Abstract
Alzheimer’s disease (AD) is the leading cause of dementia worldwide. Early detection is believed to be essential to disease management because it enables physicians to initiate treatment in patients with early-stage AD (early AD), with the possibility of stopping the disease or slowing disease progression, preserving function and ultimately reducing disease burden. The purpose of this study was to review prior research on the use of eye biomarkers and artificial intelligence (AI) for detecting AD and early AD. The PubMed database was searched to identify studies for review. Ocular biomarkers in AD research and AI research on AD were reviewed and summarized. According to numerous studies, there is a high likelihood that ocular biomarkers can be used to detect early AD: tears, corneal nerves, retina, visual function and, in particular, eye movement tracking have been identified as ocular biomarkers with the potential to detect early AD. However, there is currently no ocular biomarker that can be used to definitely detect early AD. A few studies that used AI with ocular biomarkers to detect AD reported promising results, demonstrating that using AI with ocular biomarkers through multimodal imaging could improve the accuracy of identifying AD patients. This strategy may become a screening tool for detecting early AD in older patients prior to the onset of AD symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Alzheimer’s disease (AD) is an impactful neurodegenerative disease associated with cognitive decline and functional impairment, necessitating early detection and accurate diagnosis for effective intervention and management. |
Ocular biomarkers present a non-invasive and potentially accessible approach for the detection of AD and early-stage AD (early AD). Exploring the application of artificial intelligence (AI) in analyzing these biomarkers offers a promising avenue for early AD detection and monitoring. |
The study aimed to assess the feasibility and accuracy of ocular biomarkers in detecting AD and early AD, and to investigate the use of AI algorithms for the analysis of systemic and ophthalmology biomarkers for early AD detection. |
What was learned from the study? |
The results indicated a high potential for utilizing ocular biomarkers in identifying AD-related changes in ocular structure, supporting the feasibility of using ophthalmology biomarkers and AI for early AD detection. However, strong evidence supporting the use of ocular biomarkers for early AD detection was not found. |
Despite the limited number of AI models applied to ocular biomarkers currently available, this review provides valuable insights. Further investigation into the factors underlying suboptimal performance and the refinement of AI algorithms could enhance their accuracy and applicability in future research and clinical settings. |
Introduction
Dementia is characterized by progressive cognitive deterioration, most often found in the elderly aged > 85 years. Because of longer life expectancies, the rate of dementia is expected to increase from 46.8 million in 2010 to 131.5 million in 2050. Alzheimer’s disease (AD) is the most common type of dementia worldwide and is characterized by a spectrum of cognitive and neuropsychiatric symptoms, including memory loss, behavioral changes, disorientation and loss of the ability to perform daily activities [1]. AD is associated with a specific pattern of pathological changes in the brain that result in neurodegeneration and the progressive development of dementia [2].
The World Alzheimer Report indicated that dementia is among the top chronic diseases with the highest economic impact globally [3]. There is currently no treatment modality that can cure AD; therefore, efforts have focused on identifying reliable biomarkers of AD, especially in the preclinical stages of the disease. Abundant evidence supports the likelihood that the pathophysiological process of AD begins years before the individual exhibits any clinical symptoms. The ability to detect this asymptomatic phase will enable physicians to initiate treatment in patients with early-stage AD (early AD), with the possibility of stopping the disease or slowing disease progression, preserving function and ultimately reducing disease burden [4].
Pathologic hallmarks of AD are the presence of amyloid B-protein (Aβ) plaques and neurofibrillary tangles (NFT), both of which are related to local inflammation, ganglion cell degeneration and functional deficits [5]. Biomarkers provide supporting evidence to differentiate AD from other forms of dementia and to diagnose mild cognitive impairment (MCI) due to AD. While a definitive diagnosis of AD requires post-mortem evaluations of brain tissues, cerebrospinal fluid (CSF) analysis and positron emission tomography (PET) are used in combination with new clinical criteria of AD in living patients [6]. PET scans detect the deposition of amyloid in the brain following the injection of a radiolabeled tracer. This tool therefore offers a non-invasive diagnostic approach but its high cost is a barrier to general use. In comparison, CSF examinations for Aβ42 costs less but are more invasive that PET scans [5]. Less invasive investigations, such as electroencephalogram (EEG) and brain imaging can also facilitate the diagnosis and early detection of AD, but the results are currently not comparable to those of more invasive investigations [5]. (Fig. 1).
Diagnostic tools for the detection of Alzheimer’s disease. Aβ Amyloid beta, AD Alzheimer’s disease, CSF cerebrospinal fluid, EEG electroencephalogram, MRI magnetic resonance imaging, p-tau phosphorylated tau, PET positron emission tomography, SPECT single-photon emission computerized tomography, t-tau total tau
Recently, there has been an emerging interest in the development of artificial intelligence (AI) in the identification of systemic biomarkers for AD, particularly during the preclinical stages. AI algorithms can extract both known and unknown features from images and provide a reliable diagnosis without the need for manual feature identification, as has been shown for eye diseases where AI has been used on retinal images to identify such eye diseases as age-related macular degeneration [7], glaucoma [8], papilledema [9] and diabetic retinopathy [10]. AI approaches can also recognize systemic illnesses based on eye examination [11].
The aim of this study was to review prior research on the use of eye biomarkers and AI for detecting AD and early AD, and make recommendations for potential applications of these technologies in the future.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. No specific ethical approval was required for this article.
To identify ocular biomarkers in AD, we searched the PubMed databases using the following search terms: “ocular” OR “eye” AND “Alzheimer’s disease.” All relevant English-language reviews and systematic reviews published in 2021 and 2022 and identified using these search terms—86 papers in total— were reviewed, summarized and discussed. For the application of AI in the diagnosis of AD, we systematically searched PubMed databases using the following search terms: “Alzheimer’s disease” AND “artificial intelligence” OR “deep learning.” We initially retrieved 224 titles, abstracts and/or full texts of studies published in 2022 and subsequently screened for relevant studies for further meticulous review. The findings from all relevant studies are summarized and discussed in this review.
Definition of Terms
In this article, we will define “preclinical AD” as patients who have no clinical symptoms, “early AD” as patients who have some cognitive impairment but do not fully meet the criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V) [12]. For the diagnosis of “AD” or “AD dementia”, patients had to have cognitive impairment that meet the complete DSM V criteria for AD.
Results
Overview of Ocular Biomarkers in Early Alzheimer’s Disease
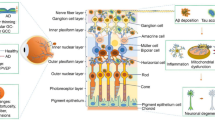
In the past few years, research in AD has increasingly focused on ocular biomarkers to facilitate the detection of early AD. Numerous structural and functional ocular biomarkers have been studied for their correlation with AD (Fig. 2; Table 1). The results show that the eye could provide potential biomarkers to detect early AD [13]. In this section, we discuss in detail ocular biomarkers according to their classification into structural biomarkers or functional biomarkers”.
Structural Biomarkers
Tears
Several studies have found that many body fluids have the potential to be biomarkers for the detection of early AD by differentiating proteome components and identifying the presence of Aβ and tau proteins [14]. Due to the accessibility and convenience of a tear sample, tears may serve as a unique source of biomarkers for AD. Some neurodegenerative and inflammatory diseases, such as Parkinson’s disease [15] and multiple sclerosis [16, 17], have been linked to differences in the proteomic composition of tears compared to that of the tears of healthy controls. The importance of tears in the diagnosis of multiple systemic diseases, including AD, was demonstrated in a study published in 2022 [18].
Kalló et al. discovered that change in the proteome components of tears can lead to the detection of AD with a sensitivity of 81% and a specificity of 77% [19]. More recent research by Gijs et al. suggests that tears with elevated levels of total tau (t-tau) and Aβ peptide 42 (Aβ42), both relatively specific biomarkers of AD, may be indicative of AD [20].
In the context that Aβ is an essential biomarker for AD and early AD, Wang et al. designed a biosensor capable of detecting Aβ42 in tear samples that could potentially be used in the future [21]. A few additional studies have identified Aβ42 and T-tau in tear fluids of AD patients at levels tenfold higher than those in serum and CSF, respectively [22, 23]. These findings suggest that tear biomarkers could potentially be used for future AD screening.
According to previous reports, microRNAs (miRNAs) play a crucial role in the etiology of AD and may be used as biomarkers to detect early AD [24], making them a topic of interest in AD research. In one study, total miRNA levels were higher in the tears of people with AD, with miRNA-200b-5p being the most promising biomarker for the disease [25].
Corneal Nerves
Alzheimer’s disease is a neuronal degenerative illness; consequently, research on the corneal nerves may be related to AD. One study on patients with AD and other neurodegenerative diseases uncovered significant reductions in corneal sensitivities [26].
Corneal confocal microscopy (CCM) has been used to examine the cornea at the cellular level with the aim to assess nerve density in the cornea. However, we identified only three studies that looked into the use of CCM in dementia; all three studies reported that AD impacts corneal nerve fiber density, branch density and fiber length [27,28,29]. In one of these studies, the corneal nerve fibers in AD were reported to be morphologically different from those in healthy controls and that all three corneal nerve fiber measures were significantly associated with cognitive function after controlling for confounders [27]. The diagnostic accuracy of CCM was high and equivalent to medial temporal lobe atrophy (MTA) rating for AD, and was superior to the MTA rating for early AD [28].
Pupil
It has been generally acknowledged that AD patients have low acetylcholine (ACh) levels, which causes pupillary system abnormalities [30]. Compared to the pupils of healthy individuals, those of AD patients are larger, respond abnormally to cholinergic antagonists and have decreased latency and amplitude of the pupillary light reflex [31]. These alterations were thought to be connected to the ACh deficiency due to the degeneration of the Edinger-Westphal nucleus found in AD patients [32]. Increased pupillary size and increased Aβ and tau levels in the CSF was found to be significantly, positively correlated in patients with the hereditary gene mutant AD [33]. At the present time, abnormal pupils have not been reported to be a sign of early AD.
Lens
The precursor of Aβ protein (APP) and Aβ are typically present in the cataractous mammalian lens. These substances are toxic to mammalian lens epithelial cells and produce cataracts. However, more recent research has demonstrated that Aβ is absent or present at extremely low levels in the human lens [34,35,36]. The accumulation of Aβ plaque in the lens of patients with AD and preclinical AD patients remains unknown. Similar to the aging process, the lens of an AD patient accumulates more misfolded, insoluble protein aggregates [37]. These results indicate that the lens may not be a highly specific biomarker for AD.
Retina and Choroid
Amyloid beta plaques were first identified on the retina in post-mortem eyes of AD patients [38]. Curcumin, which binds to Aβ plaques and fluoresces, was employed for in vivo retinal imaging of Aβ plaques [38]. These plaques cause severe ganglion cell degeneration, thinning of the retinal nerve fiber layers and loss of optic nerve axonal projections [39].
Since Aβ possesses polarized properties [40], several studies have tried to use in vivo retinal hyperspectral imaging as a biomarker of Aβ in the brain. Individuals with high Aβ load on brain PET scans and with early AD differ significantly in terms of retinal reflectance from age-matched PET-negative controls. This finding suggests that retinal imaging reflectance scores and brain Aβ accumulation are correlated and that hyperspectral imaging of the retina can predict the amount of Aβ in the brain [41].
Vascular problems also occur in AD patients due to Aβ deposits resulting in loss of the blood–brain barrier, decreased vascular density and decreased vascular blood flow in tissues of the brain [42]. Vascular structures can be investigated through retinal vessels with non-invasive retinal examinations. The most frequently used ophthalmic procedures are retinal fundus imaging, optical coherence tomography (OCT), and optical coherence tomography angiography (OCTA). Both OCT and OCTA use light waves to capture cross-section pictures of the retina and visualize vascular networks, respectively.
Visualization of retinal vessels via fundus imaging can be used as an alternative to other more invasive investigations to look for changes in the brain's blood vessels [43,44,45,46]. Narrowing or widening of vessels, low complexity and decreased density of retinal vessels can all suggest changes in the cerebral vasculature that are linked to the early stages of neurodegenerative diseases [46, 47].
Retinal nerve fiber layer (RNFL) thickness, ganglion cells-inner plexiform layer complex (GC-IPL), foveal avascular zone (FAZ) area, vessel density and perfusion density are the most common retinal parameters that have been used to identify AD with OCT and OCTA [43, 44, 48,49,50,51,52,53,54,55,56,57,58,59].
Optical coherence tomography: Numerous studies have investigated the use of retinal OCT in AD patients. A recent review [60] on OCT of the retina showed that the majority of studies in patients with AD demonstrated the presence of RNFL thinning in both the macular and peripapillary regions, along with decreases in the GC-IPL. However, these results are not exclusively found in AD patients but may also be due to aging and other factors. The authors of this review [60] concluded that OCT has the potential of being a non-invasive investigation for AD, but that additional research is required. One study reported a correlation between retinal inclusion bodies, detected by OCT imaging, and cortical amyloid deposits, detected by florbetapir PET imaging [61]. The most frequently studied OCT parameter in AD patients has been the peripapillary RNFL [44, 48, 50, 51, 53, 54], which was found to decrease in AD patients compared to healthy controls. However, a meta-analysis revealed a small range of significance [62]. Thinning of the peripapillary RNFL was seen in both early AD and AD patients, but the differences were not significant [44, 50]. A systematic review analyzing the choroidal thickness in AD patients as compared to normal controls found a significant difference [48], but a recent meta-analysis found no difference in choroidal thickness between early AD patients and normal controls [63].
Optical coherence tomography angiography: OCTA is a non-invasive imaging tool that enables a detailed angiographic view of the retinal vascular networks in different layers of the retina and the choroid. Neuroimaging studies have shown that cerebral blood flow is altered in AD patients [64]. Vascular changes in the retina may be reflected on the OCTA, making it a tool of interest. The most frequently studied OCTA parameter in AD patients is the FAZ area. Intriguingly, five studies [44, 55,56,57,58] comparing the FAZ in patients with AD to that of healthy controls indicated a significant rise in the FAZ in AD patients. However, one meta-analysis [62] revealed no difference in the FAZ of AD patients and healthy controls, while another meta-analysis [56] found that individuals with AD had significantly lower whole macular enface superficial and deep vascular density (VD) values, and lower parafoveal superficial VD than healthy controls. On the other hand, no significant difference was seen between the values for parafoveal deep VD in these two groups. Macular vessel density (m-VD) was an additional parameter utilized in detecting early AD and AD. There was evidence that m-VD was significantly lower in AD patients than in healthy controls. Interestingly, the lower the level of m-VD, the greater the level of cognitive impairment. In addition, m-VD demonstrated a correlation with cognitive function, medial temporal atrophy, Fazekas scores and the isoform 4, apolipoprotein E (APOE4) genotype [65].
Functional Biomarkers
Alzheimer’s disease might influence the visual function at both the cortical and ocular level.
Visual Acuity
Although many studies have found no appreciable difference in visual acuity between AD patients and healthy controls [31, 66, 67], some authors have reported a reduction in visual acuity in AD patients when the luminance is low [31, 68]. A large cohort follow-up study of > 15,000 older persons without dementia found that poorer visual acuity at baseline was associated with increased incidence of dementia after 6 years, even after adjusting for all factors. The authors of this study concluded that dementia could potentially be predicted by moderate-to-severe vision impairment [69]. In conclusion, visual acuity may be a biomarker that could help predict early AD.
Stereopsis
Stereopsis, or depth perception, is the ability to recognize the different distances of observed objects [70]. AD patients experience less stereopsis and depth perception of three-dimensional objects when compared to control groups [31, 37, 71]. One explanation is that successful performance on stereopsis testing requires high cognitive abilities [31]. In one study it was noted that the weakening of stereopsis in AD patients is caused by a decline in binocular disparity perception brought on by the cerebral cortex's poor visuospatial function [70]. Stereopsis may therefore be a technique for AD diagnosis but not for the diagnosis of early AD.
Saccadic Eye Movements
Saccades are quick eye movements toward touch, aural or visual stimuli [72]. Areas regulating eye movements, particularly saccades, are damaged in AD patients, resulting in abnormal eye movements. Consequently, eye movement analysis can reveal a subtle abnormality in the connection between neural and cognitive performance [73,74,75]. Recent research indicates that the anti-saccade task was the most significant abnormality in early AD and AD compared to healthy controls [76]. Anti-saccade is the task that inhibits the eyes from moving in response to stimuli. Frontal eye field and dorsolateral prefrontal cortex, which are connected to memory-related neural networks, are linked to the anti-saccadic task, possibly the result of frontal dysfunction, which is reported as an early sign of brain degeneration in AD [77]. In a recent systematic review and meta-analysis on the relationship between AD and saccadic eye movement, compared to healthy controls, both early AD and AD patients showed significant increases in saccade latencies and frequency errors [78]. It is possible, therefore, that saccadic eye movement may eventually be a biomarker for the diagnosis of AD at an earlier stage.
Non-Ophthalmic Artificial Intelligence in Alzheimer’s Disease Diagnosis
Deep learning (DL), a subset of AI, has recently been studied for the early detection of many diseases in the healthcare setting. The diseases at the forefront of DL research include those diagnosed based on the interpretation of medical images, with a focus on the medical fields of radiology, dermatology and pathology. AD, as a disease which requires imaging for diagnosis, is therefore a target for numerous DL studies.
Various approaches of AI or DL have been used to assist in AD diagnosis. Most of these AI algorithms are solely based on brain imaging. For example, magnetic resonance imaging (MRI) [79,80,81,82,83,84,85,86,87,88,89,90] and PET [91, 92] scans are able to differentiate AD from normal cognition and/or mild cognitive impairment (MCI) due to AD. In addition to brain scans, genetic information, such as DNA methylation, transcriptome, and genome-wide association studies (GWAS) also play an important role in DL-based disease detection [92,93,94,95]. Additionally, disease can be determined using DL to analyze brain immunohistochemistry sections [96] and abnormal brain metabolites from proton magnetic resonance spectroscopy (1H-MRS) studies [97]. Such tools are able to differentiate AD from other tauopathies and normal cognition plus MCI due to AD, respectively.
Overall, the performance of MRI-trained DL [79,80,81,82,83,84,85,86,87,88,89,90] to detect AD has been reported to be relatively high, with 80.0–99.79% accuracy and an area under the receiver operating characteristic (ROC) curve (AUC) of 89.21–97.3l; in addition, PET-trained DL was found to have 96.4% AUC [91] and 96.8% accuracy [92]. On the other hand, genetics-based DL [92,93,94,95] had a lower performance, with an accuracy ranging from 73.1% to 89%, and an AUC ranging from 80.5% to 99.88%.
There are also DL models computing different types of data, including demographic data, medical data, functional assessment, cognitive score and genetic and brain imaging, to discriminate AD from normal cognitive status. These models provided 93.9–96.1% AUC and 92–100% accuracy, which are comparable with MRI- and PET-based DL [98,99,100].
MCI, an early AD manifestation, can also be detected by DL models. MRI-based DL [79, 80, 84, 86], PET-based DL [91] and computing models [99] can differentiate between MCI associated with AD and normal controls, with accuracies ranging from 71.4% to 99.6% and an AUC ranging from 62.45% to 62.59%.
AI is currently an emerging tool for AD detection using various medical information, most of which are brain images. AI showed comparable performances to specialists [85] or even outperformed specialists in some models [85, 91].
Ophthalmic Artificial Intelligence to Identify Alzheimer’s Disease
The study by Wisely et al. [101] utilized multimodal retinal images as input for AI training, including GC-IPL thickness, superficial capillary plexus from OCTA images and ultra-widefield (UWF) color and fundus autofluorescence (FAF) scanning laser ophthalmoscopy images. A total of 62 eyes from AD patients and 222 eyes from healthy controls were included in this study [101]. The findings demonstrated that multimodal retinal images were highly effective at identifying AD, achieving an AUC of 0.836. The most valuable single input for disease prediction in this study was the GC-IPL. The quality of the images, such as ultra-wide field images that occasionally contained eyelashes and caused AI leading to incorrect identification of the true pathology and low numbers of input data, were identified as limitations in this study [101].
Research using a larger dataset was published by Cheung et al. [102]. They used 12,949 color fundus photos from patients from different countries for AI training. Pictures were taken from 3240 healthy individuals (7351 photos) and 648 AD patients (5598 photos). The results showed 86.3% accuracy in detecting AD in bilateral internal validation dataset. The test dataset demonstrated 79.6–92.1% accuracy in detecting AD and 80.6–89.3% accuracy in distinguishing Aβ-positive patients previously diagnosed with PET scans from Aβ-negative patients.
An attempt was made to predict cognitive function using AI on a database of 25,737 color fundus photos and metadata of healthy participants from the Canadian Longitudinal Study on Aging (CLSA). The metadata included type of drinker, type of smoker, level of education achieved, perceived mental state, among others. This study’s significant disadvantage is the absence of individuals with cognitive impairment among the enrolled subjects, which results in a relatively narrow range of cognitive scores in the training data. As a result, the results revealed a prediction accuracy of only 22.4% in cognitive function using color fundus photos and metadata [103].
Discussion
Useful biomarkers of AD, similar to biomarkers of other diseases, should be reliable and reproducible in terms of detecting or monitoring the disease, and the ideal tests or investigations to detect them should be non-invasive, easy to execute and inexpensive [104]. Since the eye is an organ with a direct connection to the brain, the pathological changes that occur in the brain may also be reflected in ocular tissues, making it an organ with the potential of containing AD biomarkers [105].
Ocular biomarkers, including those structural or functional, have been studied extensively using imaging modalities and non-invasive methods as options for detecting AD. However, not all ocular biomarkers have the same level of usefulness for the detection of early AD. The retina is the only part of the central nervous system that can be photographed non-invasively, providing sub-cellular resolutions in enface and cross-sectional views; consequently, it is the most studied tissue in the human body. Although the results from recent studies detailed in Table 1 show abnormalities and alterations in a number of ocular biomarkers, the findings are inconsistent and non-specific to AD. The retina, due to the availability of various imaging modalities that are able to explore pathological alterations, is still at the forefront as the target for studies of AI for detecting AD.
A crucial point when evaluating scientific papers is to verify the diagnostic criteria of AD employed by the authors of each quoted article, particularly whether it adheres to the DSM-5 or National Institute on Aging – Alzheimer's Association (NIA-AA) criteria. The NIA-AA criteria encompass positive biomarkers, such as the presence of Aβ/tau in the CSF, amyloid on PET and hippocampal atrophy on MRI, which contribute to increased diagnostic certainty. However, it is important to acknowledge that the authors may not have conducted a comprehensive evaluation of the criteria adopted, potentially resulting in oversight regarding the specific criteria employed in each cited article. Consequently, when cited papers assert superior sensitivity or specificity of a particular ocular biomarker, it is critical to consider that such claims may be attributed to the utilization of NIA-AA criteria rather than solely relying on the inherent quality of the ocular biomarker itself.
Retinal images have been studied for DL algorithms aimed at detecting systemic diseases other than AD, including cardiovascular disease risk, chronic kidney disease, anemia, vital signs (e.g. blood pressure) and glycated hemoglobin level, among others, with acceptable accuracy from internal validation in the same developmental datasets. These DL models have limited data on validation from new, external datasets. The deployment of these models in the real-world settings may not be feasible in the short term.
AI has been mainly applied to detect AD in various brain imaging modalities (PET and MRI). In general, these AI models can have accuracies ranging from 71% to 99%. With the addition of more data, such as genetic data, the models can increase their accuracies up to 93–99%. However, PET or MRI images are not easily accessed compared to retinal images; in particular color retinal images taken from conventional retinal cameras are difficult to obtain.
The study by Cheung et al. [102], demonstrated that AD may be identified using DL to analyze only color fundus photos. This concept provides a quick, affordable and labor-free way of detecting probable AD dementia patients with adequate sensitivity and specificity. However, although the results from the initial stages are promising, there is still a long way to go before these automated algorithms can be put to use. Using color fundus photos to diagnose AD could also be of use in the existing community eye-care center, which allows screening for common eye diseases, such as diabetic retinopathy and glaucoma, as well as screening for AD as needed. As telemedicine, non-mydriatic digital retinal cameras and smartphone cameras gain popularity in the medical field, color fundus photos will be more available. Additional research is required to see if combining color fundus photographs with blood-based or other AD biomarkers can enhance sensitivity and specificity. In addition, it would be extremely useful to employ this method to detect preclinical and prodromal AD and to predict the progression of dementia in early AD patients.
Ophthalmic AI seemed to perform better when using multimodal retinal images rather than only fundus images. Based on a review of ophthalmic AI studies to detect AD, we discovered limitations in the use of ophthalmic biomarkers for AI-based AD detection. First, AI research in ophthalmology does not have a large number of datasets that can be used to train AI. This reduces the accuracy and sensitivity of AD detection. Second, the diversity of the dataset is also essential as greater diversity could make it easier for AI to spot disparities in data. Third, the image quality in certain investigations causes AI to identify pathology inaccurately. Eliminating the artifact’s affected area is recommended, but this will allow AI to overlook the true pathology beneath the removed portion. Attention maps are proposed to determine which information the model should identify and use to make decisions from the images.
Conclusions
Alzheimer’s disease is the most common cause of dementia and has a devastating impact on patients, caregivers and societies. Although there has not yet been a standard treatment for the disease, early detection should provide awareness to those involved in patient care. To detect early onset AD, multimodal, non-invasive investigations have been utilized. Numerous biomarkers that can detect AD—and possibly early AD—are currently available, including those from the eye where many non-invasive tests and imaging modalities can be used. Ocular biomarkers are therefore among the many emerging biomarkers for detecting early AD.
Ocular biomarkers that have been found to be able to detect early AD include tears, corneal nerves, retina, visual function and, in particular, eye movement tracking. Currently, there is no ocular biomarker that can definitively detect early AD, but according to numerous studies, there is a high possibility that ocular biomarkers will be able to detect early AD in the future. The use of AI in conjunction with ocular biomarkers has been an area of interest to many researchers. However, these studies are marked with numerous limitations, including a limited number of databases, a wide variety of participants and poor ocular imaging quality.
In summary, the use of AI with ocular biomarkers through multimodal imaging could improve the accuracy of identifying AD patients, and could become a screening tool for older patients to detect preclinical AD prior to the development of AD symptoms. This topic still warrants additional research.
References
Prince AC-H, Maëlenn G, Maria K, Martin K. World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. London: Alzheimer’s Disease International (ADI); 2016.
Patterson C. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. London: Alzheimer’s Disease International (ADI); 2018.
Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer report 2021: journey through the diagnosis of dementia. London: Alzheimer’s Disease International (ADI); 2021.
Rasmussen J, Langerman H. Alzheimer’s disease—why we need early diagnosis. Degener Neurol Neuromuscul Dis. 2019;9:123–30.
Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res. 2018;7:F1000.
Budson AE, Solomon PR. New criteria for Alzheimer disease and mild cognitive impairment: implications for the practicing clinician. Neurologist. 2012;18(6):356–63.
Dong L, Yang Q, Zhang RH, Wei WB. Artificial intelligence for the detection of age-related macular degeneration in color fundus photographs: a systematic review and meta-analysis. EClinicalMedicine. 2021;35:100875.
Zheng C, Johnson TV, Garg A, Boland MV. Artificial intelligence in glaucoma. Curr Opin Ophthalmol. 2019;30(2):97–103.
Milea D, Najjar RP, Jiang Z, et al. Artificial intelligence to detect papilledema from ocular fundus photographs. N Engl J Med. 2020;382(18):1687–95.
Grzybowski A, Brona P, Lim G, et al. Artificial intelligence for diabetic retinopathy screening: a review. Eye (Lond). 2020;34(3):451–60.
Wagner SK, Fu DJ, Faes L, et al. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol. 2020;9(2):6.
Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. 2013;12(2):92–8.
Lim JK, Li Q-X, He Z, et al. The eye as a biomarker for Alzheimer’s disease. Front Neurosci. 2016;10:536.
Bălaşa AF, Chircov C, Grumezescu AMJB. Body fluid biomarkers for Alzheimer’s disease—an up-to-date overview. Biomedicines. 2020;8(10):421.
Boerger M, Funke S, Leha A, et al. Proteomic analysis of tear fluid reveals disease-specific patterns in patients with Parkinson’s disease—a pilot study. Parkinsonism Relat Disord. 2019;63:3–9.
Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteom Clin Appl. 2014;8(3–4):185–94.
Pieragostino D, Lanuti P, Cicalini I, et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J Proteom. 2019;204:103403.
Król-Grzymała A, Sienkiewicz-Szłapka E, Fiedorowicz E, Rozmus D, Cieślińska A, Grzybowski A. Tear biomarkers in Alzheimer’s and Parkinson’s diseases, and multiple sclerosis: implications for diagnosis (systematic review). Int J Mol Sci. 2022;23(17):10123.
Kalló G, Emri M, Varga Z, et al. Changes in the chemical barrier composition of tears in Alzheimer’s disease reveal potential tear diagnostic biomarkers. PLoS One. 2016;11(6):e0158000.
Gijs M, Nuijts RM, Ramakers I, Verhey F, Webers CA. Differences in tear protein biomarkers between patients with Alzheimer’s disease and controls. Invest Ophthalmol Vis Sci. 2019;60(9):1744.
Wang Y-R, Chuang H-C, Tripathi A, et al. High-sensitivity and trace-amount specimen electrochemical sensors for exploring the levels of β-amyloid in human blood and tears. Anal Chem. 2021;93(22):8099–106.
Wang X, Yu Z, Zhao X, et al. Comparative proteomic characterization of bovine milk containing β-casein variants A1A1 and A2A2, and their heterozygote A1A2. J Sci Food Agric. 2021;101(2):718–25.
Gijs M, Ramakers IHGB, Visser PJ, et al. Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep. 2021;11(1):22675.
Femminella GD, Ferrara N, Rengo G. The emerging role of microRNAs in Alzheimer’s disease. Front Physiol. 2015;6:40.
Kenny A, Jiménez-Mateos EM, Zea-Sevilla MA, et al. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci Rep. 2019;9(1):15437.
Örnek N, Dağ E, Örnek K. Corneal sensitivity and tear function in neurodegenerative diseases. Curr Eye Res. 2015;40(4):423–8.
Ponirakis G, Al Hamad H, Sankaranarayanan A, et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann Clin Transl Neurol. 2019;6(4):689–97.
Al-Janahi E, Ponirakis G, Al Hamad H, et al. Corneal nerve and brain imaging in mild cognitive impairment and dementia. J Alzheimers Dis. 2020;77(4):1533–43.
Dehghani C, Frost S, Jayasena R, et al. Morphometric changes to corneal dendritic cells in individuals with mild cognitive impairment. Front Neurosci. 2020;14:556137.
Shen J, Wu J. Nicotinic cholinergic mechanisms in Alzheimer’s disease. Int Rev Neurobiol. 2015;124:275–92.
Singh AK, Verma S. Use of ocular biomarkers as a potential tool for early diagnosis of Alzheimer’s disease. Indian J Ophthalmol. 2020;68(4):555–61.
Fotiou D, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer’s and Parkinson’s disease: evaluation with pupillometry. Int J Psychophysiol. 2009;73(2):143–9.
Frost SM, Kanagasingam Y, Sohrabi HR, et al. Pupil response biomarkers distinguish amyloid precursor protein mutation carriers from non-carriers. Curr Alzheimer Res. 2013;10(8):790–6.
Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014;24(1):25–32.
Williams EA, McGuone D, Frosch MP, Hyman BT, Laver N, Stemmer-Rachamimov A. Absence of Alzheimer disease neuropathologic changes in eyes of subjects with Alzheimer disease. J Neuropathol Exp Neurol. 2017;76(5):376–83.
Michael R, Rosandić J, Montenegro GA, et al. Absence of beta-amyloid in cortical cataracts of donors with and without Alzheimer’s disease. Exp Eye Res. 2013;106:5–13.
Dehghani C, Frost S, Jayasena R, Masters CL, Kanagasingam Y. Ocular biomarkers of Alzheimer’s disease: the role of anterior eye and potential future directions. Investig Ophthalmol Vis Sci. 2018;59(8):3554–63.
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54:S204–17.
Doustar J, Torbati T, Black KL, Koronyo Y, Koronyo-Hamaoui M. Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol. 2017;8:701.
Campbell MC, Vries DD, Emptage L, et al. Polarization properties of amyloid beta in the retina of the eye as a biomarker of Alzheimer’s disease. Bio-Optics: design and application. Optica Publishing Group. 2015;BM3A:4.
Hadoux X, Hui F, Lim JKH, et al. Non-invasive in vivo hyperspectral imaging of the retina for potential biomarker use in Alzheimer’s disease. Nat Commun. 2019;10(1):4227.
Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosc. 2011;12(12):723–38.
Alber J, Goldfarb D, Thompson LI, et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: what we know, what we don’t, and how to move forward. Alzheimers Dement. 2020;16(1):229–43.
Ge Y-J, Xu W, Ou Y-N, et al. Retinal biomarkers in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Ageing Res Rev. 2021;69: 101361.
Lemmens S, Devulder A, Van Keer K, Bierkens J, De Boever P, Stalmans I. Systematic review on fractal dimension of the retinal vasculature in neurodegeneration and stroke: assessment of a potential biomarker. Front Neurosci. 2020;14:16.
Wu H, Wang C, Chen C, et al. Association between retinal vascular geometric changes and cognitive impairment: a systematic review and meta-analysis. J Clin Neurol. 2020;16(1):19–28.
McGrory S, Cameron JR, Pellegrini E, et al. The application of retinal fundus camera imaging in dementia: a systematic review. Alzheimers Dement (Amst). 2017;6:91–107.
Chan VTT, Sun Z, Tang S, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126(4):497–510.
Chhablani PP, Ambiya V, Nair AG, Bondalapati S, Chhablani J. Retinal findings on OCT in systemic conditions. Semin Ophthalmol. 2018;33(4):525–46.
den Haan J, Verbraak FD, Visser PJ, Bouwman FH. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst). 2017;6:162–70.
Knoll B, Simonett J, Volpe NJ, et al. Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement (Amst). 2016;4:85–93.
López-de-Eguileta A, Cerveró A, de Sabando AR, Sánchez-Juan P, Casado A. Ganglion cell layer thinning in Alzheimer’s disease. Medicina (Kaunas). 2020;56(10):553.
Mejia-Vergara AJ, Restrepo-Jimenez P, Pelak VS. Optical coherence tomography in mild cognitive impairment: a systematic review and meta-analysis. Front Neurol. 2020;11: 578698.
Noah AM, Almghairbi D, Moppett IK. Optical coherence tomography in mild cognitive impairment—systematic review and meta-analysis. Clin Neurol Neurosurg. 2020;196: 106036.
Hui J, Zhao Y, Yu S, Liu J, Chiu K, Wang Y. Detection of retinal changes with optical coherence tomography angiography in mild cognitive impairment and Alzheimer’s disease patients: a meta-analysis. PLoS One. 2021;16(8): e0255362.
Jin Q, Lei Y, Wang R, Wu H, Ji K, Ling L. A systematic review and meta-analysis of retinal microvascular features in Alzheimer’s disease. Front Aging Neurosci. 2021;13: 683824.
Katsimpris A, Karamaounas A, Sideri AM, Katsimpris J, Georgalas I, Petrou P. Optical coherence tomography angiography in Alzheimer’s disease: a systematic review and meta-analysis. Eye (Lond). 2022;36(7):1419–26.
Rifai OM, McGrory S, Robbins CB, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: a systematic review. Alzheimers Dement (Amst). 2021;13(1): e12149.
Zhang J-F, Wiseman S, Valdés-Hernández MC, et al. The application of optical coherence tomography angiography in cerebral small vessel disease, ischemic stroke, and dementia: a systematic review. Front Neurol. 2020;11:1009.
Cabrera DeBuc D, Gaca-Wysocka M, Grzybowski A, Kanclerz P. Identification of retinal biomarkers in Alzheimer’s disease using optical coherence tomography: recent insights, challenges, and opportunities. J Clin Med. 2019;8(7):996.
Snyder PJ, Johnson LN, Lim YY, et al. Nonvascular retinal imaging markers of preclinical Alzheimer’s disease. Alzheimers Dement (Amst). 2016;4:169–78.
Costanzo E, Lengyel I, Parravano M, et al. Ocular biomarkers for Alzheimer disease dementia: an umbrella review of systematic reviews and meta-analyses. JAMA Ophthalmol. 2023;141(1):84–91.
Yeh T-C, Kuo C-T, Chou Y-B. Retinal microvascular changes in mild cognitive impairment and Alzheimer’s disease: a systematic review, meta-analysis, and meta-regression. Front Aging Neurosci. 2022;14: 860759.
Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis. 2014;42:S411–9.
Wang X, Wang Y, Liu H, et al. Macular microvascular density as a diagnostic biomarker for Alzheimer’s disease. J Alzheimers Dis. 2022;90:139–49.
Polo V, Rodrigo MJ, Garcia-Martin E, et al. Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease. Eye (Lond). 2017;31(7):1034–41.
Colligris P, de Lara MJP, Colligris B, Pintor J. Ocular manifestations of Alzheimer’s and other neurodegenerative diseases: the prospect of the eye as a tool for the early diagnosis of Alzheimer’s disease. J Ophthalmol. 2018;2018:8538573.
Salobrar-García E, de Hoz R, Ramírez AI, et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS One. 2019;14(8):e0220535.
Lee ATC, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW. Higher dementia incidence in older adults with poor visual acuity. J Gerontol A Biol Sci Med Sci. 2020;75(11):2162–8.
Lee C-N, Ko D, Suh Y-W, Park K-W. Cognitive functions and stereopsis in patients with Parkinson’s disease and Alzheimer’s disease using 3-dimensional television: a case controlled trial. PLoS One. 2015;10(3):e0123229.
Kim N-G, Lee H-W. Stereoscopic depth perception and visuospatial dysfunction in Alzheimer’s disease. Healthcare (Basel). 2021;9(2):157.
Binder MD, Hirokawa N, Windhorst U (eds). Encyclopedia of neuroscience. Saccadic eye movement. Springer, Berlin, p. 3564.
Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39(7):742–56.
Jamadar SD, Fielding J, Egan GF. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol. 2013;4:749.
McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68(3):255–70.
Chehrehnegar N, Shati M, Esmaeili M, Foroughan M. Executive function deficits in mild cognitive impairment: evidence from saccade tasks. Aging Ment Health. 2022;26(5):1001–9.
Alichniewicz KK, Brunner F, Klünemann HH, Greenlee MW. Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Front Psychol. 2013;4:467.
Opwonya J, Doan DNT, Kim SG, et al. Saccadic eye movement in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neuropsychol Rev. 2022;32(2):193–227.
Wang B, Lim JS. Zoom-in neural network deep-learning model for Alzheimer’s disease assessments. Sensors (Basel). 2022;22(22):8887.
Liu S, Masurkar AV, Rusinek H, et al. Generalizable deep learning model for early Alzheimer’s disease detection from structural MRIs. Sci Rep. 2022;12(1):17106.
Tinauer C, Heber S, Pirpamer L, et al. Interpretable brain disease classification and relevance-guided deep learning. Sci Rep. 2022;12(1):20254.
Tuan TA, Pham TB, Kim JY, Tavares J. Alzheimer’s diagnosis using deep learning in segmenting and classifying 3D brain MR images. Int J Neurosci. 2022;132(7):689–98.
Feng X, Provenzano FA, Small SA. A deep learning MRI approach outperforms other biomarkers of prodromal Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):45.
Houria L, Belkhamsa N, Cherfa A, Cherfa Y. Multi-modality MRI for Alzheimer’s disease detection using deep learning. Phys Eng Sci Med. 2022;45(4):1043–53.
Kim JS, Han JW, Bae JB, et al. Deep learning-based diagnosis of Alzheimer’s disease using brain magnetic resonance images: an empirical study. Sci Rep. 2022;12(1):18007.
Loddo A, Buttau S, Di Ruberto C. Deep learning based pipelines for Alzheimer’s disease diagnosis: a comparative study and a novel deep-ensemble method. Comput Biol Med. 2022;141: 105032.
Hu Y, Wen C, Cao G, Wang J, Feng Y. Brain network connectivity feature extraction using deep learning for Alzheimer’s disease classification. Neurosci Lett. 2022;782: 136673.
AlSaeed D, Omar SF. Brain MRI analysis for Alzheimer’s disease diagnosis using CNN-based feature extraction and machine learning. Sensors (Basel). 2022;22(8):2911.
Coupé P, Manjón JV, Mansencal B, Tourdias T, Catheline G, Planche V. Hippocampal-amygdalo-ventricular atrophy score: Alzheimer disease detection using normative and pathological lifespan models. Hum Brain Mapp. 2022;43(10):3270–82.
Tomassini S, Sbrollini A, Covella G, et al. Brain-on-Cloud for automatic diagnosis of Alzheimer’s disease from 3D structural magnetic resonance whole-brain scans. Comput Methods Programs Biomed. 2022;227:107191.
Etminani K, Soliman A, Davidsson A, et al. A 3D deep learning model to predict the diagnosis of dementia with Lewy bodies, Alzheimer’s disease, and mild cognitive impairment using brain 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2022;49(2):563–84.
Jo T, Nho K, Bice P, Saykin AJ. Deep learning-based identification of genetic variants: application to Alzheimer’s disease classification. Brief Bioinform. 2022;23(2):bbac022.
Chiricosta L, D’Angiolini S, Gugliandolo A, Mazzon E. Artificial intelligence predictor for Alzheimer’s disease trained on blood transcriptome: the role of oxidative stress. Int J Mol Sci. 2022;23(9):5237.
Mahendran N, Durai Raj VPM. A deep learning framework with an embedded-based feature selection approach for the early detection of the Alzheimer’s disease. Comput Biol Med. 2022;141: 105056.
Bahado-Singh RO, Radhakrishna U, Gordevičius J, et al. Artificial intelligence and circulating cell-free DNA methylation profiling: mechanism and detection of Alzheimer’s disease. Cells. 2022;11(11):1744.
Koga S, Ikeda A, Dickson DW. Deep learning-based model for diagnosing Alzheimer’s disease and tauopathies. Neuropathol Appl Neurobiol. 2022;48(1):e12759.
Kherchouche A, Ben-Ahmed O, Guillevin C, et al. Attention-guided neural network for early dementia detection using MRS data. Comput Med Imaging Graph. 2022;99:102074.
Qiu S, Miller MI, Joshi PS, et al. Multimodal deep learning for Alzheimer’s disease dementia assessment. Nat Commun. 2022;13(1):3404.
Golovanevsky M, Eickhoff C, Singh R. Multimodal attention-based deep learning for Alzheimer’s disease diagnosis. J Am Med Inform Assoc. 2022;29(12):2014–22.
Tu Y, Lin S, Qiao J, Zhuang Y, Zhang P. Alzheimer’s disease diagnosis via multimodal feature fusion. Comput Biol Med. 2022;148:105901.
Wisely CE, Wang D, Henao R, et al. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. Br J Ophthalmol. 2022;106(3):388–95.
Cheung CY, Ran AR, Wang S, et al. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health. 2022;4(11):e806–15.
Corbin D, Lesage F. Assessment of the predictive potential of cognitive scores from retinal images and retinal fundus metadata via deep learning using the CLSA database. Sci Rep. 2022;12(1):5767.
Trojanowski JQ, Growdon JH. A new consensus report on biomarkers for the early antemortem diagnosis of Alzheimer disease: current status, relevance to drug discovery, and recommendations for future research. J Neuropathol Exp Neurol. 1998;57(6):643–4.
Hays CC, Zlatar ZZ, Wierenga CE. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(2):167–79.
Acknowledgements
We would like to express our gratitude to all of the individuals who have contributed to the completion of this research paper. We extend our heartfelt appreciation to the authors of this paper, Pareena Chaitanuwong, Panisa Singhanetr, Methaphon Chainakul, Niracha Arjkongharn, Paisan Ruamviboonsuk and Andrzej Grzybowski, for their dedication and collaborative efforts in conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding or sponsorship was received for this study or publication of this article.
Author contributions
Pareena Chaitanuwong, Paisan Ruamviboonsuk and Andrzej Grzybowski contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pareena Chaitanuwong, Methaphon Chainakul and Niracha Arjkongharn. The first draft of the manuscript was written by Pareena Chaitanuwong, Panisa Singhanetr, Methaphon Chainakul and Paisan Ruamviboonsuk. All authors commented on every version of the manuscript. All authors read and approved the final manuscript.
Disclosures
All authors declare that they have no competing interests. Pareena Chaitanuwong, Panisa Singhanetr, Methaphon Chainakul and Niracha Arjkongharn have nothing to disclose. Paisan Ruamviboonsuk has received grants from Roche; is a consultant for Roche; and has received speaker fees from Novartis, Roche, Bayer, and Topcon. Andrzej Grzybowski has received grants from Alcon, Bausch&Lomb, Zeiss, Teleon, J&J, CooperVision and Hoya; has received lecture fees from Thea, Polpharma and Viatris; and is a member of the advisory boards of Nevakar, GoCheckKids, and Thea.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. No specific ethical approval was required for this article.
Data availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chaitanuwong, P., Singhanetr, P., Chainakul, M. et al. Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies. Neurol Ther 12, 1517–1532 (2023). https://doi.org/10.1007/s40120-023-00526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00526-0