Abstract
Introduction
Treatment adherence and persistence impact the effectiveness of edoxaban for the prevention of thromboembolism in patients with atrial fibrillation (AF). The objective of this analysis was to assess adherence and persistence of edoxaban vs. other non-vitamin K antagonist oral anticoagulants (NOACs) and vitamin K antagonists (VKAs).
Methods
Utilizing a German claims database, adults with AF with the first pharmacy claim identified for edoxaban, apixaban, dabigatran, rivaroxaban, or VKAs from January 2013 to December 2017 were included in a propensity score-matched analysis. The first pharmacy claim was the index claim. Adherence (i.e., proportion of days covered [PDC]) and persistence (proportion of patients who continued therapy) were compared between edoxaban and other therapies. Patients receiving once-daily (QD) vs. twice-daily (BID) NOAC were also analyzed.
Results
Overall, 21,038 patients were included (1236 edoxaban, 6053 apixaban, 1306 dabigatran, 7013 rivaroxaban, and 5430 VKA). After matching, baseline characteristics were well balanced across cohorts. Adherence was significantly higher for edoxaban vs. apixaban, dabigatran, and VKAs (all P < 0.0001). Significantly more edoxaban patients continued therapy vs. rivaroxaban (P = 0.0153), dabigatran (P < 0.0001), and VKAs (P < 0.0001). Time to discontinuation was significantly longer for edoxaban vs. dabigatran, rivaroxaban, and VKAs (all P < 0.0001). More patients receiving NOACs QD had a PDC ≥ 0.8 compared with those receiving NOACs BID (65.3 vs. 49.6%, respectively; P < 0.05); persistence rates were comparable between QD and BID groups.
Conclusions
Patients with AF receiving edoxaban had significantly higher adherence and persistence compared with those receiving VKAs. This trend was also seen in NOAC QD regimens vs. NOAC BID regimens for adherence. These results provide insight into how adherence and persistence may contribute to the effectiveness of edoxaban for stroke prevention in patients with AF in Germany.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Treatment for atrial fibrillation with oral anticoagulation relies on adherence and persistence to be effective. |
Edoxaban was associated with greater adherence and persistence compared with most other non-vitamin K antagonist oral anticoagulants and vitamin K antagonists. |
Adherence to once-daily regimens of non-vitamin K antagonist oral anticoagulants was significantly higher compared with adherence to twice-daily regimens. |
These results provide a factor to help possibly explain edoxaban effectiveness previously observed in the real world. |
Introduction
Atrial fibrillation (AF) is the most common arrythmia worldwide, with a global prevalence of 0.5% and an estimated incidence in 2017 of approximately 403 new cases per million people [1]. The incidence of AF increases with age and is greater among males [1]. Characterized by an irregular and often rapid heartbeat, AF increases the risk of ischemic stroke and mortality five-fold and two-fold, respectively [2, 3]. Throughout the world, AF is a growing public health concern, with a steadily increasing prevalence contributing significantly to healthcare expenditures [1, 4]. Among European countries, Germany has one of the highest prevalence rates of AF, estimated at 2.1%, with an incidence of 4.1 per 1000 person-years [4, 5].
Oral anticoagulants (OACs) are the standard of care for the prophylaxis of thromboembolic events in patients with AF [2]. The 2020 European Society of Cardiology guidelines for AF recommend the use of non-vitamin K antagonist OACs (NOACs) over vitamin K antagonists (VKAs) [2]. In Germany, NOACs were licensed for the prevention of thromboembolism in patients with nonvalvular AF (NVAF) between 2011 and 2015: dabigatran in 2011, rivaroxaban in 2011, apixaban in 2012, and edoxaban in 2015 [6,7,8,9]. Edoxaban was effective in the prevention of ischemic stroke or systemic embolism among German patients with AF [10].
A therapy’s effectiveness in the real world relies on both adherence and persistence. Medication adherence refers to the patient’s propensity to use it as prescribed, and persistence describes the duration of time the patient continues its use [11]. When OACs are taken twice daily (BID) rather than once daily (QD), there is an increased risk of nonadherence [12, 13]. Other factors, such as older age, the existence of certain comorbidities, and the presence of multiple cardiovascular risk factors, may contribute to nonadherence [12, 14]. The negative impacts of nonadherence on treatment outcomes in AF include an increase in the odds of combined all-cause mortality and stroke, under-anticoagulation, and other thromboembolic cardiovascular events [12, 15, 16]. The objective of this study was to assess the adherence and persistence of edoxaban compared with other NOACs and VKAs among patients with AF in Germany to better understand the utilization patterns of OACs.
Methods
Data Source
The Deutsche Analysedatenbank für Evaluation und Versorgungsforschung is an administrative database that covers approximately 3.5 million statutory health-insured lives in Germany. This database, managed by Gesundheitsforen (GFL), is compliant with the General Data Protection Regulation (GDPR) in Europe and is exempt from obtaining Institutional Review Board (IRB) approval. This database constitutes a representative sample of the German population [17]. Basic information, such as age, sex, date of death, in- and outpatient diagnoses (as determined by International Classification of Diseases [ICD] codes), prescription data (Anatomical Therapeutical Chemical codes), and healthcare cost information, is available [10]. Data from January 2013 through December 2017 were used.
Study Population
Inclusion Criteria
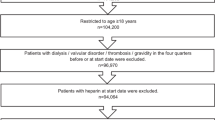
The study population included adult patients with AF in the database with at least one pharmacy claim for edoxaban, apixaban, dabigatran, rivaroxaban, or VKA during the study period. The confirmatory AF diagnosis was defined as at least one primary (hospitalization caused by AF) or secondary (patient with AF hospitalized because of a co-existing condition) hospital discharge AF diagnosis or at least one outpatient AF diagnosis before or on the index date, and at least one outpatient AF diagnosis between 12 months before to three months after the index date. Patients with continuous enrollment in the 12 months before the index date were also included. The first NOAC or VKA pharmacy claim was considered the index claim, and the date of the claim was considered the index date. Patients who initiated the index therapy were included. All inclusion criteria are summarized in Supplementary Table S1.
Exclusion Criteria
Patients who received any NOAC within 12 months of the index date, a VKA in the 12 months before the index VKA claim, and/or at least one NOAC or one NOAC plus VKAs on the index date were excluded. Additionally, patients diagnosed with certain conditions—such as valvular AF, deep vein thrombosis, pulmonary embolism, or end-stage renal disease—in the 12 months before or on the index date were excluded from the study. Patients who were pregnant in the 12 months before or on the index date or before the end of the study period were excluded, as were patients who had a joint replacement procedure in the 12 months before or on the index date. Lastly, patients who underwent other procedures that may have caused a disruption in their OAC regimen, such as heart valve replacement, were excluded from the analyses. Further details on conditions and procedures preventing inclusion and their corresponding ICD-9/ICD-10 codes are found in Supplementary Table S2. Exclusion criteria are summarized in Supplementary Table S1.
Baseline Characteristics
Baseline demographics, clinical characteristics, and pre-index healthcare resource utilization were gathered for all patients. Clinical characteristics included Charlson Comorbidity Index (type and total number) and three risk scores for patients with AF: CHADS2 (congestive heart failure, hypertension, age ≥ 75, diabetes, previous stroke/transient ischemic attack), CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75, diabetes, previous stroke/transient ischemic attack, vascular disease, age [65–74], female sex), and modified HAS-BLED (hypertension, abnormal liver/renal function, stroke history, bleeding history or predisposition, elderly, drug/alcohol use) [18,19,20]. History of stroke and medication history were also gathered.
Outcomes
Adherence
Medication adherence was assessed by the proportion of days covered (PDC) and the medication possession ratio (MPR). The PDC was the number of days covered from the first prescription to last prescription of the index therapy for the entire study period divided by the number of days in the entire study period. The MPR was calculated by the number of days of drug supply from the first prescription to last prescription of the index therapy for the entire study period divided by the number of days in the entire study period. Adherence was defined as the proportion of patients with a PDC and/or an MPR ≥ 0.8. Adherence outcomes were compared in patients on edoxaban vs. VKAs and other NOACs. Additionally, adherence in patients receiving NOAC QD vs. NOAC BID was analyzed.
Persistence
Medication persistence was assessed by time (in days) to discontinuation of the index therapy. Patients were considered persistent until a supply gap of > 90 days of the index therapy, as seen in previous studies [21, 22]. The proportions of patients continuing the index therapy at day 180 were reported. Persistence was compared in patients on edoxaban vs. VKAs and other NOACs and also in patients receiving NOACs QD vs. those receiving NOACs BID.
Adherence and persistence were assessed during the first 12-month period based on data availability and matching adjustment. Outcomes were measured during the first 12-month follow-up period, starting from the index prescriptions until (1) the first event, or (2) end of follow-up, or (3) discontinuation of the index OAC or change of OAC, or (4) death, whichever occurred first.
Statistical Analysis
Propensity Score Matching
The goal of propensity score matching (PSM) is to control for potential differences in baseline characteristics between cohorts in an observational study to reduce confounding [23,24,25,26]. Propensity score-matched cohorts with a 1:1 ratio were formed using the nearest neighbor matching algorithm and sampling without replacement. The results of the PSM analysis were substantiated with an inverse probability treatment weighting analysis, which reinforced the results [10].
Analysis
Before matching, Fisher’s exact test was used to test for differences between groups in categorical data; t tests were used for continuous variables. After matching, multivariable logistic regression was performed to quantify the odds of adhering to edoxaban vs. other OACs. Logistic regression was performed following PSM to reduce bias from potential residual confounding and identify key risk factors that affect adherence [27]. The Cox proportional-hazards model compared time to discontinuation. All data were analyzed using R Statistical Software (v3.6.3, R Core Team 2020).
Results
Baseline Characteristics
A total of 118,547 patients made a pharmacy claim for at least one NOAC or VKA in the database. Of these patients, 21,038 were eligible for the study, including 1236 edoxaban, 6053 apixaban, 1306 dabigatran, 7013 rivaroxaban, and 5430 VKA patients (Fig. S1). Of the patients receiving NOACs, 8249 patients were dosed QD (edoxaban and rivaroxaban patients) and 7359 were dosed BID (apixaban and dabigatran patients). Before matching, the mean age across all cohorts ranged from 70.9 to 74.3 years with the proportion of patients ≥ 75 years old ranging from 45.1 to 56.6%. Sex distribution was similar across cohorts, with approximately 40% female. Edoxaban patients had generally lower mean CHADS2 scores (2.0 vs. 2.3, 2.3, 2.0, and 2.2 for apixaban, dabigatran, rivaroxaban, and VKAs, respectively) and CHA2DS2-VASc scores (3.5 vs. 4.0, 3.8, and 4.0 for apixaban, dabigatran, and VKAs, respectively). Mean modified HAS-BLED scores for edoxaban patients were lower vs. most other OAC patients (2.3 vs. 2.5, 2.4, 2.3, and 2.5 for apixaban, dabigatran, rivaroxaban, and VKAs, respectively). Baseline characteristics before matching are summarized in Table 1. After matching, the cohorts were well balanced across demographics and clinical characteristics (Supplementary Tables S3–S6).
Outcomes Prior to Matching
Patients receiving edoxaban had a mean shorter follow-up duration compared with patients receiving other OACs (425 days vs. 629, 776, 782, and 870 days for apixaban, dabigatran, rivaroxaban, and VKAs, respectively) (Table 2). Among patients taking edoxaban, treatment adherence was significantly higher vs. apixaban, dabigatran, and VKAs (PDC ≥ 0.8: 63 vs. 50, 43, and 40%, respectively; all P < 0.0001; MPR ≥ 0.8: 66 vs. 54, 48, and 43%, respectively; all P < 0.0001). For edoxaban vs. rivaroxaban, the proportion of patients with a PDC ≥ 0.8 was 63 vs. 65% (P = 0.1286); the proportion of patients with an MPR ≥ 0.8 was 66 vs. 70% (P = 0.0212) (Table 2). Persistence was significantly greater among edoxaban patients compared to dabigatran, rivaroxaban, and VKAs patients (77 vs. 67, 72, and 56%, respectively; all P < 0.0001). Persistence was comparable between edoxaban and apixaban (77 vs. 77%; P = 0.6047) (Table 2).
Outcomes After Matching
Adherence
The proportion of patients with a PDC ≥ 0.8 was confirmed as significantly higher for edoxaban vs. apixaban (63 vs. 52%), dabigatran (62 vs. 41%), and VKAs (63 vs. 45%; all P < 0.0001) (Table 3). Edoxaban was associated with an increased likelihood of a PDC ≥ 0.8 vs. apixaban (odds ratio [OR] 1.64; 95% confidence interval [CI] 1.39–1.94), dabigatran (OR 2.40, 95% CI 1.99–2.88), and VKAs (OR 2.17, 95% CI 1.84–2.57; all P < 0.001) (Fig. 1). The proportion of patients with a PDC ≥ 0.8 was comparable between edoxaban and rivaroxaban (63 vs. 67%; OR 0.86, 95% CI 0.73–1.02; P = 0.0900) (Table 3 and Fig. 1).
Similarly, significantly more edoxaban patients were confirmed having an MPR ≥ 0.8 vs. apixaban (66 vs. 56%), dabigatran (65 vs. 46%), and VKAs (66 vs. 47%; all P < 0.0001) (Table 3). Edoxaban patients were more likely to have an MPR ≥ 0.8 vs. apixaban (OR 1.63, 95% CI 1.38–1.93), dabigatran (OR 2.32, 95% CI 1.93–2.79), and VKA patients (OR 2.29, 95% CI 1.94–2.72; all P < 0.001) (Fig. 1). Fewer edoxaban patients had an MPR ≥ 0.8 compared with rivaroxaban patients (66 vs. 71%; OR 0.81, 95% CI 0.68–0.97; P = 0.0190) (Table 3 and Fig. 1).
The proportion of patients with a PDC ≥ 0.8 was significantly higher for patients receiving NOACs QD compared with patients receiving NOACs BID (65.3 vs. 49.6%; P < 0.05) (Fig. 2). Correspondingly, significantly more patients receiving NOACs QD had an MPR ≥ 0.8 vs. patients receiving NOACs BID (69.5 vs. 53.3%; P < 0.05) (Fig. 2).
Post-matching logistic regression identified independent predictors of adherence outcomes. Key parameters associated with the proportion of patients with a PDC ≥ 0.8 for edoxaban vs. OAC were age, region, congestive heart failure, dementia, metastatic cancer, use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers therapy, and use of statins (Table 4). Similarly, age, region, congestive heart failure, dementia, metastatic cancer, use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers therapy, and use of statins were key independent predictors of the proportion of patients with an MPR ≥ 0.8 for edoxaban vs. OAC (Table 5).
Persistence
Persistence was significantly higher for edoxaban vs. dabigatran (76 vs. 66%; P < 0.0001), rivaroxaban (77 vs. 73%; P = 0.0153), and VKAs (77 vs. 59%; P < 0.0001) (Table 3). Persistence was comparable in the edoxaban group compared with the apixaban group (77 vs. 76%; P = 0.3658). Time to discontinuation was longer among edoxaban patients compared with dabigatran, rivaroxaban, and VKA patients (all P < 0.0001), and was similar in edoxaban and apixaban patients (Fig. 3a–d). At day 180, persistence rates were comparable between patients receiving NOACs QD (73.4%) and those receiving NOACs BID (74.2%; P = 0.13) (Fig. 2).
Discussion
This retrospective claims analysis of 21,038 patients with AF in Germany compared the adherence and persistence rates of edoxaban vs. other NOACs and VKAs. Edoxaban was associated with significantly higher adherence vs. apixaban, dabigatran, and VKAs and significantly higher persistence vs. dabigatran, rivaroxaban, and VKAs. Additionally, adherence to QD regimens of NOAC was significantly higher compared to adherence to NOAC BID regimens.
There is considerable research on the adherence and persistence of NOACs in AF patients, but few studies have comparatively assessed these measures in edoxaban. Currently published comparative adherence studies focus mainly on apixaban, dabigatran, and rivaroxaban, and all include slightly varied methodologies [28,29,30,31]. Among these studies, rivaroxaban consistently has the highest adherence rate [28,29,30,31]. The higher adherence rate for rivaroxaban compared with apixaban and dabigatran can be attributed to the lower pill burden associated with QD dosing compared to BID dosing [29, 31]. The impact of QD dosing is seen in this study as well, where adherence to edoxaban is significantly higher compared with adherence to apixaban and dabigatran and similar to that of rivaroxaban. Previous studies show that QD administration of a drug used to manage a chronic disease is associated with increased absolute adherence [9, 13, 32,33,34]. Therefore, dosing frequency may be an important factor for clinicians to consider when prescribing OACs to patients with AF. In addition to dosing frequency, other predictors of impaired adherence include older age and relevant comorbidities, mainly congestive heart failure, dementia, and metastatic cancer. Older age, dementia, and metastatic cancer are consistent with results from previous studies of other OACs [35, 36].
Apixaban, dabigatran, and rivaroxaban are associated with greater adherence vs. warfarin (47.5 vs. 40.2%; P < 0.001) [37]. The association between NOACs and increased adherence and persistence vs. VKAs may be due in part to the reliance on routine medical screening and international normalized ratio (INR) tests to calibrate VKA dosage [38,39,40]. Patients on VKA must therefore schedule regular visits to a clinic, which can be disruptive to normal life. This is especially relevant to persistence, because asking a patient to do this long-term can be burdensome. Patients receiving VKAs reported more feelings of inconvenience, frustration, and burden compared with those receiving NOACs [41]. A lower tolerability (e.g., higher occurrence of gastrointestinal discomfort for dabigatran) and safety profile (e.g., a higher incidence of bleeding events for VKAs, rivaroxaban, and 150 mg dabigatran) might also explain the impaired persistence with VKAs, rivaroxaban, and dabigatran vs. edoxaban; however, specific analyses on this topic in the real-world setting are needed. Of note, in the real-world GARFIELD-AF registry, both major and minor bleeding were predictors of OAC discontinuation in patients with newly diagnosed AF; importantly, this discontinuation was associated with a significantly higher risk of all-cause death, thromboembolic events, and myocardial infarction [42].
The largest strength of the current study is the sizable, representative sample size of included patients. Additionally, the sensitivity analyses were performed via the use of inverse probability treatment weighting (IPTW), which further verified the results of the PSM analyses. The results from IPTW and PSM were similar. Lastly, this is the only published study investigating all approved NOACs in Germany and VKAs in a comparative adherence and persistence analysis. This study provides a direct comparison of adherence between NOACs using robust real-world claims data.
However, this study had several limitations. First, primary and secondary AF diagnoses were recorded in the database, but these variables were not adjudicated and were prone to coding errors and omissions. Second, adherence and persistence were assessed using medication dispensing data (i.e., prescription and refill), however, the study did not actually determine if the patients were compliant in taking medication. This is a recognized limitation in observational studies associated with this type of health insurance data [10, 12, 16]. Third, INR results were not available in the database, therefore dosage changes in VKAs were not known to better understand the adherence/persistence pattern among VKA patients. Residual confounding may be present due to the unobserved factors. Additionally, as this analysis included about 3.5 million statutory health-insured lives, approximately 5% of the total lives covered by statutory health insurance in Germany, generalizing results to other patient populations should be cautioned. Lastly, the study only presented the 12-month adherence data for all OACs based on the available information from the database, and might not reflect long-term adherence pattern beyond 12 months, which can be addressed by future research.
Conclusions
In this study of over 20,000 German patients with AF, edoxaban was associated with increased adherence vs. apixaban, dabigatran, and VKAs and increased persistence vs. dabigatran, rivaroxaban, and VKAs. The results of this study provide a factor to help possibly explain edoxaban effectiveness previously observed in the real world. Clinicians should consider adherence and persistence findings when prescribing OACs to patients with AF.
Change history
18 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40119-023-00321-w
References
Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–21. https://doi.org/10.1177/1747493019897870.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612.
Migdady I, Russman A, Buletko AB. Atrial fibrillation and ischemic stroke: a clinical review. Semin Neurol. 2021;41(4):348–64. https://doi.org/10.1055/s-0041-1726332.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20. https://doi.org/10.2147/clep.s47385.
Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15(4):486–93. https://doi.org/10.1093/europace/eus333.
Pradaxa® (Dabigatran); 2011. https://www.akdae.de/Arzneimitteltherapie/NA/Archiv/2011030-Pradaxa.pdf.
Xarelto® (Rivaroxaban); 2013. https://www.akdae.de/Arzneimitteltherapie/NA/Archiv/201310-Xarelto.pdf.
Eliquis® (Apixaban); 2013. https://www.akdae.de/Arzneimitteltherapie/NA/Archiv/201303-Eliquis.pdf.
Lixiana® (Edoxaban); 2015. https://www.akdae.de/Arzneimitteltherapie/NA/Archiv/201504-Lixiana-DVT.pdf.
Marston XL, Wang R, Yeh YC, Zimmermann L, Ye X, Gao X, et al. Comparison of clinical outcomes of edoxaban versus apixaban, dabigatran, rivaroxaban, and vitamin K antagonists in patients with atrial fibrillation in Germany: a real-world cohort study. Int J Cardiol. 2022;346:93–9. https://doi.org/10.1016/j.ijcard.2021.11.008.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–7. https://doi.org/10.1111/j.1524-4733.2007.00213.x.
Emren SV, Senoz O, Bilgin M, Beton O, Aslan A, Taskin U, et al. Drug adherence in patients with nonvalvular atrial fibrillation taking non-vitamin K antagonist oral anticoagulants in Turkey: NOAC-TR. Clin Appl Thromb Hemost. 2018;24(3):525–31. https://doi.org/10.1177/1076029617693940.
Emren SV, Zoghi M, Berilgen R, Ozdemir IH, Celik O, Cetin N, et al. Safety of once- or twice-daily dosing of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with nonvalvular atrial fibrillation: a NOAC-TR study. Bosn J Basic Med Sci. 2018;18(2):185–90. https://doi.org/10.17305/bjbms.2017.2279.
Charlton A, Vidal X, Sabate M, Bailarin E, Martinez LML, Ibanez L. Factors associated with primary nonadherence to newly initiated direct oral anticoagulants in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2021;27(9):1210–20. https://doi.org/10.18553/jmcp.2021.27.9.1210.
Kimmel SE, Chen Z, Price M, Parker CS, Metlay JP, Christie JD, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) study. Arch Intern Med. 2007;167(3):229–35. https://doi.org/10.1001/archinte.167.3.229.
Shore S, Carey EP, Turakhia MP, Jackevicius CA, Cunningham F, Pilote L, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810–7. https://doi.org/10.1016/j.ahj.2014.03.023.
Lübbert C, Zimmermann L, Borchert J, Hörner B, Mutters R, Rodloff AC. Epidemiology and recurrence rates of clostridium difficile infections in Germany: a secondary data analysis. Infect Dis Ther. 2016;5(4):545–54. https://doi.org/10.1007/s40121-016-0135-9.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. https://doi.org/10.1001/jama.285.22.2864.
Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, Lip GY. Reliable identification of “truly low” thromboembolic risk in patients initially diagnosed with “lone” atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5(2):319–26. https://doi.org/10.1161/circep.111.966713.
Roldan V, Marin F, Fernandez H, Manzano-Fernandez S, Gallego P, Valdes M, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real-world” population with atrial fibrillation receiving anticoagulant therapy. Chest. 2013;143(1):179–84. https://doi.org/10.1378/chest.12-0608.
Banerjee A, Benedetto V, Gichuru P, Burnell J, Antoniou S, Schilling RJ, et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study. Heart. 2020;106(2):119–26. https://doi.org/10.1136/heartjnl-2019-315307.
Komen JJ, Heerdink ER, Klungel OH, Mantel-Teeuwisse AK, Forslund T, Wettermark B, et al. Long-term persistence and adherence with non-vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur Heart J Cardiovasc Pharmacother. 2021;7(FI1):f72–80. https://doi.org/10.1093/ehjcvp/pvaa017.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61. https://doi.org/10.1002/pst.433.
Lanehart RE, de Gil PR, Kim ES, Bellara AP, Kromrey JD, Lee RS. Propensity score analysis and assessment of propensity score approaches using SAS® procedures; 2012. https://support.sas.com/resources/papers/proceedings12/314-2012.pdf.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–8. https://doi.org/10.2307/2683903.
Austin PC. Double propensity-score adjustment: a solution to design bias or bias due to incomplete matching. Stat Methods Med Res. 2017;26(1):201–22. https://doi.org/10.1177/0962280214543508.
Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18(8):1150–7. https://doi.org/10.1093/europace/euv421.
McHorney CA, Ashton V, Laliberte F, Germain G, Wynant W, Crivera C, et al. Adherence to rivaroxaban compared with other oral anticoagulant agents among patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23(9):980–8. https://doi.org/10.18553/jmcp.2017.23.9.980.
McHorney CA, Crivera C, Laliberte F, Germain G, Wynant W, Lefebvre P. Adherence to rivaroxaban versus apixaban among patients with non-valvular atrial fibrillation: analysis of overall population and subgroups of prior oral anticoagulant users. PLoS One. 2018;13(4):e0194099. https://doi.org/10.1371/journal.pone.0194099.
McHorney CA, Peterson ED, Laliberte F, Germain G, Nelson WW, Crivera C, et al. Comparison of adherence to rivaroxaban versus apixaban among patients with atrial fibrillation. Clin Ther. 2016;38(11):2477–88. https://doi.org/10.1016/j.clinthera.2016.09.014.
Goette A, Hammwhöner M. How important it is for therapy adherence to be once a day? Eur Heart J Suppl. 2016;18:17–112. https://doi.org/10.1093/eurheartj/suw048.
Vrijens B, Heidbuchel H. Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace. 2015;17(4):514–23. https://doi.org/10.1093/europace/euu311.
Weeda ER, Coleman CI, McHorney CA, Crivera C, Schein JR, Sobieraj DM. Impact of once- or twice-daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta-regression analysis. Int J Cardiol. 2016;216:104–9. https://doi.org/10.1016/j.ijcard.2016.04.082.
Jankowska-Polanska B, Katarzyna L, Lidia A, Joanna J, Dudek K, Izabella U. Cognitive function and adherence to anticoagulation treatment in patients with atrial fibrillation. J Geriatr Cardiol. 2016;13(7):559–65. https://doi.org/10.11909/j.issn.1671-5411.2016.07.006.
Ording AG, Sogaard M, Nielsen PB, Lip GYH, Larsen TB, Grove EL, et al. Oral anti-coagulant treatment patterns in atrial fibrillation patients diagnosed with cancer: a Danish nationwide cohort study. Br J Haematol. 2022;197(2):223–31. https://doi.org/10.1111/bjh.18060.
Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5(2):e003074. https://doi.org/10.1161/jaha.115.003074.
Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost. 2016;115(1):31–9. https://doi.org/10.1160/th15-04-0350.
Nelson WW, Song X, Thomson E, Smith DM, Coleman CI, Damaraju CV, et al. Medication persistence and discontinuation of rivaroxaban and dabigatran etexilate among patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2015;31(10):1831–40. https://doi.org/10.1185/03007995.2015.1074064.
Kim H, Lee YS, Kim TH, Cha MJ, Lee JM, Park J, et al. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: a COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF). Korean J Intern Med. 2020;35(1):99–108. https://doi.org/10.3904/kjim.2017.415.
Coleman CI, Coleman SM, Vanderpoel J, Nelson W, Colby JA, Scholle JM, et al. Patient satisfaction with warfarin- and non-warfarin-containing thromboprophylaxis regimens for atrial fibrillation. J Investig Med. 2013;61(5):878–81. https://doi.org/10.2310/jim.0b013e31828df1bf.
Cools F, Johnson D, Camm AJ, Bassand JP, Verheugt FWA, Yang S, et al. Risks associated with discontinuation of oral anticoagulation in newly diagnosed patients with atrial fibrillation: results from the GARFIELD-AF registry. J Thromb Haemost. 2021;19(9):2322–34. https://doi.org/10.1111/jth.15415.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by Daiichi Sankyo Inc. Employees from Daiichi Sankyo Inc. participated in all aspects of this study, including its design, analysis, interpretation of data, writing of the report, and the decision to submit the article for publication. All authors had full access to study data and take responsibility for the integrity of the data and accuracy of the data analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Daiichi Sankyo Inc.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Kimberly Dent-Ferguson, MBS, MPH, and Margaret Van Horn, PhD, CMPP, of AlphaBioCom, a Red Nucleus company (King of Prussia, PA, USA), and funded by Daiichi Sankyo Inc.
Author Contributions
Giuseppe Patti, Rosa Wang, Xiaocong Li Marston, Yu-Chen Yeh, Lisa Zimmermann, Xin Ye and Xin Gao contributed to the planning, conduct, and/or reporting of the study. Giuseppe Patti, Rosa Wang, Xiaocong Li Marston, Lisa Zimmermann and Bernd Bruggenjurgen contributed to data interpretation and/or analysis. Giuseppe Patti, Rosa Wang, Xiaocong Li Marston, Yu-Chen Yeh, Lisa Zimmermann, Xin Ye, Xin Gao and Bernd Bruggenjurgen participated in writing and/or critical review of the manuscript and approved the final version for publication. Giuseppe Patti is responsible for the overall content of this work as guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
Giuseppe Patti received consulting fees; payment or honoraria for lectures, presentations, speaker’s bureaus, and manuscript writing or educational events; and support for attending meetings and/or travel from Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb-Pfizer, and Daiichi Sankyo Inc. Rosa Wang is an employee of Daiichi Sankyo Inc. Xiaocong Li Marston, Yu-Chen Yeh, and Xin Gao were employees of Pharmerit (now known as OPEN Health) at the time of the study and received consulting fees from Daiichi Sankyo Inc. Lisa Zimmermann is an employee of Gesundheitsforen Leipzig, who received consulting fees from OPEN Health. Xin Ye is an employee of Daiichi Sankyo Inc. Bernd Brüggenjürgen declares participation on an advisory board for Daiichi Sankyo Inc.
Compliance with Ethics Guidelines
Ethics committee approval was not required for this study as the data are retrospective health insurance claims data, which are exempt from obtaining IRB approval to access the database. We confirm that we had permission to access and use the data from GFL, the owner of the database. The Deutsche Analysedatenbank database is compliant with GDPR from GFL.
Data Availability
The data underlying this article were provided by GFL under license. The authors declare that data supporting the findings of this study are available within the article and its supplementary information files. Additional data may be shared upon request to the corresponding author with permission of GFL.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised: Corrections to Table 1, 2, 3 and Fig 1 updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Patti, G., Wang, R., Marston, X.L. et al. Anticoagulant Treatment Adherence and Persistence in German Patients with Atrial Fibrillation. Cardiol Ther 12, 371–391 (2023). https://doi.org/10.1007/s40119-023-00315-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00315-8