Abstract
Introduction
Acute kidney injury (AKI) is occasionally detected in patients receiving anti-tuberculosis (TB) treatment. This prospective cohort study is the first to investigate the incidence, risk factors, and renal outcomes of AKI during anti-TB treatment.
Methods
This study was conducted from January 1, 2016, to May 31, 2018. Patients with a new diagnosis of TB and on standard anti-TB treatment were enrolled, and the patients received regular laboratory monitoring. AKI was defined according to the Kidney Disease: Improving Global Outcome (KDIGO) criteria. Urinalysis, renal ultrasonography, blood erythrocyte morphology, and fractional excretion of sodium were performed at AKI onset. The TB treatment regimen was adjusted by the primary physician if necessary. Risk factors for AKI were identified through Cox regression.
Results
In total, 106 patients were recruited (mean age 52.6 years, 71.7% men). Eleven (10.3%) patients experienced AKI. Increased serum uric acid and hemoglobin levels were noted at AKI onset. All patients with AKI achieved renal recovery and completed anti-TB treatment containing rifampin. Age [hazard ratio (HR) 1.06 (1.02–1.11)], a higher baseline estimated glomerular filtration rate [eGFR; HR 1.04 (1.02–1.06)], and a blood eosinophil count > 350 (109/L) [HR 10.99 (2.28–53.02)] were associated with a higher risk of AKI during TB treatment.
Conclusion
Regular pharmacovigilant monitoring revealed an incidence of renal impairment during anti-TB treatment that was higher than expected. AKI was more common in older patients with a higher eGFR and blood eosinophil count. However, the complications had no influence on TB treatment completion, and no permanent renal impairment occurred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Retrospective data indicated an incidence of AKI about 0.05–7.1% with inconsistent definition. In addition, risk factors have not been determined. |
What was learned from the study? |
More than 10% of a series of 106 adults developed AKI during TB treatment. |
Old age, normal renal function, and an initial blood eosinophil count >350 (109/L) were risk factors for AKI. |
AKI had no influence on TB treatment completion, and no permanent renal impairment occurred. |
Introduction
More than 1.7 billion people (approximately 22% of the world population) have Mycobacterium tuberculosis (MTB) infections [1]. Tuberculosis (TB) caused 1.5 million deaths in 2020 and is the 13th leading cause of death worldwide. Age is a major risk factor for TB [2] and associated with a longer treatment delay and higher mortality rate [3]. Because of the aging global population, TB must be diagnosed and treated quickly. However, the risk of adverse reactions during anti-TB treatment is higher for older adults, particularly those receiving rifampin (RIF) or pyrazinamide (PZA) [4, 5]. The situation is exacerbated in Taiwan because Taiwan’s population is aging at a rate more than twice that of Western countries [6]. Adverse reactions may compromise drug adherence and worsen anti-TB treatment outcomes [7].
Acute kidney injury (AKI) is a rare and severe complication that can interrupt anti-TB treatment and cause permanent kidney damage [8]. Among first-line anti-TB drugs, the drug that most commonly causes AKI is RIF [8,9,10]. In rare cases, isoniazid (INH) and ethambutol (EMB) might be associated with AKI. In these cases, tubulointerstitial nephritis was diagnosed but mechanisms of AKI were not established [11, 12]. To date, no prospective studies have evaluated the incidence of AKI during anti-TB treatment. Retrospective cohort studies on TB treatment have reported an AKI incidence of 0.05–7.1% [13, 14]. However, these studies have not regularly measured renal function, and one study performed a laboratory survey only when patients exhibited gastrointestinal (such as nausea and vomiting) or flu-like symptoms [15]. Furthermore, these studies have employed various definitions of kidney injury [8, 13, 16]. Predictive factors for AKI, particularly in older adults, were also undetermined. Therefore, we conducted this prospective cohort study to evaluate the incidence of AKI during first-line anti-TB treatment and explored the risk factors for this complication.
Methods
Study Design and Participants
This prospective cohort study was conducted at the National Taiwan University Hospital Hsin-Chu branch from January 1, 2016, to May 31, 2018. Approval from the Research Ethics Committee of the National Taiwan University Hospital Hsin-Chu branch was obtained prior to study initiation (institutional review board No.:104-040-F). Patients aged ≥ 18 years who had received first-line anti-TB treatment for culture-confirmed or clinically diagnosed TB, and who could be followed up regularly according to the directives of the Taiwan Guidelines for TB Diagnosis & Treatment were included in this study [17]. All patients received standard anti-TB treatment comprising daily INH, RIF, EMB, and PZA for the first 2 months and daily INH and RIF for the next 4 months. Although the brand of anti-TB medication may have changed over the study period, the active ingredients of the medication were consistent. For patients with an estimated creatinine clearance of < 30 mL/min, the frequencies of EMB and PZA were changed to once every 2 days with the unit dose unchanged. If necessary, the regimen was modified by the primary care physician (e.g., for patients with adverse drug reactions or resistant MTB strains).
Patients were excluded if they: had urinary tract infections; had received potentially nephrotoxic drugs other than anti-TB drugs at AKI onset; had other conditions that potentially cause AKI, such as hypercalcemia, dehydration, hypotension, or other nephrogenic conditions; or had end-stage renal disease and were receiving renal replacement therapy.
Laboratory Surveillance
In accordance with the directives of the Taiwan Guidelines for TB Diagnosis & Treatment, a basic laboratory survey, including complete blood count measurements with differential counts and liver (aspartate transaminase, alanine transaminase, and total bilirubin) and renal (blood urea nitrogen, creatinine, and uric acid) marker tests were performed before anti-TB treatment and at 2, 4, and 8 weeks after initiation of anti-TB treatment [17]. Further laboratory surveys were allowed if the primary care physician deemed them necessary.
AKI Definition and Intervention
AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [18]. Urinalysis, renal ultrasonography, erythrocyte morphology, and fractional excretion of sodium (FENa) measurements were performed at AKI onset. Time to AKI was defined as the interval between anti-TB treatment initiation and AKI onset. When the initial serum creatinine level did not meet the KDIGO criterion for AKI, renal recovery was defined as the return of the serum creatinine level to the baseline level. AKI duration was defined as the interval between AKI onset and renal recovery.
Data Collection and Statistical Analysis
Data on sex, age, smoking status, alcohol consumption, comorbidities, the results of acid-fast bacillus (AFB) smear and mycobacterial culture and laboratory tests; the anti-TB regimen and anti-TB drug–related side effects, and the onset and management of AKI were collected. The estimated glomerular filtration rate (eGFR) was calculated with the Modification of Diet in Renal Disease formula [19].
All data are expressed as number (%), mean ± standard deviation, or median (interquartile range). Intergroup differences were determined using a t test or Mann–Whitney U test for continuous variables on the basis of their normality, and the chi-square test or Fisher’s exact test for categorical variables, as appropriate. The time to AKI associated with each variable was compared using the Kaplan–Meier method with the log-rank test. All variables with a p value ≤ 0.1 in univariate analysis were subjected to multivariate Cox proportional hazards regression analysis to compute adjusted hazard ratios (HRs) and 95% confidence intervals. All analyses were conducted using the SPSS 24.0 (IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
Results
Patient Characteristics
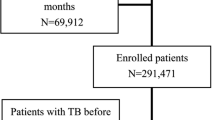
A total of 259 patients with pulmonary TB were identified during the study period, and 111 (42.9%) were enrolled (Fig. 1). Among them, three withdrew their consent, one had a diagnosis of Mycobacterium abscessus lung disease, and another died before anti-TB treatment. Therefore, 106 patients were included for further analysis. Among them, 11 (10.3%) experienced AKI during anti-TB treatment.
The mean age of the 106 patients was 52.6 ± 21.1 years, and 71.7% were men (Table 1). The average body mass index (BMI) was 21.6 ± 3.2 kg/m2, and 52 (49.1%) patients had a current or former smokers. The most common comorbidities were hypertension (22; 20.8%) and diabetes mellitus (20; 18.9%). The mycobacterial culture results of 39 (36.8%) patients were positive for AFB and 85 (80.2%) were positive for a positive culture report for MTB complex. Four (4.7%) patients had INH-resistant MTB isolates, and one (1.1%) had EMB-resistant isolate. No multidrug-resistant strains were identified. The most common side effects of anti-TB treatment were gastrointestinal symptoms (19; 17.9%) and skin rashes (16; 15.1%).
The laboratory follow-up completion rates were 95.3%, 97.2%, and 96.2% after 2, 4, and 8 weeks of treatment, respectively. Eleven (10.3%) patients experienced AKI (Table 1). Compared with patients without AKI, a greater percentage of patients with AKI were current or former smokers (72.7% vs. 46.3%, p = 0.097), and patients with AKI exhibited a higher risk of fever and gastrointestinal symptoms. The patients with and without AKI were similar in their baseline hemogram and biochemistry results; however, patients with AKI had a higher eGFR (112.3 ± 38.1 vs. 92.5 ± 29.6, p = 0.018; Table 2).
Clinical Course of AKI
Among the 11 patients with AKI, the timing of onset of AKI ranged from 6 to 68 days, and the time to renal recovery ranged from 8 to 286 days (Table 3). Most AKI events were classified as KDIGO stage 1, except for one, which was stage 3. None of them received nephrotoxic drugs other than anti-TB medication after entering the study. After AKI onset, four patients continued the previous anti-TB regimen, six discontinued all anti-TB drugs, and one discontinued EMB. Among the six patients who discontinued RIF, four restarted full-dose RIF 2–8 weeks after discontinuation. For another patient, RIF was replaced with rifabutin after 2 weeks of discontinuation. For the remaining patient, RIF was reintroduced at a daily dose of 150 mg and was increased by 150 mg every 2 days until the full dose was reached.
Urinalysis revealed no overt pyuria or hematuria; however, two patients exhibited mild proteinuria (1+; Table 3). The FENa values ranged from 0.2 to 2.9%, suggesting a prerenal AKI etiology for five (45%) patients and a renal etiology for six (55%) patients. Renal echography revealed enlarged kidneys in two patients, renal stones in two patients, and renal atrophy in one patient. Erythrocyte morphology revealed abnormal red blood cells (RBCs) in 10 patients, with anisocytosis in eight and teardrop cells, target cells, and fragmented RBCs in one patient each. All patients recovered their renal function and completed their anti-TB treatment. The laboratory findings revealed higher serum uric acid (0.58 ± 0.14 vs. 0.30 ± 0.12 mmol/L, p < 0.001) and hemoglobin (135 ± 20 vs. 127 ± 19 g/L, p = 0.034) levels at AKI onset than at baseline (Table 2).
Predictors of AKI
The Kaplan–Meier analysis revealed that a higher eGFR (HR 1.02 [1.00–1.04]) and a blood eosinophil count > 350 (109/L) (HR 4.3 [1.21–15.25]) were associated with a higher risk of AKI during anti-TB treatment. Other potential risk factors, including BMI, smoking habits, hypertension, diabetes mellitus, positive mycobacterial culture results (AFB smear or TB culture), and treatment-related side effects, were not statistically significant (Table 4).
The multivariate Cox regression analysis revealed that older age [HR 1.06 (1.02–1.11)], higher baseline eGFR [HR 1.04 (1.02–1.06) per unit increase in eGFR], and a blood eosinophil count > 350 (109/L) [HR 10.99 (1.28–53.02)] were significant predictors of AKI during anti-TB treatment (Table 4).
Discussion
This is the first prospective study to investigate the risk of AKI among patients receiving anti-TB treatment by regularly monitoring their renal function; we report three major findings. First, the incidence of AKI during anti-TB treatment was 10.3%, higher than that observed in our previous retrospective study (7.1%). Second, older age, higher eGFR, and a blood eosinophil count > 350 (109/L) were the independent predictors of AKI. Third, all patients with AKI completed anti-TB treatment regimen with no or only one drug modification, and achieved renal recovery within 1 year.
The literature review revealed four retrospective studies and two case series that focused on AKI during anti-TB treatment; the studies are summarized in Table 5 [8, 13,14,15,16, 20]. Four studies enrolled patients receiving anti-TB treatment [13,14,15, 20], and two focused on patients receiving RIF [8, 16]. The incidence of AKI in Romania [13], Japan [20], and Taiwan ranges from 0.05 to 10.4%. The low incidence of AKI in Romania may be due to underestimation because the Iasi Hemodialysis Centre database was missing data [13]. The higher incidence of AKI in Taiwan may be attributable to Taiwan’s aging population (median age between 52 and 68 years) and the application of a KDIGO definition that includes mild (stage 1) AKI [14]. Moreover, we adopted a prospective study design and performed regular follow-up of renal function; thus, we could precisely observe patients with mild AKI and without clinical symptoms and provide a true estimate of AKI incidence in patients receiving anti-TB treatment.
Among first-line anti-TB drugs, RIF, INH, and EMB are associated with AKI during treatment [8,9,10,11,12]. EMB-induced acute renal failure is rare, and only three cases of EMB-induced tubulointerstitial nephritis have been reported [11]. Similarly, only a few pediatric cases of INH-induced kidney injury have been reported [12]. Therefore, RIF is the drug most likely to cause AKI [8,9,10]. The pathophysiology of RIF-induced AKI is not well documented. However, one study suggested that RIF antigens induce either a type II or type III hypersensitivity reaction in which anti-RIF antibodies form immune complexes that are deposited in the renal vessels, glomerular endothelium, and interstitial area [16]. These reactions cause two distinct pathological changes in the kidneys: immune complex deposition in the vessels causes vascular constriction and tubular ischemia, leading to acute tubular necrosis (ATN), whereas the deposition of immune complexes in the interstitial area leads to acute tubulointerstitial nephritis (ATIN) [16]. This pathophysiology is supported by the fact that ATIN and ATN are the most common histopathological findings in anti-TB treatment-related AKI (Table 5) [8, 13, 15, 16].
In this study, the average time to AKI was 35 days, and the mean AKI duration was 89 days; these results are similar to those of our previous retrospective study [14], in which most patients experienced mild AKI (stage 1). Although anti-TB treatment was interrupted for seven (63.6%) patients, RIF was reintroduced successfully. All patients experiencing AKI completed anti-TB treatment with an RIF-containing regimen. On the basis of FENa values, the etiology of AKI was classified as prerenal (45%) or intrinsic (55%). Prerenal AKI may be caused by a decrease in food intake due to gastrointestinal side effects. Because few patients undergo renal biopsy, confirming the cause of intrinsic AKI remains challenging. In the present study, renal recovery was achieved within 1 year, and patients with prerenal or intrinsic AKI exhibited similar recovery rates (Table 3). Our recovery rate of 100% was considerably higher than those in previous studies, [8, 13,14,15,16]. Because of the prospective setting and nearly all patients’ adherence to the follow-up protocol, renal function decline could be detected early, and intervention could be performed before the patients exhibited AKI symptoms, such as oliguria or generalized edema.
Our multivariate Cox regression analysis revealed that older age, higher baseline eGFR, and a blood eosinophil count > 350 (109/L) were significant predictors of AKI during anti-TB treatment. The higher incidence of AKI in older individuals may be attributable to the following: (1) comorbidities that accumulate with age; (2) comorbidities that necessitate interventions (e.g., drugs) that function as kidney stressors or nephrotoxins; and (3) age-related transcriptomic, hemodynamic, physiologic, and structural kidney alterations [21, 22]. Therefore, clinicians should monitor renal function regularly during anti-TB treatment, particularly for older patients.
A higher eGFR was associated with a higher risk of AKI; however, previous studies have demonstrated that patients with chronic kidney disease or higher baseline serum creatinine levels (the injured kidney) are more vulnerable to AKI [23,24,25]. In a recent study of patients with TB in China, high eGFR was not determined to be a risk factor for AKI [26]. Therefore, our finding that a high eGFR is a risk factor for AKI may be due to the partial confounding by baseline kidney function of the percentage changes in serum creatinine level after AKI onset; therefore, diagnosing AKI on the basis of the KDIGO guidelines in patients with chronic kidney disease remains challenging [27]. Nevertheless, our results suggest that regular renal monitoring during anti-TB treatment is necessary, even for patients with normal renal function.
Eosinophilia is a rare presentation in drug-induced AKI, whereas urine eosinophilia is common, particularly of ATIN [28]. Although urine eosinophilia cannot be used to effectively distinguish ATIN from ATN or other kidney diseases [29], substantial eosinophilia often reflects an allergic drug reaction and may assist in the diagnosis of hospital-acquired AKI [30, 31]. Higher eosinophil counts may induce a stronger immune reaction during anti-TB treatment, thereby inducing kidney injury.
Our study has some limitations. First, a histopathological examination was not performed for a definite AKI diagnosis. Therefore, it is difficult to ascertain that the AKI was drug-related. Instead, we used FENa values to identify AKI caused by prerenal or intrinsic factors. Second, the small sample size may not be representative of the population with TB and may not fully delineate the characteristics of AKI during anti-TB treatment. Some important predictors may be overlooked because of low statistical power. Third, because all patients with AKI completed anti-TB treatment with an RIF-containing regimen, whether these episodes of AKI were drug induced remains uncertain. In our previous retrospective study, RIF was successfully reintroduced to 71% of patients [14]. The high successful reintroduction rate may be attributable to drug tolerance. A study reported that an RIF desensitization protocol led to a high rate of successful drug reintroduction (80–82%) [32, 33].
Conclusions
The incidence of AKI during anti-TB treatment is not low (10.3%), and AKI occurs frequently in older patients with normal renal function and blood eosinophil counts > 350 (109/L). The resulting kidney injury is usually mild, and patients recover without permanent renal damage. Moreover, most patients with AKI complete standard anti-TB treatment.
References
Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10): e1002152. https://doi.org/10.1371/journal.pmed.1002152.
Lee MR, Ho CM, Lee CH, et al. Tuberculosis contact investigation in an intermediate burden setting: implications from a large tuberculosis contact cohort in Taiwan. Eur Respir J. 2017;50(2):1700851. https://doi.org/10.1183/13993003.00851-2017.
Lee C-H, Wang J-Y, Lin H-C, et al. Treatment delay and fatal outcomes of pulmonary tuberculosis in advanced age: a retrospective nationwide cohort study. BMC Infect Dis. 2017;17(1):449. https://doi.org/10.1186/s12879-017-2554-y.
Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–7. https://doi.org/10.1164/rccm.200206-626OC.
Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5(2):231–49. https://doi.org/10.1517/14740338.5.2.231.
Lin Y-Y, Huang C-S. Aging in Taiwan: building a society for active aging and aging in place. Gerontologist. 2015;56(2):176–83. https://doi.org/10.1093/geront/gnv107.
Awofeso N. Anti-tuberculosis medication side-effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Organ. 2008;86(3):240. https://doi.org/10.2471/blt.07.043802.
De Vriese AS, Robbrecht DL, Vanholder RC, Vogelaers DP, Lameire NH. Rifampicin-associated acute renal failure: pathophysiologic, immunologic, and clinical features. Am J Kidney Dis. 1998;31(1):108–15. https://doi.org/10.1053/ajkd.1998.v31.pm9428460.
Kumar S, Mehta JA, Trivedi HL. Light-chain proteinuria and reversible renal failure in rifampin-treated patients with tuberculosis. Chest. 1976;70(4):564–5. https://doi.org/10.1378/chest.70.4.564.
Winter RJ, Banks RA, Collins CM, Hoffbrand BI. Rifampicin induced light chain proteinuria and renal failure. Thorax. 1984;39(12):952–3. https://doi.org/10.1136/thx.39.12.952.
Kwon SH, Kim JH, Yang JO, Lee EY, Hong SY. Ethambutol-induced acute renal failure. Nephrol Dial Transplant. 2004;19(5):1335–6. https://doi.org/10.1093/ndt/gfh166.
Trainin EB, Turin RD, Gomez-Leon G. Acute renal insufficiency complicating isoniazid therapy. Int J Pediatr Nephrol. 1981;2(1):53–4.
Covic A, Goldsmith DJ, Segall L, et al. Rifampicin-induced acute renal failure: a series of 60 patients. Nephrol Dial Transplant. 1998;13(4):924–9. https://doi.org/10.1093/ndt/13.4.924.
Chang CH, Chen YF, Wu VC, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis. 2014;14:23. https://doi.org/10.1186/1471-2334-14-23.
Schubert C, Bates WD, Moosa MR. Acute tubulointerstitial nephritis related to antituberculous drug therapy. Clin Nephrol. 2010;73(6):413–9. https://doi.org/10.5414/cnp73413.
Muthukumar T, Jayakumar M, Fernando EM, Muthusethupathi MA. Acute renal failure due to rifampicin: a study of 25 patients. Am J Kidney Dis. 2002;40(4):690–6. https://doi.org/10.1053/ajkd.2002.35675.
Centers for Disease Control MoHaW, R.O.C. (Taiwan). Taiwan Guidelines for TB Diagnosis & Treatment (6E). 2017. https://www.cdc.gov.tw/En/File/Get/Ptat7PMqyrzuqYbiPlZ4VQ.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. https://doi.org/10.1159/000339789.
Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. https://doi.org/10.7326/0003-4819-145-4-200608150-00004.
Sakashita K, Murata K, Takahashi Y, et al. A case series of acute kidney injury during anti-tuberculosis treatment. Intern Med (Tokyo, Japan). 2019;58(4):521–7. https://doi.org/10.2169/internalmedicine.0813-18.
O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28(2):407–20. https://doi.org/10.1681/asn.2015121308.
Coca SG. Acute kidney injury in elderly persons. Am J Kidney Dis. 2010;56(1):122–31. https://doi.org/10.1053/j.ajkd.2009.12.034.
Finlay S, Bray B, Lewington AJ, et al. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med (Lond). 2013;13(3):233–8. https://doi.org/10.7861/clinmedicine.13-3-233.
Cheng Y, Luo R, Wang X, et al. the incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394–402. https://doi.org/10.2215/cjn.04650420.
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. https://doi.org/10.1038/s41572-021-00284-z.
Du ZX, Chang FQ, Wang ZJ, Zhou DM, Li Y, Yang JH. A risk prediction model for acute kidney injury in patients with pulmonary tuberculosis during anti-tuberculosis treatment. Ren Fail. 2022;44(1):625–35. https://doi.org/10.1080/0886022x.2022.2058405.
Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–9. https://doi.org/10.1681/asn.2008070669.
Perazella MA, Rosner MH. Drug-induced acute kidney injury. Clin J Am Soc Nephrol. 2022. https://doi.org/10.2215/cjn.11290821.
Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–62. https://doi.org/10.2215/cjn.01330213.
Perazella MA. AKI in a hospitalized patient with cellulitis. Clin J Am Soc Nephrol. 2013;8(4):658–64. https://doi.org/10.2215/cjn.09370912.
Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6(8):461–70. https://doi.org/10.1038/nrneph.2010.71.
Matz J, Borish LC, Routes JM, Rosenwasser LJ. Oral desensitization to rifampin and ethambutol in mycobacterial disease. Am J Respir Crit Care Med. 1994;149(3 Pt 1):815–7. https://doi.org/10.1164/ajrccm.149.3.8118654.
Kim JH, Kim HB, Kim BS, Hong SJ. Rapid oral desensitization to isoniazid, rifampin, and ethambutol. Allergy. 2003;58(6):540–1. https://doi.org/10.1034/j.1398-9995.2003.00152.x.
Acknowledgements
We thank the National Taiwan University Hospital Hsin-Chu Branch Research Program supported this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The journal's Rapid Service Fee was funded by the authors.
Author contributions
Chia-Hao Chang, Lih-Yu Chang, Jen-Chung Ko, and Jann-Yuan Wang: study concept and design, analysis of data, and drafting of manuscript. Chia-Hao Chang, Yueh-Feng Wen, Chien-Jen Chang, Li-Ta Keng, and Ping-Hsien Tsou: statistical analysis and interpretation of data. Chia-Hao Chang, Lih-Yu Chang, and Jann-Yuan Wang: interpretation of data and review of manuscript. Chong-Jen Yu: review of manuscript. All of the authors have approved final version of the manuscript.
Disclosures
Chia-Hao Chang, Lih-Yu Chang, Jen-Chung Ko, Yueh-Feng Wen, Chien-Jen Chang, Li-Ta Keng, Ping-Hsien Tsou, Kai-Lun Yu, Jann-Yuan Wang, and Chong-Jen Yu have no relevant disclosures.
Compliance with Ethics Guidelines
The study was approved by the institutional review board at all participating institutions (IRB No.104–040-F), and provided written informed consent at enrolment.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chang, CH., Chang, LY., Ko, JC. et al. Incidence of and Risk Factors for Acute Kidney Injury During Antituberculosis Treatment: A Prospective Cohort Study and Literature Review. Infect Dis Ther 12, 919–931 (2023). https://doi.org/10.1007/s40121-023-00761-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00761-w