Abstract
Introduction
Observational studies have indicated widespread comorbidity of white matter (WM) lesions and Alzheimer’s disease (AD) in the elderly, but the causality and direction of their relationship remained unclear. Our study aims to examine the bidirectional causal relationship between WM change and AD using a genetically informed method.
Methods
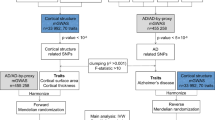
We performed a bidirectional two-sample mendelian randomization (MR) study to investigate the correlation of three WM phenotypes—white matter hyperintensities (WMH, N = 18,381), fractional anisotropy (FA, N = 17,673), and mean diffusivity (MD, N = 17,467)—with AD (N = 63,926) using summary statistics from genome-wide association studies (GWAS). The inverse variance weighted method (IVW) was used to evaluate the causal estimate and alternative methods to test the heterogeneity, horizontal pleiotropy, and outliers.
Results
There was no significant causal evidence of WM MRI markers on AD across all MR methods. We identified significant evidence of causal effects of AD on the risk of WMH (OR 1.06, 95% CI 1.03–1.10, p < 0.01). The same direction of effects was observed in MR-Egger, weighted median, and weighted mode analysis. Besides, we also observed a risk causal relationship between AD with MD in MR-Egger, weighted median, and weighted mode-based methods (MR-Egger OR 1.38, 95% CI 1.07–1.79, p = 0.02; weighted median OR 1.21, 95% CI 1.02–1.45, p = 0.03; weighted mode-based OR 1.32, 95% CI 1.14–1.53, p < 0.01). However, the general significance of the causal effect of AD on WMH and MD disappeared when we removed the single nucleotide polymorphisms (SNPs) near the APOE regions, revealing that the ability of AD to increase the risk of white matter damage might be mediated by APOE to some extent. Unfortunately, we did not observe significant causal evidence of AD on FA across all MR analyses.
Conclusions
In this bidirectional MR study, we did not observe that WM injuries were associated with a higher risk of AD. Likewise, genetically predicted AD did not result in a causal effect on white matter damage. However, our research revealed that underlying mechanisms linking AD and white matter lesions might be related to the SNPs near APOE regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Observational studies have indicated widespread comorbidity of white matter (WM) lesions and Alzheimer’s disease (AD) in the elderly, but a direct clue of causation between WM lesions and AD remained unclear. |
In this study, we examined the bidirectional causal relationship between WM change and AD using a genetically informed method. |
What was learned from the study? |
In this bidirectional mendelian randomization study, we did not observe that WM injuries were associated with a higher risk of AD. Likewise, genetically predicted AD did not result in a causal effect on WM damage. |
Our research revealed that underlying mechanisms linking AD and WM lesions might be related to the single nucleotide polymorphisms (SNPs) near APOE regions. |
This study suggested that SNPs near APOE regions might participate in the specific biological processes underlying the comorbid etiology of AD and WM damage. |
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia, chiefly marked by amyloid plaques and neurofibrillary tangles [1, 2]. However, several large anti-amyloid trials for mild-to-moderate AD have yielded disappointing results [2, 3], which made researchers gradually move away from simple assumptions to more broad causality. Substantial evidence showed that the vascular hypothesis might be an alternative theory for AD etiology [4]. Cerebral small vessel disease (CSVD) is a disorder of cerebral microvessels that always leads to white matter lesions and other abnormalities [5]. As an assessment, CSVD contributed to about 50% of dementia worldwide [5,6,7]. Recent meta-analyses have investigated and suggested that white matter hyperintensities (WMH) at baseline conferred a 25% elevated risk of AD, and periventricular WMH conferred a 1.51-fold excess risk for dementia [8]. Besides, diffusion tensor imaging (DTI) measures and assesses white matter microstructure integrity and white matter damage via estimation of fractional anisotropy (FA) and mean diffusivity (MD) [9]. Observational studies reported abnormalities in DTI, such as decreased fractional anisotropy (FA) and increased mean diffusivity (MD), in AD and mild cognitive impairment within a diversity of white matter regions [10,11,12]. Moreover, AD pathology was more likely to have a detrimental impact on WM lesions. Previous studies detected that Aβ pathology developed early cerebral blood flow reductions [13] and brain amyloid could increase the posterior WMH loads [14]. Amyloid accumulation also had a worse effect on white matter integrity in the absence of cognitive impairment, particularly in amyloid stage I–II [15]. Current evidence about a possible relationship between AD and WM damage was mainly based on observational studies, but a direct clue of causation between white matter lesions and AD remained uncertain.
Mendelian randomization (MR) is an alternative means to obtain unconfounded causal inference for the association between white matter change and Alzheimer’s disease as the MR approach takes advantage of genetic variants as instruments [16]. To this end, we extracted instruments from summary statistics of genome-wide association studies (GWAS) for white matter MRI markers and AD and applied a bidirectional two-sample MR design to assess the potential causal relationship of white matter lesions with the risk of AD, and vice versa.
Methods
Data Source and Instruments
MRI Markers of WM
We drew all summary GWAS statistics of MRI markers of WM from UK Biobank, from patients aged between 40 and 69 years at recruitment [17]. This GWAS examined the following three MRI markers: white matter hyperintensities (WMH, N = 18,381), fractional anisotropy (FA, N = 17,673), and mean diffusivity (MD, N = 17,467). In this GWAS, individuals diagnosed with major central nervous system (CNS) diseases that could be related to white matter changes (e.g., stroke, Parkinson’s disease, multiple sclerosis, dementia, or any other CNS neurodegenerative condition) were excluded from the analysis. WMH trait was log-transformed and normalized for brain volume. Principal component analysis (PCA) was performed on the FA and MD measures of each of the 48 different brain tracts to obtain a single global white matter FA and MD measure. Summary level data for white matter markers that consisted of full sets of association results were available from Cerebrovascular Disease Knowledge Portal (www.cerebrovascularportal.org). In order to avoid bias introduced by overlapping cohorts between AD and WM traits, we did not apply the additional cohorts for WMH, such as Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE) and a study from patients with ischemic stroke, which were used in the published WMH GWAS. We selected the significant variants with a threshold of p < 5 × 10−8, and clumped single nucleotide polymorphisms (SNPs) for independence with SNPs correlated at r2 < 0.001 within 500 kb based on European ancestry reference data from the 1000 Genomes Projects.
Alzheimer’s Disease
We drew on clinically diagnosed LOAD summary data from a recent GWAS of International Genomics of Alzheimer’s Project (IGAP) stage 1 discovery study consisting of 21,982 cases and 41,944 controls [18]. All stage 1 samples are from four consortia: Alzheimer Disease Genetics Consortium (ADGC; 14,428 cases and 14,562 controls), CHARGE Consortium (2137 cases and 13,474 controls), The European Alzheimer’s Disease Initiative (EADI; 2240 cases and 6631 controls), and Genetic and Environmental Risk in AD/Defining Genetic, Polygenic and Environmental Risk for Alzheimer’s Disease Consortium (GERAD/PERADES; 3177 cases and 7277 controls). More detailed information about summary demographics was available in the original works. For MR analysis, independent SNPs were clumped meeting a threshold of p < 5 × 10−8.
Standard Protocol Approvals, Registrations, and Patient Consent
Studies contributing to WM MRI markers and AD were approved by an institutional review board in the original GWAS [17, 18]. In our study, we only applied summary data.
Statistical Analysis
Before MR analysis, we calculated the proportion of variance (R2) explained by the instrumental SNPs. The strength of instruments was judged by F statistics, with a strong instrument defined as an F statistic greater than 10 [19]. For each direction of potential influence, inverse-variance weighted (IVW) was performed for the primary MR estimates [20]. This method will return a biased result if SNPs present horizontal pleiotropy, which will be contrary to the MR assumption [21]. Therefore, we used other MR methods, (e.g., MR-Egger regression [21], weighted mode-based method [22], and weighted median approach [23]) as complementary analyses to decrease the bias of horizontal pleiotropy, which would result in loss of robust statistical power [24]. In addition to these four methods, we also applied Pleiotropy Residual Sum and Outlier (MR-PRESSO) to identify horizontal pleiotropic outliers [25] assuming that horizontal pleiotropy occurred in less than 50% of instruments. Finally, since SNPs on the apolipoprotein E (APOE) were highly associated with AD and some clues also indicated that APOE was a top hit in the white matter damage [17, 26], MR analysis without SNPs near the APOE regions (Chr19:45,116,911–46,318,605) was considered as supplementary results to reduce the horizontal pleiotropy. Results are reported with odds ratios (ORs) per an approximate 1 standard deviation (SD) increment of each exposure. We estimated statistical power for our MR analyses using an online calculator (https://sb452.shinyapps.io/power). All analyses were performed using R version 4.0.3 with “TwosampleMR” and “MR-PRESSO” packages.
Results
Statistical Power
The F statistic for each SNP was greater than 10, indicating less weak instrument bias (Table S1 in the supplementary material). When we considered all instruments, all statistical power for our MR estimates was more than 80%, except for the effect of AD on WMH (Table S2 in the supplementary material). However, the statistical power for the causal relationship of AD on WM sharply decreased if we removed the SNPs in the APOE regions (Table S2).
Estimates of the Causal Effect of WM MRI Markers on AD
We did not find any statistically significant causal evidence of WM MRI markers on AD across all MR methods. MR estimates remained null even though we removed the outlier using MR-PRESSO methods (Fig. 1). The presence of heterogeneity and pleiotropic effect are shown in Table S2. In addition, we found that no single genetic variant influenced the results in leave-one-out analyses (Figs. S1–S3 in the supplementary material). Summary statistics for the genetic instruments used to assess the effect of WM on the risk of AD are shown in Table S3 in the supplementary material.
Causal effect estimates of genetically predicted white matter change on Alzheimer’s disease. For the causal effect of FA on Alzheimer’s disease (AD), we detected two outliers (rs4150221 and rs76122535) using the MR-PRESSO (Pleiotropy Residual Sum and Outlier) method. For the causal effect of MD on AD, we detected one outlier (rs4150221) using the MR-PRESSO. SNP single nucleotide polymorphism, N number, OR odds ratios, CI confidence interval, WM white matter, AD Alzheimer’s disease, WMH white matter hyperintensities, FA fractional anisotropy, MD mean diffusivity, IVW inverse variance weighted
Estimates of the Causal Effect of AD on WM MRI Markers
We identified significant evidence that genetically predicted AD was associated with a greater WMH load (IVW OR 1.06, 95% CI 1.03–1.10, p < 0.01). The same direction of effects was observed in MR-Egger, weighted median, and weighted mode analysis (Fig. 2). There was significant heterogeneity in the IVW method and possible pleiotropy in the MR-Egger analysis of WMH (p < 0.05) (Table S2). Besides, we also observed a risk causal relationship between AD with MD in MR-Egger, weighted median, and weighted mode-based methods (MR-Egger OR 1.38, 95% CI 1.07–1.79, p = 0.02; weighted median OR 1.21, 95% CI 1.02–1.45, p = 0.03; weighted mode-based OR 1.32, 95% CI 1.14–1.53, p < 0.01) (Fig. 2). However, analyses leaving out each SNP revealed that rs679515 drove this association (Fig. S6 in the supplementary material). There was significant heterogeneity and possible pleiotropy in both analyses of WMH (IVW Cochran Q, 33.22, p = 0.03; MR-Egger intercept, − 0.01, p < 0.01; MR PRESSO Global test, p < 0.01) and MD (IVW Cochran Q, 55.47, p < 0.01; MR-Egger Cochran Q, 46.34, p < 0.01; MR PRESSO Global test, p < 0.01). There was no difference between estimates from MR-PRESSO before and after the outlier’s correction for WMH (MR PRESSO-Raw, p = 0.13; MR PRESSO-Corrected, p = 0.07) and MD (MR PRESSO-Raw, p = 0.39; MR PRESSO-Corrected, p = 0.96) (Fig. 2). Most importantly, when we removed the SNPs in the APOE regions, the general significance of the causal effect of AD on WMH and MD disappeared, revealing that the ability of AD to increase the risk of white matter damage might be mediated by APOE to some extent. Unfortunately, we did not observe significant causal evidence of AD on FA across all MR analyses (Fig. 2). The presence of heterogeneity and the pleiotropic effect is shown in Table S2. Summary statistics for the genetic instruments used to assess the effect of AD on the risk of WM are shown in Table S4 in the supplementary material. Leave-one-out analyses for AD on the WM lesions are shown in Figs. S4–S6 in the supplementary material.
Causal effect estimates of genetically predicted Alzheimer’s disease on white matter lesions. For the causal effect of Alzheimer’s disease (AD) on white matter lesions without APOE, we removed five SNPs near the APOE regions (Chr19:45,116,911–46,318,605), namely rs10416500, rs150685845, rs7412, rs1081105, and rs147711004. For the causal effect of AD on WMH in the total group, two outliers (rs1081105 and rs147711004) were detected by the MR-PRESSO (Pleiotropy Residual Sum and Outlier) method. For the causal effect of AD on FA in the total group, two outliers (rs11218343 and rs679515) were detected by the MR-PRESSO method. For the causal effect of AD on MD in the total group, two outliers (rs147711004 and rs679515) were detected by the MR-PRESSO method. SNP single nucleotide polymorphism, N number, OR odds ratios, CI confidence interval, WM white matter, AD Alzheimer’s disease, WMH white matter hyperintensities, FA fractional anisotropy, MD mean diffusivity, IVW inverse variance weighted
Discussion
In this bidirectional MR study, a comprehensive MR analysis was performed to assess the association between AD and white matter lesions using a large sample size of GWAS pooled data involving more than 63,000 subjects in AD cohorts and over 17,000 individuals for WMH load and WM microstructural changes. Regrettably, we did not observe that WM injuries were associated with a higher risk of AD. Likewise, genetically predicted AD did not result in a causal effect on white matter damage. However, our research suggested that underlying mechanisms linking AD and white matter lesions might be related to the SNPs near APOE.
Some lines of evidence suggested the comorbidity of abnormalities in the brain microvascular system and AD [1, 13, 27, 28]. In the general population, the prevalence of white matter lesions increased exponentially with age, ranging from 11% to 21% at age 64 and 94% aged 82 [29]. Current findings indicated that WMH might predict AD a decade before the clinical stage [30]. In addition to the positive association between WMH and clinical AD [8], systematic reviews and meta-analysis studies also have shown a relationship between WMH and a higher risk of specific cognitive domains in patients with AD or MCI [31]. Moreover, a recent review has suggested colocalized widespread disrupted white matter integrity and AD predominant pathologies (Aβ42 or tau) in patients with subjective cognitive impairment (SCI), MCI, or AD [32]. Furthermore, oxidative stress and microglia-mediated inflammation might be common possible pathogenesis of AD progression and white matter damage [33,34,35,36,37,38,39,40,41]. However, observational studies could not exactly distinguish consequences from causes because of the influence of confounding, as accumulating evidence suggested that cardiovascular disease and other lifestyle-related disorders, including diabetes, smoking, and obesity, might contribute to the progression of dementia and WM lesions [2, 34].
Using MR approaches, our results suggested that white matter damage containing WMH and white matter integrity could not elevate the risk of AD, which was consistent with a previous randomized controlled trial (RCT) showing that hypertension treatment with nilvadipine did not slow the decline in cognition or function in patients with mild- and moderate-stage AD [42], although a meta-analysis of RCT studies suggested blood pressure control prevented WMH progression [43, 44]. Moreover, intensive blood pressure control did not result in a significant reduction in the risk of probable dementia relative to standard blood pressure [45]. However, these findings were less consistent with a recent MR study showing a positive association of WMH volume with AD [46]. This inconsistency might be due to the selection bias because the population recruited in our study was limited to European ancestry and the age seemed to be more severe when at recruitment. Similarly, a recent MR analysis leveraging GWAS summary statistics for 110 DTI measurement revealed that the higher risk of AD was causally associated with genetically determined WM integrity in the corpus callosum [47], but not overall contribution of white matter connectivity. Therefore, white matter changes that increased the risk of AD from the observational study might be better explained by other factors rather than the direct effect. For example, underlying conditions with cardiovascular disease [48] could interfere with WM lesions and cause AD.
For the relationship between AD and white matter damage, the true causal relationship of AD to the risk of WM injuries was obscured by APOE. In our research, we observed a false positive result that AD could increase the WMH load and damage the WM microstructural integrity when we included all the instruments of AD, whereas the positive result disappeared when we removed the SNPs in the APOE region. On the basis of this, we reasonably inferred that SNPs near APOE might explain the genetic correlation of AD with WM pathologies. As we all know, the presence of the ε4 allele of APOE had the strongest association with sporadic AD [49]. Meanwhile, APOE has been reported to destroy blood–brain barrier integrity, affect cerebral blood flow, and cause neuronal-vascular coupling disorders [50, 51]. In line with our results, a recent study confirmed that a higher genetic risk score for AD, especially driven by APOE, was associated with WM lesion burden by examining the polygenic overlap between AD and vascular pathologies. Additionally, the effect of APOE on memory and global cognition might be partly mediated by WM damage in the mediation analysis [26]. Additionally, the APOE ε4 allele could modulate brain WM structure before any impending cognitive or clinical manifestations of the disease [52] in an age-independent manner [53]. Besides, APOE genotype might influence the interaction of WM function with AD pathology. WMH was correlated with amyloid burden especially in the posterior brain regions in APOE ε4 non-carriers but not in the APOE ε4 carriers, suggesting that the influence of APOE might override the effect of WMH on amyloid burden [54]. However, our MR analyses might not be powerful enough to detect the small effect of impact of AD on WMH after removing the SNPs near the APOE regions, which needs expanded future discovery GWAS. More evidence is needed to further investigate the mechanisms that underline the influence of AD on white matter.
Limitations
A typical MR study should be designed following three core assumptions [55]: (1) instruments should be associated with exposure; (2) instruments should influence the outcome only through the exposure, rather than any other pathways; (3) instruments should not be associated with any confounders. To completely rule out all confounders was still a challenge for an MR study as it might not be possible to measure all confounders in the absence of an exact understanding of the complex biology of their relationship with the exposure [55]. In our study, in addition to the conventional IVW method, we applied four methods as sensitivity analysis, namely the MR-Egger method, weighted median, weighted mode-base method, and MR-PRESSO. A potential bias in our MR study was the presence of horizontal pleiotropy mainly caused by APOE when we assessed the causal effect of AD on the risk of white matter injuries. To solve this, we removed the instruments in the APOE regions. In addition, the causal relationship of genetically determined AD with white matter lesions was null after removing the SNPs near the APOE regions. However, the low power might be the reason for the null results. As population stratification might affect the genetic associations, we restricted the population to European ancestry.
Conclusions
In the present study, we did not provide evidence to support a direct clue of causation between white matter lesions and AD risk using a bidirectional MR approach. However, we held that SNPs near the APOE region might explain the genetic correlation of AD with WM pathologies. Further research is necessary to provide insight into specific biological processes underlying the comorbid etiology of AD and white matter damage.
References
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403.
Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer’s disease. Lancet. 2016;388:505–17.
Liu PP, Xie Y, Meng XY, Kang JS. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther. 2019;4:29.
Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–67.
Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–96.
Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–86.
Bos D, Wolters FJ, Darweesh SKL, et al. Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018;14:1482–92.
Hu HY, Ou YN, Shen XN, et al. White matter hyperintensities and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev. 2021;120:16–27.
Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–65.
Reginold W, Luedke AC, Itorralba J, Fernandez-Ruiz J, Islam O, Garcia A. Altered superficial white matter on tractography MRI in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2016;6:233–41.
Carlson ML, Toueg TN, Khalighi MM, et al. Hippocampal subfield imaging and fractional anisotropy show parallel changes in Alzheimer’s disease tau progression using simultaneous tau-PET/MRI at 3T. Alzheimers Dement (Amst). 2021;13:e12218.
Papma JM, de Groot M, de Koning I, et al. Cerebral small vessel disease affects white matter microstructure in mild cognitive impairment. Hum Brain Mapp. 2014;35:2836–51.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–34.
Palhaugen L, Sudre CH, Tecelao S, et al. Brain amyloid and vascular risk are related to distinct white matter hyperintensity patterns. J Cereb Blood Flow Metab. 2021;41:1162–74.
Wang YJ, Hu H, Yang YX, et al. Regional amyloid accumulation and white matter integrity in cognitively normal individuals. J Alzheimers Dis. 2020;74:1261–70.
Choi KW, Chen CY, Stein MB, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiat. 2019;76:399–408.
Persyn E, Hanscombe KB, Howson JMM, Lewis CM, Traylor M, Markus HS. Genome-wide association study of MRI markers of cerebral small vessel disease in 42,310 participants. Nat Commun. 2020;11:2175.
Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30.
Huang SY, Yang YX, Kuo K, et al. Herpesvirus infections and Alzheimer’s disease: a Mendelian randomization study. Alzheimers Res Ther. 2021;13:158.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Hemani G, Bowden J, Davey SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195–208.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Lin YF, Smith AV, Aspelund T, et al. Genetic overlap between vascular pathologies and Alzheimer’s dementia and potential causal mechanisms. Alzheimers Dement. 2019;15:65–75.
Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–7.
Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:942–51.
Hase Y, Horsburgh K, Ihara M, Kalaria RN. White matter degeneration in vascular and other ageing-related dementias. J Neurochem. 2018;144:617–33.
Mortamais M, Artero S, Ritchie K. White matter hyperintensities as early and independent predictors of Alzheimer’s disease risk. J Alzheimers Dis. 2014;42(Suppl 4):S393-400.
van den Berg E, Geerlings MI, Biessels GJ, Nederkoorn PJ, Kloppenborg RP. White matter hyperintensities and cognition in mild cognitive impairment and Alzheimer’s disease: a domain-specific meta-analysis. J Alzheimers Dis. 2018;63:515–27.
Alm KH, Bakker A. Relationships between diffusion tensor imaging and cerebrospinal fluid metrics in early stages of the Alzheimer’s disease continuum. J Alzheimers Dis. 2019;70:965–81.
Simpson JE, Ince PG, Higham CE, et al. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol Appl Neurobiol. 2007;33:670–83.
Alber J, Alladi S, Bae HJ, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): knowledge gaps and opportunities. Alzheimers Dement (N Y). 2019;5:107–17.
Swardfager W, Yu D, Scola G, et al. Peripheral lipid oxidative stress markers are related to vascular risk factors and subcortical small vessel disease. Neurobiol Aging. 2017;59:91–7.
Aytac B, Coskun O, Alioglu B, et al. Decreased antioxidant status in migraine patients with brain white matter hyperintensities. Neurol Sci. 2014;35:1925–9.
Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–60.
Wendeln AC, Degenhardt K, Kaurani L, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–8.
Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53:6709–15.
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–19.
Amor S, McNamara NB, Gerrits E, et al. White matter microglia heterogeneity in the CNS. Acta Neuropathol. 2022;143:125–41.
Lawlor B, Segurado R, Kennelly S, et al. Nilvadipine in mild to moderate Alzheimer disease: a randomised controlled trial. PLoS Med. 2018;15:e1002660.
Lai Y, Jiang C, Du X, et al. Effect of intensive blood pressure control on the prevention of white matter hyperintensity: systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich). 2020;22:1968–73.
Su C, Wu H, Yang X, Zhao B, Zhao R. The relation between antihypertensive treatment and progression of cerebral small vessel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100:e26749.
Group SMIftSR, Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–61.
Sargurupremraj M, Suzuki H, Jian X, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun. 2020;11:6285.
Song W, Qian W, Wang W, Yu S, Lin GN. Mendelian randomization studies of brain MRI yield insights into the pathogenesis of neuropsychiatric disorders. BMC Genom. 2021;22:342.
Ferreira JP, Kearney Schwartz A, Watfa G, et al. Memory alterations and white matter hyperintensities in elderly patients with hypertension: the ADELAHYDE-2 study. J Am Med Dir Assoc. 2017;18(451):e413-451e425.
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90.
Tai LM, Thomas R, Marottoli FM, et al. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 2016;131:709–23.
Koizumi K, Hattori Y, Ahn SJ, et al. Apoepsilon4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat Commun. 2018;9:3816.
Kljajevic V, Meyer P, Holzmann C, et al. The epsilon4 genotype of apolipoprotein E and white matter integrity in Alzheimer’s disease. Alzheimers Dement. 2014;10:401–4.
Heise V, Filippini N, Ebmeier KP, Mackay CE. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. 2011;16:908–16.
Noh Y, Seo SW, Jeon S, et al. White matter hyperintensities are associated with amyloid burden in APOE4 non-carriers. J Alzheimers Dis. 2014;40:877–86.
Grover S, Del Greco MF, Stein CM, Ziegler A. Mendelian randomization. Methods Mol Biol. 2017;1666:581–628.
Acknowledgements
This work was made possible by the generous sharing of GWAS summary statistics. We thank the participants, researchers, and staff associated with the many other studies from which we used data for this report. We thank the Hugh Markus group for providing summary data for MRI markers. We also thank the IGAP for providing summary results data for AD. We thank the China Scholarship Council (CSC) [No.201906270200] to fund Li Yaqing to visit the University of Cambridge through the PhD Exchange Scheme.
Funding
The study was financially supported by National Natural Science Foundation of China (No. 81771151 and No. 82071210) and the Central Guidance on Local Science and Technology Development Fund of Hubei Province (No. 2020ZYYD014). The journal’s Rapid Service Fee was funded by Junjian Zhang.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Yaqing Li was involved in study design, acquisition, statistical analysis, drafting and revising the manuscript. Tian Li and Jiaxin Zheng were involved in acquisition and statistical analysis. Junjian Zhang was involved in study design and obtaining funding. All authors contributed to manuscript revision, read and approved the submitted version.
Disclosures
Yaqing Li, Jiaxin Zheng, Tian Li and Junjian Zhang have nothing to disclose.
Compliance with Ethics Guidelines
All data sources used in the MR study received approval from an ethics standards committee on human experimentation and obtained informed consent from all participants, which could be obtained in the original GWAS.
Data Availability
The authors hereby declare that the generated datasets in this study will be presented upon request from the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Y., Zheng, J., Li, T. et al. White Matter and Alzheimer’s Disease: A Bidirectional Mendelian Randomization Study. Neurol Ther 11, 881–892 (2022). https://doi.org/10.1007/s40120-022-00353-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00353-9