Abstract
Yellow subthreshold micropulse laser (YSML) is a retinal laser capable of inducing a biologic response without causing thermal damage to the targeted tissue. The 577-nm YSML is delivered to the retina abiding by different protocols in which wavelength, power, duration, spot size and number of spots can be properly set to achieve the most effective and safe treatment response in various chorioretinal disorders. The ultrashort trains of power modulate the activation of the retinal pigment epithelium cells and intraretinal cells, such as Müller cells, causing no visible retinal scars. Subthreshold energy delivered by YSML stimulates the production of the heat-shock proteins, highly conserved molecules that protect cells against any sort of stress by blocking apoptotic and inflammatory pathways that cause cell damage. YSML treatment allows resorption of the subretinal fluid in central serous chorioretinopathy and intraretinal fluid in various conditions including diabetic macular edema, postoperative cystoid macular edema and other miscellaneous conditions. YSML also seems to modulate the development and progression of reticular pseudodrusen in dry age-related macular degeneration. The aim of this review is to discuss and summarize the safety and efficacy of YSML treatment in retinal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conventional laser photocoagulation delivers continuous waves of energy, which gets dissipated and absorbed as heat within the target tissue and produces visible burns associated with retinal scars and scotomas. | |
Over the past 30 years, there has been ongoing development and increasing interest in looking for an effective and safer alternative to continuous wave laser. | |
Subthreshold laser, which adopts several wavelengths, has been conceived to preserve retinal pigment epithelium from laser burns while effectively targeting the underlying disease by regulating heat-shock proteins and cytokine expression within the tissue. | |
Yellow subthreshold laser treatment appears to be a safe and effective therapeutic option for several diseases, including diabetic macular edema, central serous chorioretinopathy and other miscellaneous conditions. | |
A more accurate standardization of yellow subthreshold laser setting protocols is still desired, and further randomized, prospective studies with longer follow-up are warranted to confirm the role in chorioretinal diseases. |
Introduction
Conventional threshold laser photocoagulation, first developed between 1950 and the 1970s, became a valuable treatment option for several retinal diseases over the last decades [1]. Its exact mechanism of action remains unknown, but several studies in animal models have demonstrated a local rise in preretinal and intraretinal oxygen tensions within areas overlying the photocoagulation spots [2, 3]. The energy delivered by laser treatment affects ocular tissues depending on wavelength, impulse duration, power and effective tissue energy absorption.
Conventional laser photocoagulation delivers energy to the target area as continuous waves (CW) throughout the entire pulse duration. Light energy gets dissipated as heat, and pigmented tissue absorbs the energy wavelengths. Heat absorption leads unequivocally to a rise in tissue temperature, leading often to collateral damage to the neighboring areas [2, 3]. In the past decades, coagulative necrosis and visible grayish/whitish burns within the target tissue were considered necessary to achieve successful treatment results [4]. Unfortunately, this was often associated with development of retinal scars, laser-related scotomas and complications such as subretinal neovascular membranes and subretinal fibrosis [5].
For these reasons, over the past 30 years, there have been ongoing development and increasing interest in looking for an effective and safer alternative to CW laser known as subthreshold laser therapy. This procedure provides therapeutic effects for several chorioretinal diseases while avoiding the typical laser-related damaging effects [1, 6].
Several subthreshold laser wavelengths have been adopted, ranging from green at 532 nm, yellow at 577 nm and 810 nm at near-infrared spectrum ranges [1, 6, 7]. This review aims to specifically analyze and summarize indications, efficacy and safety of the yellow subthreshold micropulse laser (YSML) treatment in retinal diseases.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods of Literature Search
We carried out a review of literature regarding the efficacy and safety of YSML in retinal diseases using PubMed and Embase database up to August 2022 with the following terms: conventional laser, laser treatment, micropulse laser, navigated laser, subthreshold laser, yellow subthreshold laser and combination of the terms. All relevant publications written in English were sourced, including prospective and retrospective clinical studies and laboratory experimental studies. We included case reports only if they contributed new and relevant information about efficacy and safety of YSML.
YSML Application Strategy
In YSML, the standard wave of energy is chopped into series of repetitive micropulses (ON-time) that persist between 0.1 and 0.5 s and are separated by relatively long off-times. This allows for heat dissipation and reduces the typical threshold laser side effects.
The ratio between ON-time and total exposure time (ON + OFF) is called the duty cycle (DC) and represents the effective laser delivery time, which can be adjusted individually to achieve fine control and efficient spatial confining of photothermal effects [8].
The single spots on the retina are invisible, either ophthalmoscopically or using any current retinal multimodal imaging technique. Moreover, they are not characterized by any microperimetric retinal sensitivity reduction [8, 9].
Single-spot repetitive series of short duration micropulses, instead of a CW laser pulse, were demonstrated to deliver a total amount of energy insufficient to cause tissue damage [10].
Of note, substantial differences exist in protocols of subthreshold laser delivery on which wavelength, power, duration, spot size and number of spots can be set differently to achieve the most effective and safe treatment response. Moreover, Navilas® Laser System is an advanced focal/panretinal photocoagulation device revolutionizing the treatment of vision-threatening retinal diseases by integrating diagnostics with laser therapy and allowing pre-planned, computer-guided treatments.
Some physicians use a fixed power for laser applications, and some prefer different strategies based on the least invasive setting producing a visible lesion, defined as “threshold.” Duty cycle and power are then reduced accordingly to lower the applied energy to a “subthreshold” target [11]. Of course, if laser settings are too low, the treatment will be subtherapeutic, and in case settings are too high, there is a high risk of damaging the retinal pigment epithelium (RPE) and/or the neural retina.
From this perspective, EndPoint Management (EpM) protocol was thought to set a proper therapeutic window and titration procedure and to standardize subthreshold parameters. In the EpM algorithm, laser power is titrated to induce a barely visible spot, which allows defining the corresponding energy at 100%. It was demonstrated that no tissue damage could be detected below 30% of the EpM energy [12].
Potential YSML-Induced Biologic Mechanisms
Several lines of evidence claim that threshold burns may not be necessary to achieve photocoagulative therapeutic benefits [12].
From this perspective, subthreshold laser has been conceived to preserve RPE while effectively targeting the underlying disease by regulating heat-shock proteins (HSPs) and cytokine expression within the tissue [7].
HSPs are a group of ubiquitary, highly conserved molecules that can be triggered by a variety of stressful stimuli to protect cells against any sort of stress by blocking apoptotic and inflammatory pathways that cause cell damage [13]. Destructive suprathreshold energy is unnecessary to achieve biologic response and to maximize anti-inflammatory HSP release. In fact, it was recently shown that HSP expression begins when just above 20% EpM energy is released within the tissue [12, 14]. Furthermore, it was demonstrated that the still-viable cells induce a gene expression leading to a healing response to sublethal laser insults rather than the laser-killed cells [15, 16].
In addition, subthreshold energy stimulates cells to restore the blood-retinal barrier, which is now known to be regulated by the retinal glial cell population [17, 18]. It downregulates a series of local growth factors, inhibitors and permeability factors that were shown to be causative of underlying pathologic pathways elicited by retinal chronic hyperglycemia [17, 18].
Because of all the aforementioned characteristics, YSML allows treating lesions with large numbers of spots and offers the possibility to treat and retreat all retinal areas, including the fovea. Furthermore, it was shown to grant color vision preservation and maintain contrast sensitivity [19] compared to conventional photocoagulation [9].
Clinical Applications
YSML and Diabetic Macular Edema
Conventional laser treatment in diabetic macular edema (DME) is routinely performed according to the modified Early Treatment Diabetic Retinopathy Study (ETDRS) protocol [20]. It was demonstrated to be effective in reducing vision loss incidence, but at the same time it is a destructive treatment associated with tissue impairment and many side effects, including choroidal neovascularization, permanent photoreceptors loss, laser scars and subretinal fibrosis [21,22,23,24].
Beneficial therapeutic effects are believed to be promoted by the destructive burn of oxygen-consuming photoreceptors and retinal pigmented cells that, in turn, produce pro-angiogenic mediators [8].
This long-standing belief has been slowly controverted by a better understanding of the inducible changes in retinal gene expression even with “lighter” laser treatments and by growing evidence of papers showing promising morpho-functional outcomes with much more gentle, subvisible, micropulse treatment [25, 26].
Many patients with early DME display no symptoms at all and often have excellent visual acuity. Indeed, the risks of performing conventional photocoagulation in such eyes are objectionable and the same can apply to intravitreal drugs injections [27].
Currently, the treatment of clinical and subclinical DME, which comprises the largest number of diabetic patients with macular involvement, also includes YSML [28].
Safety and Efficacy of YSML
Most of the literature on safety and efficacy and YSML in DME is based on evaluation of best-corrected visual acuity (BCVA) and central macular thickness (CMT) outcomes after treatment. A summary of the data collected is reported in Table 1.
The mean follow-up of all the studies ranged between 2 and 16.6 months [29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Overall, a worsening of both BCVA and CMT was not observed in any of the cohorts evaluated [29,30,31,32,33,34,35,36,37,38,39,40,41,42]. In particular, a significant CMT decrease and BCVA improvement in two thirds were demonstrated [29,30,31,32,33,34,35,36,37] and in half of the studies [29, 30, 33, 36, 38, 40, 41], respectively, whereas a stabilization of these features was described in the remaining cases throughout the entire follow-up [31, 32, 34, 35, 37,38,39,40,41,42].
No visible retinal or choroidal lesions were described in any studies on either fundus examination or fundus imaging [fundus autofluorescence (FAF), fluorescein angiography (FA) or color fundus photograph]. On optical coherence tomography (OCT), no integrity or reflectivity changes of the outer retina (external limiting membrane, inner segment/outer segment junction, RPE) were described. No side effects were reported [29,30,31,32,33,34,35,36,37,38,39,40,41,42].
An interesting stratification of the data was done in two studies by Citirik et al., who allocated the study subjects into different groups according to their initial CMT: group 1 ranged between 250 and 300 μm, group 2 between 301 and 400 μm and group 3 had a baseline CMT > 401 μm [29, 30]. The results indicated that the anatomical severity of DME may affect the YSML outcome, with a statistically significant improvement of BCVA and CMT observed only in the group 1 patients with a baseline CMT < 300 μm. The cause of YSML lack of response in patients with severe anatomical disease is not known; however, it was speculated that severe edema could dilute and reduce the concentration of cytokines released by YSML-stimulated RPE cells that might be responsible for the beneficial effects of the treatment [29].
Another independent variable that was extensively found to affect the therapy outcome was the timing of response. Indeed, it was shown that the vast majority of patients with DME were characterized by a significant CMT shrinkage within the first 3 months of therapy and that from the 4th month onwards no further CMT reduction could be observed [43, 44] In other words, if no improvement is achieved within the first 4 months, waiting longer is unlikely to result in any significant beneficial YSML effect.
Kikushima et al. and Vujosevic et al. drew a comparison of morphologic and visual function safety parameters between YSML versus the subthreshold micropulse infrared (810 nm) laser, which is a widely adopted wavelength in published studies to test subthreshold laser efficacy [35, 45]. No significant differences were found in terms of either efficacy outcomes or safety profile between the two SLTs, suggesting the two lasers to be comparable in treating mild center involving DME [35, 45].
To date, there is great variability in the choice of laser power, titration, DC and pulse duration for DME eyes [31, 46]. A group of experts recently published the YSML consensus guideline settings for DME suggesting a DC of 5%, pulse duration 200 ms, spot size 150–200 μm with no spacing between spots and titration power of 50% of threshold power [11, 46].
Donati et al. evaluated efficacy and safety of morphologic and functional outcomes of diabetic patients affected by mild center involving DME treated with two different settings of yellow subthreshold laser: a fixed and a variable regimen delivered with the same DC (5%) [31]. The main considered outcomes were BCVA and CMT changes in both groups. Fixed regimen consisted of 100 μm spot size, 250 mW power and a variable number of confluent spots based on the center involving DME extension. Regarding the variable regimen, instead, micropulse laser power was selected starting with a 200-μm CW test burn in non-edematous areas outside the vascular arcades. The preferential starting power was 70 mW, slowly increased by 10–20 mW until a hardly visible burn was seen, at which point the laser was switched to micropulse mode multiplying the test burn power by 4 and keeping the spot size of 200 μm. Both YSML treatment regimens were found equally effective in terms of BCVA stabilization and center involving DME reduction [31].
To support the safety of YSML, Wells-Gray et al. performed an observational study investigating the integrity of individual cones after YSML treatment using high-resolution retinal imaging [47]. Cones that were evident before the treatment remained visible, whereas cones that were initially hidden by the DME became even more distinguishable after treatment. In addition, total retinal thickness displayed a statistically significant thinning in 50% of the patients, and no subject showed any sort of photoreceptor impairment after the therapy [47].
Optical coherence tomography angiography (OCTA) was also used to investigate parameters that could be potentially affected in DME patients after YSML treatment [41, 48]. The area of foveal avascular zone, number of microaneurysms (MAs), cyst area and presence of capillary network alterations were investigated within the superficial capillary plexus (SCP) and deep capillary plexus (DCP). The most peculiar finding of this study was the early decrease of MA number within the DCP, considered a hallmark of DR [49]. Leaking MAs are believed to be one of the main causes of DME development; therefore, their reduction within the DCP may ultimately lead to an inner nuclear layer (INL) thickness decrease and consequently DME shrinkage [50, 51].
Of note, YSML is not directly targeted to MAs, as it is instead the modified conventional ETDRS laser treatment, which determines MA clotting. Indeed, the RPE is considered the main site of action of YSML, but the exact mechanism leading to MA closure is still unknown [51].
Two relatively recent papers assessing retinal thickness changes after repeated YSML sessions in DR patients demonstrated an important INL thickness reduction as a result of Müller cell (MC) downregulation and return to normal size [40, 52].
Furthermore, Midena et al. demonstrated a marked reduction of diabetes-induced glial fibrillary acidic protein (GFAP), an important biomarker of MCs activity, following YSML [17]. The association of all these molecular findings suggests that YSML induces morpho-functional recovery of MCs with a substantial reduction of their inflammatory pathologic biomarkers [17, 52].
YSML Versus Intravitreal Injections
Anti-VEGF injections have emerged as the first-choice treatment for DME [53]. As widely demonstrated in clinical trials and real-life studies, protracted series of anti-VEGF injections are proven to efficiently manage DME [53]. However, the cost of recurrent injections and ophthalmologic check-ups imposes a significant economic burden on these patients and seriously hinders provision of optimal treatment [54].
Currently, in real-life routine practice, ophthalmologists may also consider other choices for DME management, including YSML.
Several studies in the literature have compared YSML with anti-VEGF therapy for DME. A summary of the data collected is reported in Table 2.
The mean follow-up of the studies shown ranged between 6 and 24 months [55,56,57,58,59,60,61,62,63,64]. Most of the studies reported indicated a common positive result for the usefulness of YSML, which appeared to have complimentary effects to the anti-VEGF therapy [55,56,57,58,59,60,61,62,63,64]. Their combined use was demonstrated, in fact, to significantly reduce the number of anti-VEGF injections while preserving or even improving morpho-functional outcomes. BCVA improvement and a CMT decrease were illustrated in two thirds [55, 56, 58,59,60,61, 63] and in half of the studies considered [55, 58,59,60,61], respectively, and a stabilization of these features was described in the remaining cases throughout the entire follow-up [56, 57, 62,63,64].
Of note, only two studies reported no significant differences within the cohorts treated with anti-VEGF therapy and anti-VEGF + YSML in terms of number of injections needed [62, 64].
All these studies highlighted the advantages of performing YSML in mild DME cases, including the easy administration, laser treatment management and lower costs of the procedure [55,56,57,58,59,60,61,62,63,64].
The efficacy of YSML over anti-VEGF therapy was also evaluated in terms of OCTA changes in a retrospective analysis carried out by Karasu et al. [65]. Data of 44 eyes of 44 patients with DME refractory to anti-VEGF were reported in a 6-month single-center follow-up study. A significant decrease (p < 0.05) occurred in the SCP, choriocapillaris and DCP, which caused a substantial decrease in vessel densities. In parallel, BCVA improved and CMT decreased significantly [65].
YSML Versus Conventional Laser
Laser photocoagulation was suggested as preferential therapy for DME after ETDRS, much before the anti-VEGF era [66]. Contrast sensitivity reduction, accidental foveal impairment, poor color vision and expansion of macular scars were common complications of laser photocoagulation, which led this procedure to take a backseat over the years [67].
The efficacy and safety of YSML were compared to conventional retinal laser photocoagulation by Chhablani et al. who conducted a 3-month prospective randomized study including 30 eyes of 20 patients who received either YSML or standard CW laser [67]. All patients underwent microperimetry, thickness measurements and visual acuity examinations. While both treatment arms achieved a stabilization of BCVA, the main differences between the two groups involved retinal volume and sensitivity. These two parameters were demonstrated to be heavily and negatively impaired by CW therapy, whereas positively preserved when treated with YSML [67].
Li et al. quantitatively investigated the combined effect of YSML with panretinal laser photocoagulation (PRP) on 86 eyes of 86 patients previously diagnosed with severe non-proliferative diabetic retinopathy (NPDR) with a center involving DME [68]. Several OCTA parameters, including foveal avascular zone (FAZ), capillary density (CD), CMT, choriocapillary flow area (ChF) and BCVA, were evaluated during a 6-month observational retrospective study. Overall, BCVA remained stable throughout the entire follow-up. CMT, macular edema, blood flow and capillary density were characterized by a decreasing trend, whereas FAZ tended to increase during the 6-month period [68].
A limitation of these studies includes the relatively small sample sizes and short-term follow-ups unable to highlight the effects of DME recurrences. In addition, visual function examinations such as visual field test were not performed. From this perspective, further prospective studies with more patients would help to better understand the compared effect of YSML with conventional laser.
YSML After Pars Plana Vitrectomy
Application of YSML in patients who previously underwent pars plana vitrectomy (PPV) for tractional DME was explored by Bonfiglio et al. [69]. They reported data of a consecutive comparative prospective study on 95 eyes of 95 patients in which 54 eyes were treated 6 months after PPV with YSML and 41 eyes were assigned to the control group only for observation. In the treatment group, mean BCVA increased and CMT decreased in parallel more significantly than in the control group. In addition, vessel densities evaluated with OCTA in the SCP and DCP were substantially higher and FAZ significantly smaller in the YSML group. No adverse effects were described in YSML patients [69].
YSML and Central Serous Chorioretinopathy
Several treatment strategies have been proposed for central serous chorioretinopathy (CSC) including observation, diuretics, anti-VEGF intravitreal injections, photodynamic therapy (PDT) and different kind of laser treatments [70]. The goals of an ideal treatment should consider the subretinal fluid (SRF) resolution, vascular permeability alteration restoration and photoreceptor and RPE cell recovery [71, 72].
Safety and Efficacy of YSML
To assess the efficacy of YSML in chronic CSC, special focus was placed on the SRF and CMT reduction and BCVA variation after treatment. A summary of the data collected is reported in Table 3.
Overall, a BCVA improvement was reported [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91], except for one study displaying a visual acuity stabilization [92].
All the studies that explored a SRF variation showed a reduction of the fluid [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]. No visible retinal or choroidal lesions were described in any study, and no side effects were reported [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]. Wood et al. hypothesized that, since laser energy of visible wavelengths is mainly absorbed by the RPE, the efficacy of YSML could be related to RPE cell vitality and trophism [9].
Chen et al. further subcategorized the cohort of patients based on three phenotypes classified according to FA: (1) focal point of iuxtafoveal leakage with no RPE atrophy, (2) focal point of iuxtafoveal leakage with RPE atrophy and (3) diffuse RPE impairment with indistinct source of iuxtafoveal leakage. They reported an increasing trend of fluid reabsorption and decreasing SRF recurrence in patients with limited RPE atrophy and focal leakage [93].
Other anatomic features have been investigated as predictors of higher YSML effectiveness.
In particular, Kiraly et al. demonstrated the amount of SRF to be an important biomarker in predicting treatment response by observing worse outcomes in cases with greater SRF [82]. They also investigated the role of pigment epithelium detachments (PEDs), concluding that wider PEDs correlated with poor response to YSML [82].
Several OCT parameters were demonstrated to influence the YSML response.
Altinel et al. observed 39 eyes of 39 patients for 24 months, focusing on the ellipsoid zone (EZ) band integrity and analyzing its correlation with YSML success status [94]. The eyes were allocated into three groups: complete remission, partial remission and failure. Baseline EZ was found significantly intact in 71.4% of eyes in the complete remission group, and these rates were progressively inferior in the partial remission and failure groups, with 38.5% and 25%, respectively [94].
Hyperreflective dots were also found in all the retinal layers, and subretinal fibrinous exudates were seen more commonly in the failure group than in the complete remission group (p > 0.05) [94]. These findings suggest that hyperreflective dots may help predict the need for early YSML treatment.
There is only one report in the literature showing the use of 577-nm YSML to successfully treat a patient with chronic CSC with subretinal fibrin deposition with complete subretinal fluid and fibrin reabsorption and no visible retinal damage [95].
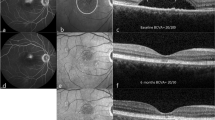
A representative case of chronic CSC successfully treated with YSML is shown in Fig. 1.
Multimodal imaging of a 41-year-old patient with an 11-month history of chronic central serous chorioretinopathy (cCSC) treated with yellow subthreshold micropulse laser (YSML). A–B Baseline color fundus photography (A) and red-free light picture (B) show neurosensory retinal detachment in the macular area. C–D Early and late phase fluoresceine angiography displays the characteristic ink-blot pattern with a single point leakage that gradually increases in size over time. The area of leakage (red circle, D) is the target of YSML. E–G Tracked spectral domain optical coherence tomography scans with corresponding near infrared images acquired with enhanced depth imaging mode at baseline (E) and at 1 month (F) and 6 months (G) after YSML show resolution of the subretinal fluid over time
YSML Versus Photodynamic Therapy
The PLACE Trial confirmed photodynamic therapy (PDT) to be superior to high-density subthreshold laser (HDSL) treatment for chronic CSC in terms of patients’ percentage with complete SRF resorption, BCVA increase and higher mean retinal sensitivity increase on microperimetry [96]. HDSL treatment protocol consisted of a DC of 5%, spot size of 125 µm, power starting from 1800 mW with lowering of 300 mW if any retinal discoloration was visible and pulse duration of 200 ms. PDT consisted of half-dose verteporfin (3 mg/m2) infused over 10 min followed by laser activation for 83 s [97].
Considering that PDT is an invasive procedure that can rarely carry risks of collateral retinal damage, several authors suggest YSML as a competitive alternative for chronic CSC [75, 97]. Moreover, in the last 2 years there has been a verteporfin shortage worldwide that still represents a problem in many countries [98].
PDT and YSML have different mechanisms of action. While YSML targets the RPE, the treatment effect of PDT is believed to be the result of remodeling of the choroidal vascular endothelium through the formation of free radicals after photoactivation, resulting in occlusion, thrombosis and choroidal hypoperfusion in the treated areas [99].
Roca et al. treated leakage sites identified by FA and/or ICGA in a cohort of 92 eyes followed up for 12 months with either YSML or PDT. The first consisted of a DC of 5%, spot sizes from 100 to 200 µm, power from 320 to 660 mW and pulse duration of 200 ms; the latter consisted in half-dose verteporfin (3 mg/m2) infused over 10 min, followed by laser activation for 83 s. The authors described a significant CMT decrease from baseline in both YSML and PDT groups [100]. BCVA paralleled this improvement only in patients treated with YSML with a gain of ≥ 3 lines. Contrarily, in the PDT group after the 12-month follow-up, only 19% of eyes had such a visual acuity increase, with the vast majority (73%) recuperating no more that two lines from baseline BCVA. Notably, no adverse events attributable to the YSML were observed, whereas one eye within the half-dose PDT group developed choroidal neovascularization 4 weeks after the treatment and was treated with three intravitreal bevacizumab injections [100].
Ntomoka et al. retrospectively assessed 45 eyes of 39 patients who underwent either one of the two treatments with a minimum follow-up of 6 months [101]. PDT was performed over areas of choroidal hyper-permeability on ICGA by injecting a normal dose of verteporfin infused over 8 min followed by laser activation for 83 s. YSML was carried out in confluent spots directed to areas of focal leakage in the earliest phase of FA with a DC of 5%, spot size of 100 µm, pulse duration of 200 ms and 30% threshold power. The group treated with YSML demonstrated a significantly greater increase in BCVA compared to PDT [101]. The trend was confirmed anatomically, with a CMT decrease significantly higher in the YSML group as well. In addition, 13 (59%) eyes treated with subthreshold laser showed complete SRF reabsorption compared to only 5 (21%) in the PDT cohort [101].
In a study with a long-term follow-up, Scholz et al. made a comparison by retrospectively analyzing 100 patients with a mean follow-up of 2.6 years (SD ± 3.3), of which 42 received YSML while the rest were treated with PDT [102]. Hyperfluorescent areas on mid-phase ICGA and the corresponding “hot spots” on mid-phase FA were treated. YSML treatment protocol consisted of a DC of 5%, spot size of 160 µm, pulse duration of 200 ms, 50% threshold power and PDT consisting of half-dose verteporfin (3 mg/m2) infused over 10 min followed by laser activation for 83 s. Results observed showed similar anatomic outcomes with comparable CMT decrease (p < 0.05) and SRF resorption (p > 0.05) within the two treatment arms; BCVA, instead, improved more in the YSML group (p > 0.05). They noted a significant difference between the two groups in treatment response regarding the duration, more or less than 1 year, of the disease. Indeed, a significant higher number of patients with a disease duration < 1 year showed treatment response to YSML (92% vs. 58%). Another OCT variable demonstrated to have an impact on both treatment responses was central retinal thickness (CRT): non-responders showed a statistically significant lower CRT at baseline compared to responders (337 ± 81 μm vs. 442 ± 131 μm). Regarding safety, only the PDT group displayed side effects: one patient developed CNV, and one patient suffered from a moderate allergic reaction to verteporfin with tachycardia, dyspnea, flushing and hypotension [102].
Altinel et al. retrospectively compared 52 eyes of 46 patients over 8.42 (± 3.34 SD) months [92]. They found that the YSML group was characterized by longer SRF resolution duration and a slower trend of BCVA improvement compared to the fellow group. EZ band integrity was explored as well, with higher SRF resolution rates observed in both treatment arms when EZ was intact [92].
Ozmert et al. retrospectively evaluated 33 eyes of 30 patients during a 12-month follow-up [103]. Their results reached similar findings: mean BCVA (p > 0.05), CMT and SRF (p < 0.05) outcomes improved significantly with YSML treatment, consisting of a DC of 5%, spot size of 160 µm, pulse duration of 200 ms and 50% threshold power [103].
Ho et al. investigated alterations in choriocapillaris blood flow with OCTA and choroidal volume with en-face OCT [104]. Eighteen patients were randomized into YSML and PDT groups. YSML was set with a DC of 5%, spot size of 200 µm, pulse duration of 200 ms and power of 340–400 mW, whereas PDT was performed with half-dose verteporfin (3 mg/m2) infused over 10 min followed by laser activation for 83 s. Results were extremely positive: flow deficit areas with suspected choriocapillaris hypoperfusion were found in all CSC cases at baseline, and a progressive reduction of such areas was found in both YSML and PDT groups (p < 0.05), with the latter showing better results. Mean choroidal volume decreased as well at all time points (1, 3 and 6 months), but only in the PDT arm [104].
Van Rijssen et al. prospectively analyzed 29 eyes of 29 patients from the PLACE trial cohort for 8 weeks to assess whether choroidal vascularity index (CVI) changes could be responsible for therapeutic efficacy of both PDT and YSML [105]. No significant correlations were demonstrated between CVI and the two treatment options, leading the authors to conclude that any CVI change may not be primarily responsible for the treatment effect [105].
YSML Versus Conventional Laser
The efficacy of YSML was also compared to conventional laser treatment.
Sun et al. carried out a prospective, double-masked, 12-week trial, randomizing 88 patients with a diagnosis of chronic CSC to one of the two lasers [106]. At the end of follow-up, YSML demonstrated non-inferiority to CW laser regarding BCVA improvement. In contrast, anatomical outcomes appeared to be more prominent in the cohort treated with threshold laser, which displayed a proportion of patients with complete SRF reabsorption of 81.82% compared to 63.63% of YSML [106].
Maruko et al. retrospectively investigated 28 patients over 3.4 months in the CW laser group and 2.2 months in the YSML group [107]. BCVA showed no improvement compared to baseline, whereas SRF resolution outcomes were equivalent between the two lasers (66% and 64% in CW laser and YSML, respectively). Importantly, despite comparable therapeutic effects, CW laser treatment resulted in RPE damage at the site of laser delivery in all eyes treated, while only one eye that underwent YSML developed some sort of RPE modification on FAF [107].
YSML Versus Eplerenone
Oral mineralocorticoid receptor inhibitors (MRIs) such as eplerenone and spironolactone were found to be associated with SRF resolution, choroidal thickening decrease and BCVA improvement in the short term [108].
However, Lotery et al., running a randomized, double-blind, placebo-controlled trial over a 12-month follow-up on 114 patients, assigned each patient to receive either eplerenone (57) or placebo (57) and found that MRI was not superior to placebo in BCVA improvement [109].
A few other studies in the literature compared eplerenone to YSML effectiveness.
Toto et al. retrospectively enrolled 36 eyes of 30 patients into subthreshold and eplerenone groups and followed them up for 3 months [110]. Mean BCVA, CMT and SRF improved significantly by the end of the follow-up (p < 0.001) in both cohorts. In particular, 55.6% and 66.7% of patients showed a complete reabsorption of SRF over the period of interest in the YSML and eplerenone groups, respectively [110].
Vignesh et al. retrospectively evaluated 48 eyes over a median follow-up of 8 months in YSL and 4.5 months in the eplerenone treatment arm [111]. Complete SRF resorption was observed in 12/28 (42.8%) eyes in YSML, a much higher proportion compared to eplerenone (4/20, 20%). BVCA paralleled the anatomical outcomes, showing a greater improvement in YSML versus eplerenone group (0.14 vs. 0.05 logMAR) [111].
No paper compared YSML's efficacy to spironolactone or evaluated its effects on CSC.
YSML in Miscellaneous Disorders
YSML in Age-Related Macular Degeneration
In the last decades, anti- VEGF injections dramatically decreased the incidence of vision loss due to CNV in neovascular age-related macular degeneration (AMD) [112]. Contrarily, no therapy is available to prevent dry AMD from progressing into its latest stage, geographic atrophy (GA) [113]. Reticular pseudodrusen (RPDs) are associated with an increased progression to both forms of late AMD [114, 115]. In this light, a therapy that targets RPD progression could be pivotal for AMD management. Considering that the RPE dysfunction was suggested as the main causative element in RPD pathogenesis, a subthreshold laser that preserves and stimulates RPE function could play a crucial role [116, 117].
Querques et al. prospectively enrolled 20 eyes of 20 patients with a RPD finding secondary to a diagnosis of dry AMD and treated them with YSLT, following them up over a 3-month period [118]. No changes in BCVA were observed from baseline. YSML-treated areas did not display any sort of worsening in macular sensitivity, whereas RPD distribution and concentration appeared to be affected (p < 0.05). In particular, a significant increase of stage-1 RPDs [characterized by a diffuse deposition of hyperreflective material between the RPE and the inner/outer segments (IS/OS) boundary] was observed (p < 0.05) and associated with a decrease of stage 3 RPDs (featured by a thicker and conical appearance of deposited material passing through the IS/OS boundary). A statistically significant association was also found between RPD regression and ONL thickness increase (p < 0.05) [118].
Huang et al. focused their research on evaluating long-term outcomes of YSML on drusenoid PEDs [119]. A total of 21 eyes of 16 patients were consecutively included and followed up for a mean of 25.3 (SD ± 12.6) months and categorized in two groups based on presence (6 eyes) or absence (15 eyes) of drusenoid PED collapse after YSML treatment. Height, area and volume of dPEDs were positively correlated with the collapse, suggesting that larger lesions are more likely to collapse after YSML treatment. Moreover, the collapse group showed faster growth and regression rates of dPEDs compared to the natural course of these features, therefore reducing the RPE separation from the underlying Bruch’s membrane/choriocapillaris complex and consequently mitigating the RPE damage. More importantly, at the end of the follow-up, BCVA was stable compared to baseline and similar within the two groups, suggesting that YSML could alleviate not only the natural course of the disease but also its related vision impairment [119].
Further prospective studies with larger sample size are needed to further confirm these data.
YSML in Pseudophakic Cystoid Macular Edema
Pseudophakic cystoid macular edema (PCME), also known as Irvine-Gass syndrome, is a major cause of unexpected postoperative vision loss [120, 121]. Surgical insults along with postoperative inflammation are widely shown to be the important risk factors for this condition, which commonly tends to resolve spontaneously [122]. However, for chronic presentations treatment is often mandatory, requiring steroidal or non-steroidal anti-inflammatory (NSAI) drugs or even anti-VEGFs or steroid intravitreal injections [123, 124].
Verdina et al. retrospectively included ten eyes of ten patients with refractory PCME to standard treatments, namely NSAI eyedrops, topical steroids, oral indomethacin, sub-Tenon triamcinolone injections and dexamethasone intravitreal implant [125, 126]. All underwent YSML and were followed up for 6 months. Five cases occurred after uncomplicated cataract surgery, two cases after complicated cataract surgery with posterior capsule rupture and three cases subsequent to retinal detachment. At the end of the follow-up, BCVA had improved significantly from baseline. Anatomic restoration was also demonstrated with complete resorption of cystoid macular edema and a statistically significant CMT reduction (p = < 0.005) [125, 126].
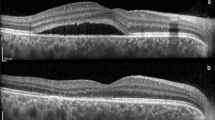
A representative case of postsurgical CME successfully managed with a single YSML session in a patient operated on for a rhegmatogenous retinal detachment is shown in Fig. 2.
Persistent post-surgical macular edema following a combined cataract and pars plana vitrectomy (PPV) surgery for rhegmatogenous retinal detachment (RD) successfully treated with yellow subthreshold micropulse laser (YSML). A Color fundus picture 3 months after combined cataract and PPV surgery for rhegmatogenous RD shows a reattached retina. B Spectral domain optical coherence tomography (SD-OCT) image shows persistent intraretinal cysts not responsive to any topical and/or oral medications including anti-inflammatory and steroids eye drops. C Color fundus picture 6 months after YSML session. D Corresponding tracked SD-OCT reveals resolution of the intraretinal fluid
YSML in Radiation Retinopathy
Radiation retinopathy is a progressive and chronic vasculopathy secondary to exposure to radiation. Current treatment includes thermal laser photocoagulation, intravitreal anti-VEGF and steroid injections, and hyperbaric oxygen [127]. Despite treatments patients often may have progressive visual impairment secondary to ischemic retinal damage. Wong et al. reported a case of a 60-year-old man who developed retinopathy in his left eye 23 years after radiotherapy for nasopharyngeal carcinoma treated with YSML [128]. Baseline BCVA was 20/40, and FA showed macular leak compatible with CME, which was confirmed on OCT. Ten months after one single treatment, BCVA improved to 20/20, and OCT examination demonstrated complete reduction of the cystoid macular edema [128].
YSML in Branch Retinal Vein Occlusion
Several treatment modalities for branch retinal vein occlusion (BRVO) have been proposed over the years; among them, anti-VEGF is recognized as the treatment of choice for BRVO-induced CME [129].
Terashima et al. retrospectively enrolled 46 eyes of 46 patients with BRVO and allocated them to two groups: the first received intravitreal ranibizumab (IVR) + YSML, whereas the second underwent IVR monotherapy [130]. BCVA and CMT improved in both groups, and results did not differ significantly between the two. However, the number of intravitreal injections decreased significantly in the IVR + YSML group compared to the IVR-only cohort (2.3 ± 0.9 vs. 1.9 ± 0.9) at the end of the 6-month follow-up [130].
YSML in Idiopathic Macular Telangiectasia Type 1
Type 1 macular telangiectasia (MacTel) is an aneurysmal telangiectasia, most commonly unilateral and typically found in the temporal half of the macula. It commonly occurs in middle-aged males, and it is thought to be a variant of Coats’ disease [131]. Therapeutic options consist of laser photocoagulation, intravitreal injections of steroids or anti-VEGF agents [132]. Kang et al. reported a case of a 54-year-old man with type 1 MacTel in the left eye [133]. BCVA was 20/800 at baseline, and spectral domain (SD)-OCT showed severe CME, for which he underwent two ineffective intravitreal injections of bevacizumab before receiving three YSML sessions. One month after the last treatment, SD-OCT demonstrated complete CME resorption and absence of any macular damage. However, after 1 year the treated area showed focal atrophic changes, which the authors claimed to potentially be associated with the chronic CME but they could not exclude that it was triggered by repeated YSLT. Nevertheless, at 3-year follow-up BCVA still improved to 20/40 in the treated eye [133].
Conclusions
The YSML has progressively been recognized as an effective treatment option for several chorioretinal diseases. A growing body of evidence highlights its efficacy and safety in both short- and long-term follow-ups. Based on the literature, YSML can be considered a safe, cost-effective and non-invasive therapeutic procedure. It is less destructive with fewer potential adverse effects like CNV, RPE atrophy or choroidal ischemia compared to CW laser. Nevertheless, a more accurate standardization of laser setting protocols is still desired, and a better understanding of the underlying molecular mechanisms would be pivotal for the future advancement and optimization of this relatively new treatment approach. Further randomized prospective studies with longer follow-up and larger sample size studies are warranted to confirm its role in chorioretinal diseases management.
References
Scholz P, Altay L, Fauser S. A review of subthreshold micropulse laser for treatment of macular disorders. Adv Ther. 2017;34:1528–55.
Molnar I, Poitry S, Tsacopoulos M, Gilodi N, Leuenberger PM. Effect of laser photocoagulation on oxygenation of the retina in miniature pigs. Invest Ophthalmol Vis Sci. 1985;26:1410–4.
Novack RL, Stefånsson E, Hatchell DL. The effect of photocoagulation on the oxygenation and ultrastructure of avascular retina. Exp Eye Res. 1990;50:289–96.
Mainster MA. Ophthalmic laser surgery: principles, technology, and technique. Trans New Orleans Acad Ophthalmol. 1985;33:81–101.
Inagaki K, Shuo T, Katakura K, Ebihara N, Murakami A, Ohkoshi K. Sublethal photothermal stimulation with a micropulse laser induces heat shock protein expression in ARPE-19 cells. J Ophthalmol. 2015;2015:729792.
Brader HS, Young LHY. Subthreshold diode micropulse laser: a review. Semin Ophthalmol. 2016;31:30–9.
Roider J. Laser treatment of retinal diseases by subthreshold laser effects. Semin Ophthalmol. 1999;14:19–26.
Sivaprasad S, Dorin G. Subthreshold diode laser micropulse photocoagulation for the treatment of diabetic macular edema. Expert Rev Med Devices. 2012;9:189–97.
Wood EH, Karth PA, Sanislo SR, Moshfeghi DM, Palanker Dv. Nondamaging retinal laser therapy for treatment of central serous chorioretinopathy: what is the evidence? Retina. 2017;37:1021–33.
Stanga PE, Reck AC, Hamilton AMP. Micropulse laser in the treatment of diabetic macular edema. Semin Ophthalmol. 1999;14:210–3.
Chhablani J, Chhablani J, Ong J, Rajendran A, Zhang X, Parolini B, et al. Subthreshold laser therapy guidelines for retinal diseases. Eye (Lond). 2022;12:1–2.
Lavinsky D, Wang J, Huie P, Dalal R, Lee SJ, Lee DY, et al. Nondamaging retinal laser therapy: rationale and applications to the macula. Invest Ophthalmol Vis Sci. 2016;57:2488–500.
Piri N, Kwong JMK, Gu L, Caprioli J. Heat shock proteins in the retina: focus on HSP70 and alpha crystallins in ganglion cell survival. Prog Retin Eye Res. 2016;52:22–46.
Dorin G. Evolution of retinal laser therapy: minimum intensity photocoagulation (MIP). Can the laser heal the retina without harming it? Semin Ophthalmol. 2004;19:62–8.
Sramek C, Mackanos M, Spitler R, Leung LS, Nomoto H, Contag CH, et al. Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2011;52:1780–7.
Lavinsky D, Cardillo JA, Melo LAS, Dare A, Farah ME, Belfort R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:4314–23.
Midena E, Micera A, Frizziero L, Pilotto E, Esposito G, Bini S. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-46515-y.
Midena E, Bini S, Martini F, Enrica C, Pilotto E, Micera A, et al. Changes of aqueous humor müller cells’ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina. 2020;40:126–34.
Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol. 2010;55:516–30.
Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, et al. Comparison of the modified early treatment diabetic retinopathy study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80.
Striph GG, Hart WM, Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988;95:1673–9.
Lewis H, Schachat AP, Haimann MH, Haller JA, Quinlan P, von Fricken MA, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990;97:503–11.
Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:652–6.
Everett LA, Paulus YM. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2021. https://doi.org/10.1007/s11892-021-01403-6.
Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–4.
Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30:908–16.
Busch C, Fraser-Bell S, Zur D, Rodríguez-Valdés PJ, Cebeci Z, Lupidi M, et al. Real-world outcomes of observation and treatment in diabetic macular edema with very good visual acuity: the OBTAIN study. Acta Diabetol. 2019. https://doi.org/10.1007/s00592-019-01310-z.
Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012;8:274–84.
Citirik M. The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med Sci. 2019;34:907–12.
Çitirik M. Non-damaging retinal laser therapy in recurrent diabetic macular edema after anti-VEGF injections. Turk J Med Sci. 2021;51:2616–20.
Donati MC, Murro V, Mucciolo DP, Giorgio D, Cinotti G, Virgili G, et al. Subthreshold yellow micropulse laser for treatment of diabetic macular edema: comparison between fixed and variable treatment regimen. Eur J Ophthalmol. 2021;31:1254–60.
Filloy A, Chong V, Solé E. Subthreshold yellow laser for fovea-involving diabetic macular edema in a series of patients with good vision: effectiveness and safety of a fovea-sparing technique. BMC Ophthalmol. 2020. https://doi.org/10.1186/s12886-020-01536-4.
Frizziero L, Calciati A, Torresin T, Midena G, Parrozzani R, Pilotto E, et al. Diabetic macular edema treated with 577-nm subthreshold micropulse laser: a real-life, long-term study. J Pers Med. 2021. https://doi.org/10.3390/jpm11050405.
Hamada M, Ohkoshi K, Inagaki K, Ebihara N, Murakami A. Subthreshold photocoagulation using endpoint management in the PASCAL® system for diffuse diabetic macular edema. J Ophthalmol. 2018. https://doi.org/10.1155/2018/7465794.
Kikushima W, Shijo T, Furuhata Y, Sakurada Y, Kashiwagi K. Comparison of the 1-year visual and anatomical outcomes between subthreshold red (670 nm) and yellow (577 nm) micro-pulse laser treatment for diabetic macular edema. Pharmaceuticals (Basel). 2021. https://doi.org/10.3390/ph14111100.
Kwon YH, Lee DK, Kwon OW. The short-term efficacy of subthreshold micropulse yellow (577-nm) laser photocoagulation for diabetic macular edema. Korean J Ophthalmol. 2014;28:379–85.
Latalska M, Prokopiuk A, Wróbel-Dudzińska D, Mackiewicz J. Subthreshold micropulse yellow 577 nm laser therapy of diabetic macular oedema in rural and urban patients of south-eastern Poland. Ann Agric Environ Med. 2017;24:96–9.
Passos RM, Malerbi FK, Rocha M, Maia M, Farah ME. Real-life outcomes of subthreshold laser therapy for diabetic macular edema. Int J Retina Vitreous. 2021. https://doi.org/10.1186/s40942-020-00268-3.
Valera-Cornejo DA, García-Roa M, Quiroz-Mendoza J, Arias-Gómez A, Ramírez-Neria P, Villalpando-Gómez Y, et al. Micropulse laser in patients with refractory and treatment-naïve center-involved diabetic macular edema: short terms visual and anatomic outcomes. Ther Adv Ophthalmol. 2021;13:251584142097911.
Vujosevic S, Frizziero L, Martini F, Bini S, Convento E, Cavarzeran F, et al. Single retinal layer changes after subthreshold micropulse yellow laser in diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2018;49:E218–25.
Vujosevic S, Toma C, Villani E, Brambilla M, Torti E, Leporati F, et al. Subthreshold micropulse laser in diabetic macular edema: 1-year improvement in OCT/OCT-angiography biomarkers. Transl Vis Sci Technol. 2020;9:31.
Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Aqueous humor biomarkers of müller cell activation in diabetic eyes. Invest Ophthalmol Vis Sci. 2015;56:3913–8.
Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80.
Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37:370–7.
Vujosevic S, Martini F, Longhin E, Convento E, Cavarzeran F, Midena E. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema. Retina. 2015;35:1594–603.
Lavinsky D, Sramek C, Wang J, Huie P, Dalal R, Mandel Y, et al. Subvisible retinal laser therapy: titration algorithm and tissue response. Retina. 2014;34:87–97.
Wells-Gray EM, Doble N, Ohr MP, Choi SS. Structural integrity of individual cone photoreceptors after short-wavelength subthreshold micropulse laser therapy for diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2018;49:946–54.
Vujosevic S, Gatti V, Muraca A, Brambilla M, Villani E, Nucci P, et al. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina. 2020;40(2):312–21.
Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond). 2002;16:242–60.
Blair NP, Shahidi M, Lai WW, Zelkha R. Correlation between microaneurysms and retinal thickness in diabetic macular edema. Retina. 2008;28:1097–103.
Maltsev DS, Kulikov AN, Burnasheva MA, Kazak AA, Chhablani J. Structural en face optical coherence tomography imaging for identification of leaky microaneurysms in diabetic macular edema. Int Ophthalmol. 2020;40:787–94.
Sarthy V. Focus on molecules: glial fibrillary acidic protein (GFAP). Exp Eye Res. 2007;84:381–2.
Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of diabetic macular edema. Curr Diab Rep. 2019. https://doi.org/10.1007/s11892-019-1188-4.
Jaki Mekjavić P, Balčiūnienė VJ, Ćeklić L, Ernest J, Jamrichova Z, Nagy ZZ, et al. The burden of macular diseases in central and eastern Europe-implications for healthcare systems. Value Health Reg Issues. 2019;19:1–6.
Akkaya S, Açikalin B, Dogan YE, Çoban F. Subthreshold micropulse laser versus intravitreal anti-VEGF for diabetic macular edema patients with relatively better visual acuity. Int J Ophthalmol. 2020;13:1606–11.
Altınel MG, Acikalin B, Alis MG, Demir G, Mutibayraktaroglu KM, Totuk OMG, et al. Comparison of the efficacy and safety of anti-VEGF monotherapy versus anti-VEGF therapy combined with subthreshold micropulse laser therapy for diabetic macular edema. Lasers Med Sci. 2021;36:1545–53.
Ecsedy M, Kovács I, Gergely R, Gombos K, Meisel J, Kovács A, et al. First experiences with Navilas® 577s micropulse laser in the treatment of diabetic maculopathy. Orv Hetil. 2020;161:2078–85.
el Matri L, Chebil A, el Matri K, Falfoul Y, Chebbi Z. Subthreshold micropulse laser adjuvant to bevacizumab versus bevacizumab monotherapy in treating diabetic macular edema: one- year- follow-up. Ther Adv Ophthalmol. 2021;13:251584142110408.
Elhamid AHA. Combined intravitreal dexamethasone implant and micropulse yellow laser for treatment of anti-VEGF resistant diabetic macular edema. Open Ophthalmol J. 2017;11:164–72.
Kanar H, Arsan A, Altun A, Aki S, Hacisalihoglu A. Can subthreshold micropulse yellow laser treatment change the anti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian J Ophthalmol. 2020;68:145–51.
Khattab AM, Hagras SM, AbdElhamid AH, Torky MA, Awad EA, Abdelhameed AG. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:1373–80.
Lai FHP, Chan RPS, Lai ACH, Tsang S, Woo TTY, Lam RF, et al. Comparison of two-year treatment outcomes between subthreshold micropulse (577 nm) laser and aflibercept for diabetic macular edema. Jpn J Ophthalmol. 2021;65:680–8.
Moisseiev E, Abbassi S, Thinda S, Yoon J, Yiu G, Morse LS. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur J Ophthalmol. 2018;28:68–73.
Tatsumi T, Takatsuna Y, Oshitari T, Kaiho T, Kawasaki Y, Shiko Y, et al. Randomized clinical trial comparing intravitreal aflibercept combined with subthreshold laser to intravitreal aflibercept monotherapy for diabetic macular edema. Sci Rep. 2022. https://doi.org/10.1038/s41598-022-14444-y.
Karasu B, Akbas YB, Aykut A, Çelebi ARC. Subthreshold photocoagulation, laser endpoint management based on optical coherence tomography angiography in cases of diabetic macular edema refractory to anti-VEGF. Klin Monbl Augenheilkd. 2022. https://doi.org/10.1055/a-1792-3009.
Photocoagulation for Diabetic Macular Edema: Early Treatment Diabetic Retinopathy Study Report Number 1 Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1985;103(12):1796–1806. https://doi.org/10.1001/archopht.1985.01050120030015
Chhablani J, Alshareef R, Kim DT, Narayanan R, Goud A, Mathai A. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol. 2018. https://doi.org/10.1186/s12886-018-0841-z.
Li ZJ, Xiao JH, Zeng P, Zeng R, Gao X, Zhang YC, et al. Optical coherence tomography angiography assessment of 577 nm laser effect on severe non-proliferative diabetic retinopathy with diabetic macular edema. Int J Ophthalmol. 2020;13:1257–65.
Bonfiglio V, Rejdak R, Nowomiejska K, Zweifel SA, Justus Wiest MR, Romano GL, et al. Efficacy and safety of subthreshold micropulse yellow laser for persistent diabetic macular edema after vitrectomy: a pilot study. Front Pharmacol Front Med SA. 2022. https://doi.org/10.3389/fphar.2022.832448.
van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019. https://doi.org/10.1016/j.preteyeres.2019.07.003.
Iacono P, Battaglia Parodi M, Falcomatà B, Bandello F. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res. 2015;55:76–83.
Parodi MB, Arrigo A, Iacono P, Falcomatà B, Bandello F. Central serous chorioretinopathy: treatment with laser. Pharmaceuticals (Basel). 2020;13:1–15.
Ambiya V, Goud A, Mathai A, Rani PK, Chhablani J. Microsecond yellow laser for subfoveal leaks in central serous chorioretinopathy. Dove Med Press Ltd. 2016;10:1513–9.
Arsan A, Kanar HS, Sonmez A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577-nm) treatment for chronic central serous chorioretinopathy: long-term follow-up. Eye (Lond). 2018;32:726–33.
Chhablani J, Kalra G, Alkwatli L, Fassbender B, Amoroso F, Chandra K, et al. Safety of various parameter sets with navigated microsecond pulsing laser in central serous chorioretinopathy. Int J Retina Vitreous. 2021;7:62.
Elhamid AHA. Subthreshold micropulse yellow laser treatment for nonresolving central serous chorioretinopathy. Clin Ophthalmol. 2015;9:2277–83.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Kneba M, Grzybowski A. Transfoveal micropulse laser treatment of central serous chorioretinopathy within six months of disease onset. J Clin Med. 2019. https://doi.org/10.3390/jcm8091398.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Grzybowski A. Functional and morphological outcome in patients with chronic central serous chorioretinopathy treated by subthreshold micropulse laser. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:2299–306.
Işık MU, Değirmenci MFK, Sağlık A. Efficacy of the subthreshold micropulse yellow wavelength laser photostimulation in the treatment of chronic central serous chorioretinopathy. Int J Ophthalmol. 2020;13:1404–10.
Kim JY, Park HS, Kim SY. Short-term efficacy of subthreshold micropulse yellow laser (577-nm) photocoagulation for chronic central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2015;253:2129–35.
Kim YJ, Kim SY, Ha S, Moon D, Seong S, Kwon OW, et al. Short-duration multiple-session subthreshold micropulse yellow laser (577 nm) for chronic central serous chorioretinopathy: results at 3 years. Eye (Lond). 2019;33:819–25.
Kiraly P, Smrekar J, Jaki MP. Morphological parameters predicting subthreshold micropulse laser effectiveness in central serous chorioretinopathy. Lasers Med Sci. 2022;37(8):3129–36.
Long H, Liu M, Hu Q, Li X. 577 nm subthreshold micropulse laser treatment for acute central serous chorioretinopathy: a comparative study. BMC Ophthalmol. 2022. https://doi.org/10.1186/s12886-022-02330-0.
Prasuhn M, Miura Y, Tura A, Rommel F, Kakkassery V, Sonntag S, et al. Influence of retinal microsecond pulse laser treatment in central serous chorioretinopathy: a short-term optical coherence tomography angiography study. J Clin Med. 2021. https://doi.org/10.3390/jcm10112418.
Scholz P, Ersoy L, Boon CJF, Fauser S. Subthreshold micropulse laser (577 nm) treatment in chronic central serous chorioretinopathy. Ophthalmologica. 2015;234:189–94.
Schworm B, Siedlecki J, Keidel LF, Herold TR, Luft N, Priglinger SG. Subthreshold laser therapy with a standardized macular treatment pattern in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2021;259:3271–81.
Uzlu D, Erdöl H, Kola M, Özbay AD. The efficacy of subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy. Lasers Med Sci. 2021;36:981–8.
Yadav NK, Jayadev C, Mohan A, Vijayan P, Battu R, Dabir S, et al. Subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy: safety profile and treatment outcome. Eye (Basingstoke). 2015;29:258–65.
Zeng M, Chen X, Song Y, Cai C. Subthreshold micropulse laser photocoagulation versus half-dose photodynamic therapy for acute central serous chorioretinopathy. BMC Ophthalmol. 2022. https://doi.org/10.1186/s12886-022-02331-.
Zhou L, Chong V, Lai K, Huang C, Xu F, Gong Y, et al. A pilot prospective study of 577-nm yellow subthreshold micropulse laser treatment with two different power settings for acute central serous chorioretinopathy. Lasers Med Sci. 2019;34:1345–51.
Zhou L, Lai K, Jin L, Huang C, Xu F, Gong Y, et al. Subthreshold micropulse laser vs. conventional laser for central serous chorioretinopathy: a randomized controlled clinical trial. Front Med (Lausanne). 2021. https://doi.org/10.3389/fmed.2021.682264.
Altinel MG, Kanra AY, Totuk OMG, Ardagil A, Turkmen OF. Comparison of the efficacy and safety between subthreshold micropulse laser, standard-fluence and low-fluence photodynamic therapy for chronic central serous chorioretinopathy. J Fr Ophtalmol. 2021;44:499–508.
Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229–34.
Altınel MG, Acikalin B, Gunes H, Demir G. Optical coherence tomography parameters as predictors of treatment response to a 577-nm subthreshold micropulse laser in chronic central serous chorioretinopathy. Lasers Med Sci. 2021;36:1505–14.
Zhou L, Li T, Lai K, Huang C, Xu F, Zhu Z, et al. Subretinal fibrin absorption after 577-nm subthreshold micropulse laser therapy in a CSC case: a brief report. Lasers Med Sci. 2018;33:1175–8.
van Dijk EHC, Fauser S, Breukink MB, Blanco-Garavito R, Groenewoud JMM, Keunen JEE, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125:1547–55.
Wu Z, Wang H, An J. Comparison of the efficacy and safety of subthreshold micropulse laser with photodynamic therapy for the treatment of chronic central serous chorioretinopathy: a meta-analysis. Medicine (Baltimore). 2021;100:e25722.
Sirks MJ, van Dijk EHC, Rosenberg N, Hollak CEM, Aslanis S, Cheung CMG, et al. Clinical impact of the worldwide shortage of verteporfin (Visudyne®) on ophthalmic care. Acta Ophthalmol. 2022. https://doi.org/10.1111/aos.15148.
Iovino C, Au A, Chhablani J, Parameswarappa DC, Rasheed MA, Cennamo G, et al. Choroidal anatomic alterations after photodynamic therapy for chronic central serous chorioretinopathy: a multicenter study. Am J Ophthalmol. 2020;217:104–13.
Roca JA, Wu L, Fromow-Guerra J, Rodríguez FJ, Berrocal MH, Rojas S, et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: results of the Pan-American Collaborative Retina Study (PACORES) Group. Br J Ophthalmol. 2018;102:1696–700.
Ntomoka CG, Rajesh B, Muriithi GM, Goud A, Chhablani J. Comparison of photodynamic therapy and navigated microsecond laser for chronic central serous chorioretinopathy. Eye (Lond). 2018;32:1079–86.
Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye (Basingstoke). 2016;30:1371–7.
Özmert E, Demirel S, Yanik Ö, Batioǧlu F. Low-fluence photodynamic therapy versus subthreshold micropulse yellow wavelength laser in the treatment of chronic central serous chorioretinopathy. J Ophthalmol. 2016. https://doi.org/10.1155/2016/3513794.
Ho M, Lai FHP, Ng DSC, Iu LPL, Chen LJ, Mak ACY, et al. Analysis of choriocapillaris perfusion and choroidal layer changes in patients with chronic central serous chorioretinopathy randomised to micropulse laser or photodynamic therapy. Br J Ophthalmol. 2021;105:555–60.
van Rijssen TJ, Singh SR, van Dijk EHC, Rasheed MA, Vupparaboina KK, Boon CJF, et al. Prospective evaluation of changes in choroidal vascularity index after half-dose photodynamic therapy versus micropulse laser treatment in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1191–7.
Sun Z, Huang Y, Nie C, Wang Z, Pei J, Lin B, et al. Efficacy and safety of subthreshold micropulse laser compared with threshold conventional laser in central serous chorioretinopathy. Eye (Lond). 2020;34:1592–9.
Maruko I, Koizumi H, Hasegawa T, Arakawa H, Iida T. Subthreshold 577 nm micropulse laser treatment for central serous chorioretinopathy. PLoS One. 2017. https://doi.org/10.1371/journal.pone.0184112.
Iacono P, Toto L, Costanzo E, Varano M, Parravano MC. Pharmacotherapy of central serous chorioretinopathy: a review of the current treatments. Curr Pharm Des. 2018;24:4864–73.
Lotery A, Sivaprasad S, O’Connell A, Harris RA, Culliford L, Ellis L, et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:294–303.
Toto L, D’Aloisio R, de Nicola C, Evangelista F, Ruggeri ML, Cerino L, et al. Short-term comparison between navigated subthreshold microsecond pulse laser and oral eplerenone for chronic central serous chorioretinopathy. Sci Rep. 2022. https://doi.org/10.1038/s41598-022-08764-2.
Vignesh TP, Maitray A, Sen S, Chakrabarti A, Kannan NB, Ramasamy K. Subthreshold micro-pulse yellow laser and eplerenone drug therapy in chronic central serous chorio-retinopathy patients: a comparative study. Semin Ophthalmol. 2020;35:237–45.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Sacconi R, Corbelli E, Querques L, Bandello F, Querques G. A review of current and future management of geographic atrophy. Ophthalmol Ther. 2017;6:69–77.
Querques G, Querques L, Martinelli D, Massamba N, Coscas G, Soubrane G, et al. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 2011;31:518–26.
Borrelli E, Costanzo E, Parravano M, Viggiano P, Varano M, Giorno P, et al. Impact of bleaching on photoreceptors in different intermediate AMD phenotypes. Transl Vis Sci Technol. 2019. https://doi.org/10.1167/tvst.8.6.5.
Luttrull JK, Chang DB, Margolis BWL, Dorin G, Luttrull DK. Laser resensitization of medically unresponsive neovascular age-related macular degeneration: efficacy and Implications. Retina. 2015;35:1184–94.
Fragiotta S, Scuderi L, Iodice CM, Rullo D, di Pippo M, Maugliani E, et al. Choroidal vasculature changes in age-related macular degeneration: from a molecular to a clinical perspective. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms231912010.
Querques G, Sacconi R, Gelormini F, Borrelli E, Prascina F, Zucchiatti I, et al. Subthreshold laser treatment for reticular pseudodrusen secondary to age-related macular degeneration. Sci Rep. 2021;11:2193.
Huang Z, Deng KY, Deng YM, Hui YN, Song YP. Long-term outcomes of drusenoid pigment epithelium detachment in intermediate AMD treated with 577 nm subthreshold micropulse laser: a preliminary clinical study. Int J Ophthalmol. 2022;15:474–82.
Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557.
Erichev VP, Kozlova IV, Kosova JV. Frequency and type of macular edema after cataract surgery in patients with glaucoma. Vestn Oftalmol. 2019;135:241–7.
Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012. https://doi.org/10.1097/ICU.0b013e32834cd5f8.
Grzybowski A, Sikorski BL, Ascaso FJ, Huerva V. Pseudophakic cystoid macular edema: update 2016. Clin Interv Aging. 2016. https://doi.org/10.2147/CIA.S111761.
Demirel S, Batioǧlu F, Özmert E. Intravitreal ranibizumab for the treatment of cystoid macular edema in irvine-gass syndrome. J Ocul Pharmacol Ther. 2012;28:636–9.
Verdina T, Ferrari C, Valerio E, Brombin A, Lazzerini A, Mastropasqua R, et al. Subthreshold micropulse yellow laser for the management of refractory cystoid macular edema consequent to complicated cataract surgery. Eur J Ophthalmol. 2021;31:NP93-8.
Verdina T, D’Aloisio R, Lazzerini A, Ferrari C, Valerio E, Mastropasqua R, et al. The role of subthreshold micropulse yellow laser as an alternative option for the treatment of refractory postoperative cystoid macular edema. J Clin Med. 2020. https://doi.org/10.3390/jcm9041066.
Giuliari GP, Sadaka A, Hinkle DM, Simpson ER. Current treatments for radiation retinopathy. Acta Oncol. 2011;50:6–13.
Wong JG, Nguyen TTH. Yellow pattern 577-nm micropulse laser: treatment of macular edema from radiation retinopathy—a case report. Case Rep Ophthalmol. 2017;8:81–6.
Chatziralli IP, Jaulim A, Peponis VG, Mitropoulos PG, Moschos MM. Branch retinal vein occlusion: treatment modalities: an update of the literature. Semin Ophthalmol. 2014;29:85–107.
Terashima H, Hasebe H, Okamoto F, Matsuoka N, Sato Y, Fukuchi T. Combination therapy of intravitreal ranibizumab and subthreshold micropulse photocoagulation for macular edema secondary to branch retinal vein occlusion: 6-month result. Retina. 2019;39:1377–84.
Yannuzzi LA, Bardal AMC, Freund, Bailey K, Chen K-J, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124(4):450–60.
Nowilaty S, Al-Shamsi H, Al-Khars W. Idiopathic juxtafoveolar retinal telangiectasis: a current review. Middle East Afr J Ophthalmol. 2010;17:224.
Kang YK, Park HS. Subthreshold micropulse yellow laser (577 nm) for idiopathic macular telangiectasia type 1 resistant to intravitreal injection. Korean J Ophthalmol. 2020;34(2):168–9.
Acknowledgements
Funding
No funding or sponsorship was received for this study or the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Claudio Iovino, Jay Chhablani; Methodology: Clemente Maria Iodice, Danila Pisani, Andrea Rosolia; Writing - original draft preparation: Claudio Iovino, Clemente Maria Iodice, Danila Pisani; Writing - review and editing: Claudio Iovino, Clemente Maria Iodice, Francesco Testa, Giuseppe Giannaccare, Jay Chhablani, Francesca Simonelli; Resources: Claudio Iovino, Francesca Simonelli, Jay Chhablani; Supervision: Francesco Testa, Francesca Simonelli.
Disclosures
Claudio Iovino, Clemente Maria Iodice, Danila Pisani, Andrea Rosolia, Francesco Testa, Giuseppe Giannaccare, Jay Chhablani and Francesca Simonelli declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Iovino, C., Iodice, C.M., Pisani, D. et al. Yellow Subthreshold Micropulse Laser in Retinal Diseases: An In-Depth Analysis and Review of the Literature. Ophthalmol Ther 12, 1479–1500 (2023). https://doi.org/10.1007/s40123-023-00698-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00698-w