficacy of home-based PBM in treating DME in patients with type 1 and type 2 diabetes. A total of 134 eyes mainly from patients with type 2 diabetes (93%) were randomly assigned to twice daily use for 90 s of either a PBM device (Retilux Eye Patch, PhotoOpTx, USA) that emitted red light (670 nm) or a placebo device that emitted low-power white light. The study had two phases: a primary outcome phase (4 months) and a post-outcome phase (4 months). The primary outcome measure was the change in central subfoveal thickness (CST) on OCT from baseline to 4 months. Secondary outcomes included variations in retinal volume on OCT, the percentage of DME at 4 months, and the percentage of 5-letter loss in BCVA from baseline to 4 months. No statistically significant changes in any of these parameters were found between the PBM and placebo groups at 4 months. Thus, Protocol AE did not support the effectiveness of PBM in treating DME.
Photobiomodulation in Pachychoroid Disorders
There is still a lack of evidence on PBM in pachychoroid disorders, although the study conducted by Servillo et al. on no-dose photodynamic therapy (PDT) served as the closest reference point. This treatment consisted of PDT without the infusion of verteporfin, which is commonly used in conventional PDT. The authors suggested that no-dose PDT may involve a mechanism of thermally enhanced PBM, similar to that of photobiomodulation in AMD. The benefits of no-dose PDT included its affordability, patient comfort, and the widespread availability of PDT laser systems. Promising results were found in terms of functional and morphological outcomes in patients treated with no-dose PDT, suggesting PBM as a potential future therapy for pachychoroid diseases as well. Since this study showed some limitations (e.g., retrospective design, small sample size, and short follow-up duration), further studies are needed to confirm the efficacy of PBM in pachychoroid disorders.
Conclusions and Future Perspectives
A thorough investigation of the potential of PBM to halt the progression of intermediate AMD is warranted, given both the biochemical basis for the treatment's effectiveness and the encouraging preliminary findings. Although regulatory agencies have already approved several devices to treat intermediate AMD, there are lingering questions regarding the true effectiveness of this treatment. Additional randomized controlled trials are needed with larger sample sizes and long-term follow-up. These studies would provide a more reliable assessment of the feasibility of PBM and the optimal parameters for its application. Finally, the identification of new functional imaging techniques capable of visualizing the tissue effects of PBM would be helpful. For instance, no current trial has specifically explored the alterations in autofluorescence of retinal fluorophores targeted by PBM. Consequently, the activation of specific cellular processes remains largely speculative. The introduction of new imaging techniques would enable a more comprehensive evaluation of the mechanisms of action of PBM, including the underlying biochemical pathways. By bridging this knowledge gap, we can further advance our understanding of the potential of PBM and pave the way for more effective treatments for AMD.
Data Availability Statement
There are no datasets generated or analyzed in this study; therefore, data sharing is not applicable to this article.
References
Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59:74–7.
Klein R, Klein BE, Cruickshanks KJ. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog Retin Eye Res. 1999;18:371–89. https://doi.org/10.1016/s1350-9462(98)00025-1.
Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains eye study. Ophthalmology. 1995;102:1450–60. https://doi.org/10.1016/s0161-6420(95)30846-9.
Klaver CC, Assink JJ, van Leeuwen R, Wolfs RC, Vingerling JR, Stijnen T, Hofman A, de Jong PT. Incidence and progression rates of age-related maculopathy: the Rotterdam study. Invest Ophthalmol Vis Sci. 2001;42:2237–41.
Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, Mitchell P, Wong TY. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117:921–7. https://doi.org/10.1016/j.ophtha.2009.10.007.
Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26. https://doi.org/10.1016/j.ophtha.2007.03.008.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106-116. https://doi.org/10.1016/S2214-109X(13)70145-1.
Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. https://doi.org/10.1001/jama.2013.4997.
Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–17. https://doi.org/10.1016/S1011-1344(98)00219-X.
Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–9. https://doi.org/10.1016/0014-5793(84)80577-3.
Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B. 1995;27:219–23. https://doi.org/10.1016/1011-1344(94)07078-3.
Glass GE. Photobiomodulation: a review of the molecular evidence for low level light therapy. J Plast Reconstr Aesthet Surg. 2021;74:1050–60. https://doi.org/10.1016/j.bjps.2020.12.059.
Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81:98–106. https://doi.org/10.1016/j.jphotobiol.2005.07.002.
Lan CC, Ho PY, Wu CS, Yang RC, Yu HS. LED 590 nm photomodulation reduces UVA-induced metalloproteinase-1 expression via upregulation of antioxidant enzyme catalase. J Dermatol Sci. 2015;78:125–32. https://doi.org/10.1016/j.jdermsci.2015.02.018.
Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Damasceno Maia F, do Nascimento NR, Driusso P, Parizotto NA. Low-level laser therapy (904nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96–102. https://doi.org/10.1016/j.jphotobiol.2016.09.017.
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37:293–300. https://doi.org/10.1002/lsm.20225.
Dos Santos SS, de Oliveira HA, Antonio EL, Teixeira ILA, Mansano B, Silva FA, de Carvalho PTC, Tucci PJF, Serra AJ. Low-level laser therapy prevents muscle oxidative stress in rats subjected to high-intensity resistance exercise in a dose-dependent manner. Lasers Med Sci. 2020;35:1689–94. https://doi.org/10.1007/s10103-020-02951-1.
Torres-Silva R, Lopes-Martins RA, Bjordal JM, Frigo L, Rahouadj R, Arnold G, Leal-Junior EC, Magdalou J, Pallotta R, Marcos RL. The low level laser therapy (LLLT) operating in 660 nm reduce gene expression of inflammatory mediators in the experimental model of collagenase-induced rat tendinitis. Lasers Med Sci. 2015;30:1985–90. https://doi.org/10.1007/s10103-014-1676-3.
Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA Jr, Salgado MA, Aimbire F, Albertini R. Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med. 2010;42:553–8. https://doi.org/10.1002/lsm.20896.
Boschi ES, Leite CE, Saciura VC, Caberlon E, Lunardelli A, Bitencourt S, Melo DA, Oliveira JR. Anti-Inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg Med. 2008;40:500–8. https://doi.org/10.1002/lsm.20658.
Aimbire F, Albertini R, Pacheco MT, Castro-Faria-Neto HC, Leonardo PS, Iversen VV, Lopes-Martins RA, Bjordal JM. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed Laser Surg. 2006;24:33–7. https://doi.org/10.1089/pho.2006.24.33.
Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36:307–14. https://doi.org/10.1002/lsm.20148.
Rizzi CF, Mauriz JL, Freitas Correa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, Gonzalez-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38:704–13. https://doi.org/10.1002/lsm.20371.
Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 2003;120:849–57. https://doi.org/10.1046/j.1523-1747.2003.12133.x.
Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16:4. https://doi.org/10.1186/1423-0127-16-4.
Wu YH, Wang J, Gong DX, Gu HY, Hu SS, Zhang H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: a microarray analysis. Lasers Med Sci. 2012;27:509–19. https://doi.org/10.1007/s10103-011-0995-x.
Martignago CC, Oliveira RF, Pires-Oliveira DA, Oliveira PD, Pacheco Soares C, Monzani PS, Poli-Frederico RC. Effect of low-level laser therapy on the gene expression of collagen and vascular endothelial growth factor in a culture of fibroblast cells in mice. Lasers Med Sci. 2015;30:203–8. https://doi.org/10.1007/s10103-014-1644-y.
McDaniel DH, Weiss RA, Geronemus RG, Mazur C, Wilson S, Weiss MA. Varying ratios of wavelengths in dual wavelength LED photomodulation alters gene expression profiles in human skin fibroblasts. Lasers Surg Med. 2010;42:540–5. https://doi.org/10.1002/lsm.20947.
Gkotsi D, Begum R, Salt T, Lascaratos G, Hogg C, Chau KY, Schapira AH, Jeffery G. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp Eye Res. 2014;122:50–3. https://doi.org/10.1016/j.exer.2014.02.023.
Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–44. https://doi.org/10.1073/pnas.0534746100.
Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS ONE. 2013;8: e57828. https://doi.org/10.1371/journal.pone.0057828.
Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52:3582–92. https://doi.org/10.1167/iovs.10-6664.
Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol Aging. 2013;34:602–9. https://doi.org/10.1016/j.neurobiolaging.2012.04.014.
Albarracin R, Valter K. 670 nm red light preconditioning supports Muller cell function: evidence from the white light-induced damage model in the rat retina. Photochem Photobiol. 2012;88:1418–27. https://doi.org/10.1111/j.1751-1097.2012.01130.x.
Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26:241–5. https://doi.org/10.1089/pho.2007.2132.
Merry G, Dotson R, Devenyi R, Markowitz S, Reyes S. Photobiomodulation as a new treatment for dry age related macular degeneration. Results from the Toronto and Oak Ridge Photobimodulation Study in AMD (TORPA). Invest Ophthalmol Vis Sci. 2012;53:2049–2049.
Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017;95:e270–7. https://doi.org/10.1111/aos.13354.
Markowitz SN, Devenyi RG, Munk MR, Croissant CL, Tedford SE, Ruckert R, Walker MG, Patino BE, Chen L, Nido M, Tedford CE. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40:1471–82. https://doi.org/10.1097/IAE.0000000000002632.
Burton B, Parodi MB, Jurgens I, Zanlonghi X, Hornan D, Roider J, Lorenz K, Munk MR, Croissant CL, Tedford SE, Walker M, Ruckert R, Tedford CE. LIGHTSITE II randomized multicenter trial: evaluation of multiwavelength photobiomodulation in non-exudative age-related macular degeneration. Ophthalmol Ther. 2023;12:953–68. https://doi.org/10.1007/s40123-022-00640-6.
Grewal MK, Sivapathasuntharam C, Chandra S, Gurudas S, Chong V, Bird A, Jeffery G, Sivaprasad S. A pilot study evaluating the effects of 670 nm photobiomodulation in healthy ageing and age-related macular degeneration. J Clin Med. 2020. https://doi.org/10.3390/jcm9041001.
Kim JE, Glassman AR, Josic K, Melia M, Aiello LP, Baker C, Eells JT, Jampol LM, Kern TS, Marcus D, Salehi-Had H, Shah SN, Martin DF, Stockdale CR, Sun JK, Network DR. A randomized trial of photobiomodulation therapy for center-involved diabetic macular edema with good visual acuity (Protocol AE). Ophthalmol Retina. 2022;6:298–307. https://doi.org/10.1016/j.oret.2021.10.003.
Cheng Y, Du Y, Liu H, Tang J, Veenstra A, Kern TS. Photobiomodulation inhibits long-term structural and functional lesions of diabetic retinopathy. Diabetes. 2018;67:291–8. https://doi.org/10.2337/db17-0803.
Tang J, Herda AA, Kern TS. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol. 2014;98:1013–5. https://doi.org/10.1136/bjophthalmol-2013-304477.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Federico Fantaguzzi: conception, design supervision, data collection and/or processing, analysis and/or interpretation, literature review, writer; Beatrice Tombolini: analysis and/or interpretation, literature review, writer, critical review; Andrea Servillo: analysis and/or interpretation, literature review, writer, critical review; Ilaria Zucchiatti: analysis and/or interpretation, literature review, writer, critical review; Riccardo Sacconi: analysis and/or interpretation, literature review, writer, critical review; Francesco Bandello: analysis and/or interpretation, literature review, critical review; Giuseppe Querques: analysis and/or interpretation, literature review, critical review.
Corresponding author
Ethics declarations
Conflict of Interest
Federico Fantaguzzi, MD: has nothing to disclose; Beatrice Tombolini, MD: has nothing to disclose; Andrea Servillo, MD: has nothing to disclose; Ilaria Zucchiatti MD has nothing to disclose; Riccardo Sacconi: is a consultant for: Allergan Inc (Irvine, California,USA), Bayer Shering-Pharma (Berlin, Germany), Carl Zeiss Meditec (Dublin, USA), Novartis (Basel, Switzerland). Francesco Bandello is a consultant for Alcon (Fort Worth,Texas,USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee,Belgium), Zeiss (Dublin, USA). Giuseppe Querques is a consultant for Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California,USA), Amgen (Thousand Oaks, USA), Heidelberg (Germany), KBH (Chengdu, China), LEH Pharma (London, UK), Lumithera (Poulsbo, USA), Novartis (Basel, Switzerland), Bayer Shering-Pharma (Berlin, Germany), Sandoz (Berlin, Germany), Sifi (Catania, Italy), Soof-Fidia (Albano, Italy), Zeiss (Dublin, USA).
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fantaguzzi, F., Tombolini, B., Servillo, A. et al. Shedding Light on Photobiomodulation Therapy for Age-Related Macular Degeneration: A Narrative Review. Ophthalmol Ther 12, 2903–2915 (2023). https://doi.org/10.1007/s40123-023-00812-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00812-y