Abstract
Background
Non-vitamin K antagonist oral anticoagulants (NOACs) are at least as effective and safe as vitamin K antagonists (VKAs) for stroke prevention in atrial fibrillation (AF). All pivotal trials have compared NOACs to warfarin. However, other VKAs are commonly used, for instance phenprocoumon.
Patients and methods
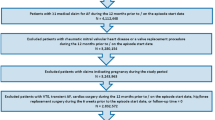
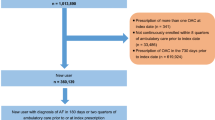
A retrospective cohort study using a German claims database assessed the comparative risks of bleeding leading to hospitalization during therapy with NOACs and phenprocoumon in AF patients. Endpoints consisted of major bleeding, gastrointestinal bleeding, and any bleeding. Data were collected from January 1, 2013 to March 31, 2015. Patients newly initiated on dabigatran, apixaban, rivaroxaban, or phenprocoumon were included. Hazard Ratios for bleeding events were derived from Cox proportional hazard models, adjusting for differences in baseline characteristics. Propensity score matching was performed as a sensitivity analysis.
Results
A total of 35,013 patients were identified, including 3138 on dabigatran, 3633 on apixaban, 12,063 on rivaroxaban, and 16,179 on phenprocoumon. Patients prescribed apixaban or phenprocoumon were older compared to those on dabigatran or rivaroxaban and had a higher CHA2DS2-VASc score. After adjusting for baseline confounders, apixaban was associated with lower risks of major bleeding (HR 0.68, 95% CI 0.51–0.90, p = 0.008), gastrointestinal bleeding (HR 0.53, 95% CI 0.39–0.72, p < 0.001), and any bleeding (HR 0.80, 95% CI 0.70–0.92, p = 0.002) compared to phenprocoumon. There were no significant differences in bleeding risk between dabigatran and phenprocoumon. Rivaroxaban was associated with more gastrointestinal bleeding (HR 1.39, 95% CI 1.21–1.60, p < 0.001) and any bleeding (HR 1.19, 95% CI 1.10–1.28, p < 0.001). Sensitivity analysis using propensity score matching confirmed these observations.
Conclusions
Apixaban therapy is associated with a significantly reduced risk of bleeding compared to phenprocoumon. Bleeding risk with dabigatran was similar to that of phenprocoumon but bleeding risk with rivaroxaban was higher.





Similar content being viewed by others
References
Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G (2013) Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace 15:486–493
Connolly SJ, Ezekowitz MD, Yusuf S et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Patel MR, Mahaffey KW, Garg J, Pan G et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Granger CB, Alexander JH, McMurray JJ et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–992
Giugliano RP, Ruff CT, Braunwald E et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369:2093–2104
Verhoef TI, Redekop WK, Daly AK et al (2013) Pharmacogenetic-guided dosing coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br J Clin Pharmacol 77(4):626–641
Andersohn F, Walker J (2016) Characteristics and external validity of the German Health Risk Institute (HRI) database. Pharmacoepidemiol Drug Saf. doi:10.1002/pds.3895
Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, Ihle P (2015) German Society for Social Medicine and Prevention; German Society for Epidemiology. Good Practice of Secondary Data Analysis (GPS): guidelines and recommendations. Gesundheitswesen 777:120–126
Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning: data mining, inference and prediction, 2nd edn. Springer, Berlin
Schoenfeld D (1982) Partial residuals for the proportional hazards regression model. Biometrika 69:239–241
Robins JM, Hernán MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology 11:550–560
Hernán MA, Brumback B, Robins JM (2002) Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat Med 2:689–170913
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
Jahnchen E, Meinertz T, Gilfrich HJ, Groth U, Martini A (1976) The enantiomers of phenprocoumon: pharmacodynamic and pharmacokinetic studies. Clin Pharmacol Ther 20:342–349
Alan SG, Daniel S et al. (2015) Mini-sentinel medical product assessment: a protocol for assessment of dabigatran. https://www.sentinelsystem.org/sites/default/files/Drugs/Assessments/Mini-Sentinel_Protocol-for-Assessment-of-Dabigatran_0.pdf
Lip GY, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, Hamilton M (2016) Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost 116:975–986
Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA (2016) Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. doi:10.1161/JAHA.116.003725
Graham DJ, Reichman ME, Wernecke M et al (2015) Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 131:157–164
Hernandez I, Baik SH, Piñera A, Zhang Y (2015) Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med 175:18–24
Acknowledgements
This analysis was funded by Bristol-Myers Squibb and Pfizer. Part of this work has been presented at the European Society of Cardiology Congress 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Professor Hohnloser has served as a consultant for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Jansen. Professor Nabauer has received lecture fees from Bayer, BMS/Pfizer, Boehringer Ingelheim, and Daiichi Sankyo. Dr. Basic is an employee of Pfizer Deutschland GmbH. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hohnloser, S.H., Basic, E. & Nabauer, M. Comparative risk of major bleeding with new oral anticoagulants (NOACs) and phenprocoumon in patients with atrial fibrillation: a post-marketing surveillance study. Clin Res Cardiol 106, 618–628 (2017). https://doi.org/10.1007/s00392-017-1098-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-017-1098-x