Abstract
Cancer and cardiovascular disease are two of the leading causes of global mortality and morbidity. Medical research has generated powerful lifesaving treatments for patients with cancer; however, such treatments may sometimes be at the expense of the patient’s myocardium, leading to heart failure. Anti-cancer drugs, including anthracyclines, can result in deleterious cardiac effects, significantly impacting patients’ functional capacity, mental well-being, and quality of life. Recognizing this, recent international guidelines and expert papers published recommendations on risk stratification and care delivery, including that of cardio-oncology services. This review will summarize key evidence with a focus on anthracycline therapy, providing clinical guidance for the non-oncology professional caring for a patient with cancer and heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiotoxicity can disrupt cancer treatment, resulting in adverse patient outcomes. |
Recently published international guidelines outline strategies for risk stratification and care delivery. |
Communication and collaborative working across cardiology and oncology specialisms, with input from medical, nursing and allied professionals, can promote a tailored patient- and family-centred experience. |
This review aims to provide a holistic, multidisciplinary overview of the most common issues in cardio-oncology. |
Introduction
Cardiovascular disease and cancer are the two main causes of morbidity and mortality worldwide [1]. Medical treatment for patients with cancer has significantly improved survival; however, some treatment modalities can lead to the development of serious cardiovascular complications, including heart failure (HF). The occurrence of such complications may result in temporary or permanent cessation of cancer treatment, depending on severity, with consequential short and long-term health implications [2,3,4]. Over the last decade there has been growing interest in the unique specialism known as cardio-oncology, with professionals seeking to ensure patients receive optimum cardiac treatment following a cancer diagnosis. Early identification of risk, with the introduction of integrated care provided by multidisciplinary cardio-oncology teams, was recommended in recent expert guidelines and a position statement [4,5,6]. The aim of this review is to provide non-oncology specialists with practical guidance on risk stratification with a focus on surveillance pathways for patients who have received anthracycline. In addition, an overview of pertinent topics, including the valuable contributions of cardio-oncology services, exercise rehabilitation and patient-reported outcomes, will be presented. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Scale of the Problem
A causal relationship has been noted between HF and cancer; they share not only common risk factors, such as ageing, male sex and diabetes mellitus, but also pathophysiological mechanisms, including inflammation, neuro-hormonal activation, oxidative stress and a dysfunctional immune system [1]. A proportion of today’s patients who survive a cancer diagnosis proceed to develop HF due to their chemotherapy, radiotherapy or immunotherapy.
Several chemotherapy drugs are recognized as being ‘cardiotoxic’ or causing cardiovascular injury affecting myocardial function [7, 8]. Differing definitions of cardiotoxicity have been used over the past 3 decades, leading to heterogeneity in diagnosis and treatment [9, 10]. To harmonize definitions, the International Cardio-Oncology Society released a consensus statement in 2022 classifying cancer-therapeutics-related cardiac dysfunction (CTRCD) into symptomatic heart failure (including a reduced ejection fraction and supportive diagnostic biomarkers in line with current HF guidance) and asymptomatic categories [11].
Mild asymptomatic CTRCD was defined as a new relative decline in global longitudinal strain (GLS) of more than 15% from baseline and or a new rise in biomarkers (with a preserved ejection fraction of 50% or more). Moderate asymptomatic CTRCD is defined as a reduction in ejection fraction of 10 percentage points or more to an ejection fraction of 40–49%. Alternatively, moderate asymptomatic CTRCD is diagnosed in patients with a reduction of less than 10 percentage points (to an ejection fraction of 40–49%) with a new decline in global longitudinal strain of more than 15% from baseline and/or a new rise in cardiac biomarkers. Severe asymptomatic CTRCD is defined as a new ejection fraction reduction to below 40%. The implementation of these definitions is supported by guidance from the European Haematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO), the International Cardio-Oncology Society (IC-OS) and the task force on cardio-oncology of the European Society of Cardiology (ESC) [4]. Well-known cardiotoxic drugs include anthracyclines, as well as many targeted therapies such as small molecule tyrosine kinase inhibitors (sunitinib) and proteasome inhibitors (carfilzomib). The position statement from the Cardio-Oncology Study Group of the Heart Failure Association of the ESC in collaboration with the IC-OS provides a table outlining cancer therapy classes and their associated cardiovascular toxicities [5].
Anthracyclines are the most studied cardiotoxic drugs, accomplishing their effective antitumour activity by infiltrating DNA, impairing transcription and cell division, inhibiting topoisomerase II activity, producing reactive oxygen species, and damaging DNA as well as cell membranes and mitochondria [12]. Human epidermal growth factor receptor-2 (HER2) is therefore required for cell proliferation, differentiation and survival when HER2-targeted therapies such as trastuzumab bind to these receptors and cause downregulation of action [13]. In a population study including over 12,000 females, those treated with anthracycline plus trastuzumab had an increased risk of HF and or cardiomyopathy [14]. Furthermore, Bowles found that cardiotoxic treatments, such as anthracycline and trastuzumab, were more likely to be administered to young healthy females [14] (see “Clinical Case 1” below).
Clinical Case 1
Mrs MT, a 45-year-old lady, was diagnosed with left breast ductal carcinoma in situ (DCIS) in 2006, which became recurrent invasive ductal carcinoma in 2017. She underwent a left mastectomy and chemotherapy with agents including anthracycline, followed by long-term letrozole.
In 2019 she presented to her GP with abdominal distension and dyspnea and was immediately referred to a cardiologist. Investigations at the cardiac consultation included ECG, showing sinus tachycardia, and echo, showing severe systolic dysfunction (EF: 30%) with severe mitral regurgitation. She was prescribed evidence-based HF medication (ACE inhibitor, B-blocker, spironolactone and loop diuretic) and referred to the regional cardio-oncology clinic.
Mrs MT was not initially informed about possible cardio-toxicity due to chemotherapy for cancer and therefore did not recognize symptoms. She was traumatized by the heart failure diagnosis. Comprehensive education and psychological support were provided, albeit late, to help her adapt to and manage this diagnosis.
In 2020, the European Society of Medical Oncology (ESMO) consensus guidance recommended surveillance for potentially cardio-toxic anticancer treatments, including radiotherapy, chemotherapy drugs or targeted therapies [15]. Indeed, cardio-oncology surveillance can improve cancer outcomes by minimizing therapy delays and treating cardiotoxicity at an early, potentially reversible stage.
Monitoring and Assessing Risk
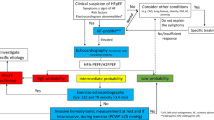
Cardiotoxicity risk changes with time and, as such, an assessment of risk should be conducted periodically. Baseline stratification aims to facilitate timely mitigation of potential risk factors and individualize cancer therapeutic and cardiotoxicity surveillance strategies without imposing any delay on treatment. This requires a comprehensive clinical history (including previous cancer treatments) and examination. Pareek et al. and, more recently, Cuomo et al. showed the importance of risk stratification prior to commencing cancer treatment, enabling high rates of oncologic treatment with improved health outcomes, i.e. improvements in ejection fraction and functional New York Heart Association (NYHA) classification. In 2020, the Heart Failure Association (HFA) and the International Cardio-Oncology Society (ICOS) published a formal risk stratification tool based on both expert consensus and contemporary data [4]. The tool stratified patients into low (< 2%), moderate (2–9%), high (10–19%) or very high (≥ 20%) cardiovascular risk [29]. The ESC 2022 cardio-oncology guidelines formally advocated the use of this HFA-ICOS tool, on which it based a detailed surveillance programme spanning from a pre-treatment baseline to post-treatment and long-term surveillance [4]. This guidance informs Fig. 1, which consolidates baseline assessment and scoring along with end of treatment, 1 year post-treatment and long-term follow-up. The American Heart Association also recommended the monitoring of cardiac function, supporting the use of key investigations for risk stratification—serum biomarkers (troponin) and imaging [3].
Surveillance strategy for anthracycline-treated patients. Adapted from the ESC 2022 cardio-oncology guidelines. If Mean Heart Dose (MHD) is not available from patient records, the prescribed dose may be utilised. A MHD ≥15 Gy equates to ≥35Gy prescribed dose; A MHD 5-15 Gy equates to 15-34Gy prescribed dose; A MHD <5 Gy equates to <15 Gy prescribed dose [4]. AC anthracycline, BP blood pressure, BMI body mass index, CV cardiovascular, D.E. doxorubicin equivalent, ECG electrocardiogram, Gy grays, Hx history, M months, MHD mean heart dose, NP natriuretic peptide, RTx radiotherapy, Tx treatment, U&E urea and electrolytes, Y years. * If abnormal, refer to cardio-oncology;(*) consider cardio-oncology referral
Serum Biomarkers
Troponins and natriuretic peptides are the most widely studied, informing risk stratification, diagnosis, and prognosis. In 2020, the Cardio-Oncology Study Group of the HFA collaborated with the Cardio-Oncology Council of the ESC to review evidence on the role of troponin and natriuretic peptides before, during and after cardiotoxic cancer therapies [16].
Troponin
Troponins are markers of acute cardiomyocyte injury and can help identify toxicity in the early stages of cancer treatment. Cardinale et al. studied over 200 breast cancer patients treated with high-dose chemotherapy and observed that large elevations in troponin I could predict significant and persistent deteriorations in LVEF up to 1 year [17, 18]. High and ultrasensitive troponins can improve the prediction of early cardiotoxicity and mortality in patients receiving anthracyclines and HER2-targeted therapies [19,20,21]. Their increased sensitivity is, however, associated with reduced specificity, as multiple non-cardiovascular complications during cancer therapy (i.e. renal dysfunction, pulmonary embolism, sepsis) can elevate troponin levels [22].
Peri-therapeutic biomarker assessment has been shown to facilitate the planning of successive downstream therapies. The Herceptin Adjuvant Study Cardiac Marker Substudy (HERA) included 452 patients, with results demonstrating that an elevated ultrasensitive troponin post-anthracycline therapy could identify patients at risk of cardiotoxicity prior to subsequent HER2-targeted treatment [23].
Evidence is less convincing on the use of troponin monitoring for long-term surveillance of cardiotoxicity. In a meta-analysis of childhood cancer survivors involving 1651 survivors, Leerink et al. demonstrated echocardiographic evidence of LV dysfunction in approximately 12% of the population. However, in five of the relevant studies, elevated troponin levels were not associated with left ventricular dysfunction [24, 25].
Natriuretic Peptides
Natriuretic peptides are produced from the heart in response to increased myocardial wall strain, typically due to systolic dysfunction. This may therefore be used to identify at-risk patient groups [21]. Specifically in cardio-oncology populations, there is some evidence that persistent peri-treatment elevations of B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are associated with cardiac dysfunction at 1 year [26, 27]. In a large Danish study of 333 patients, Skovgaard et al. demonstrated an association between elevated peri-treatment BNP and late congestive HF and mortality [26]. Similarly, persistently increased NT-proBNP was associated with abnormal diastolic function in a study by Sandri et al. [27]. Conversely, in a study by Daugaard et al., BNP levels at baseline or during therapy failed to predict dysfunction [28].
Evidence for NT-proBNP is therefore heterogeneous, with a moderate predictive ability in adult and childhood cancer survivors [24, 25, 29]. Furthermore, as natriuretic peptide levels may be affected by patients with metastatic disease, as well as in those with an elevated or low body mass index, imaging should fundamentally be a part of a surveillance programme [30]. Based on current evidence, the ESC recommended an annual assessment of natriuretic peptides alone for long-term post-treatment surveillance [4].
Combined blood and imaging biomarker approaches have also been explored. For example, Sawaya et al. studied 43 patients treated for breast cancer. Concurrent global longitudinal strain imaging and ultrasensitive troponin-I assessment during treatment with anthracycline and trastuzumab were found to predict subsequent cardiotoxicity [20].
Imaging
Echocardiogram
Echocardiography is the mainstay of imaging techniques in cardiotoxicity surveillance. The LVEF is measured by tracing the endocardial border in diastole and systole using 2D images in two planes; however, this method can be susceptible to high temporal variability [31]. Newer techniques such as three-dimensional (3D) echocardiography are more sensitive than the two-dimensional (2D) measures and have superior accuracy and reproducibility [31]. Furthermore, abnormalities in myocardial strain, a measure of deformation, precede deteriorations in ejection fraction, and values have been found to correlate with fibrosis [32, 33]. In a systematic review of 1504 patients, Thavendiranathan et al. found that a peri-therapeutic strain decline of 10–15% was predictive of subsequent cardiotoxicity [32]. Whilst evidence remains limited on the long-term outcomes in chemotherapy patients with abnormal strain, abnormal strain in non-cancer populations is an independent predictor of cardiovascular morbidity and mortality [34, 35]. In addition to the 3D ejection fraction and strain imaging, there is emerging evidence on the role of additional indicators such as diastolic function and right heart assessment.
Historic guidelines advocated echocardiographic screening when a threshold dose of anthracycline had been reached; however, dose thresholds varied widely, therefore resulting in variance of screening practice [5, 21, 24]. Consensus guidelines recommend risk stratification for childhood cancer survivors according to the dose of anthracycline and radiotherapy. Accordingly, echocardiography should be considered every 2 and 5 years for those at high and moderate risk respectively [36]. In addition to cardiomyopathy, patients who have received radiation to the mediastinum are at risk of valvular disease. ESC guidelines recommend that asymptomatic patients who have received more than 15 Gy mean heart dose or combination therapy of more than 5 Gy mean heart dose and 100 mg/m2 doxorubicin equivalent have an echocardiogram at 5-yearly intervals after treatment [4].
In 2020, the HFA, the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the ESC called for the development of treatment algorithms for all patients receiving anthracycline and HER2 therapies to inform clinical practice [37]. The following year, the British Society of Echocardiography (BSE) and British Cardio-Oncology Society (BCOS) provided targeted imaging surveillance protocols for use during cancer treatment [38].
Cardiac Magnetic Resonance Imaging (MRI)
As the gold standard for function and volumetric assessment, cardiac MRI offers an alternative imaging modality, especially for patients with poor-quality images. Mapping techniques and MRI-derived strain imaging may offer additional imaging biomarkers of cardiotoxicity in the future [39,40,41]. Early decreases in T1 times after an initial anthracycline dose were found by Muehlberg et al. to predict the subsequent cardiotoxicity in 30 patients treated for sarcoma [40]. Conversely, Jordan et al. showed that a late increase in T1 times may predict cardiotoxicity, reflective of interstitial fibrosis [39]; however, such techniques are in an early phase of investigation. MRI, whilst being the gold standard for evaluating myocardial function and volumes, remains expensive and not widely available, and is therefore recommended when echocardiographic imaging is suboptimal [38].
HFA-ICOS Risk Stratification Tool
This HFA–ICOS tool risk stratifies patients based on their cardiovascular history, cardiovascular risk profile, previous chemotherapy and baseline imaging/biomarker status (see Fig. 1) [5]. This risk categorization enables decisions regarding cardiology input, cancer therapeutic strategy and use of cardioprotective agents. In high and very high risk patients, minimizing the use of cardiotoxic agents is advised where possible, along with the initiation and use of specific chemotherapeutic cardioprotective agents, such as dexrazoxane and liposomal anthracyclines, alongside cardioprotective agents, for example angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers and statins. Cardiovascular disease and modifiable risk factors should be treated as outlined within the guidelines [4].
The first year post cancer therapy is believed to be of particular importance in cardiotoxicity surveillance. Research by Cardinale et al. noted that the majority (98%) of cardiotoxicity occurs within this first year (median follow-up 5.2 years) [10]. In addition, for a patient group considered to be relatively treatment resistant, early initiation of treatment was frequently found to be associated with recovery of cardiac function. At the end of treatment, repeat risk stratification should consider the treatment strategy along with the dose used and biomarker and imaging data, in addition to baseline risk.
Due to the high rates of early cardiotoxicity, risk stratification should be repeated at 1 year post treatment and repeated 5-yearly until end of life. In addition, due to the elevated risk of proximal coronary artery disease, patients who have received high-dose radiotherapy may be considered for non-invasive coronary artery disease surveillance at 5- to 10-year intervals [4].
There is no safe dose of anthracycline therapy, and every cancer survivor, regardless of age at treatment, who has received potentially cardiotoxic treatment should have an annual clinical review that includes a cardiovascular risk factor assessment [4].
Regarding childhood cancer survivors, it is important to remember that a ‘developing’ heart is at particular risk of toxicity, which sometimes occurs decades after the initial treatment. Lifelong surveillance of children who undergo cancer treatment should be considered. Moderate risk patients should be considered for echocardiographic screening at least every 5 years and high risk patients should be screened at least every 2 years [36]. The ESC use anthracycline and radiotherapy dose alone to classify childhood cancer survivor (CCS) risk (see treatment risk factors in Fig. 1); however, other risk calculators exist, such as those developed from the Childhood Cancer Survivor Study (CCSS) data (N = 22,643) and validated in additional multinational childhood cancer cohorts (https://ccss.stjude.org/cvcalc) [4, 42]. Similar to the HFA-ICOS proforma, this risk calculator incorporates treatment strategy, demographics, and traditional cardiovascular risk factors; however, it is only validated for patients currently aged below 40 years [42]. Each point of contact offers an opportunity for patient education, lifestyle education and management of risk factors, which are fundamental to optimal patient care.
Cardioprotective Treatment
The early initiation of cardioprotective medication is particularly important as recovery in myocardial function appears to be limited and temporary in patients with established cardiomyopathy [43, 44]. There is evidence from several small, randomized control trials suggesting that angiotensin-converting-enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), or selected beta blockers (BBs, such as carvedilol and nebivolol) administered during anthracycline chemotherapy (with or without subsequent trastuzumab treatment) can reduce the risk of significant left ventricular dysfunction during follow-up [4, 15, 45]. A period of subclinical cardiotoxicity often precedes overt cardiotoxicity, providing an important opportunity to introduce cardioprotective medications. As with all patients with HF, evidence-based medication (including ACEi and BB) can be initiated at a low dose in the acute phase. At a later stage, patients should be reviewed and uptitrated to optimal tolerated doses, with additional renin-angiotensin-aldosterone therapies added [6].
Cancer Patients and Exercise Rehabilitation
A central component of cardiac rehabilitation programmes for patients with HF is exercise training. Acknowledged in a class 1, level A recommendation within recent European HF guidelines, the benefits of exercise are well known: it improves cardiovascular reserve capacity, leading to concomitant reductions in cardiovascular morbidity, symptoms and quality of life [6, 46]. Patients presenting with HF following cancer treatment experience similar effectiveness [47, 48]. Exercise training can improve the patient's functional capacity, reliably assessed by measuring peak oxygen consumption (VO2max) [49, 51]. However, improved functional capacity can also be identified by reduction in the patient's heart rate [47] or performance in a 6 minute walking test [52]. Evidence is commonly related to breast cancer, colorectal cancer, lung resection, some leukaemias and lymphomas [52,53,54,55]. The recently published Breast Cancer Randomized Exercise Intervention study (BREXIT) included 104 females, with results concluding that exercise training can improve VO2peak and cardiac reserve [56]. Finally, in an observational study conducted by Williamson et al., the 361 patients who completed a 12-week exercise-based cardiac rehabilitation programme experienced an improvement in their cardiorespiratory fitness and survival [57]. This emphasizes the need for improved access to and support for patients with HF and cancer from multidisciplinary cardio-oncology teams.
Exercise prior to [58] or after a cancer diagnosis, both during the chemotherapy period [48, 59] and in the following weeks [47, 49], was associated with preventing cardiovascular disease, including HF and coronary heart disease [51]. Tsai et al. conducted a feasibility study of a home-based and clinic-based exercise intervention. Results found the intervention to be safe, with adherence and satisfaction improving when it was provided in the patient’s home [49]. Further longitudinal studies are warranted [60]; however, for many patients, exercise can ameliorate the functionality lost as a side-effect of cancer itself (such as sarcopenia and cachexia) and as a result of cardiotoxicity [61,62,63].
Aerobic exercise training at a moderate intensity performed at least 3 to 4 times a week for 30–45 min appears to be the best type and quantity to improve patients’ functional capacity [49, 50, 53, 54]. Supervised exercise training is the most common delivery; however, home-based training can provide equally good results [49, 54]. Some studies indicate the inclusion of strength training to improve patients’ muscle mass during and after chemotherapy [52, 64, 65]. Other studies, mainly including patients with breast cancer, found that high-intensity interval training (HIIT) had positive results [66, 67]; however, further supportive studies are needed. Finally, in a systematic review and meta-analysis of 33 studies, Chen et al. concluded the potential benefit of tai chi in improving physical ability in patients with four chronic conditions, one of which was HF [68].
As stated for other populations of patients, exercise training for cancer patients must be individualized [69] and take account of the patient’s previous history of exercise, their current fitness state, and their motivation and preferences.
Contribution of Cardio-Oncology Services
In recognition of the interplay between cardiovascular disease and cancer treatments, specialized cardio-oncology services have emerged with a view to providing an integrated multidisciplinary approach to cancer patients at risk of cardiotoxicity. The primary goal of cardio-oncology services is to deliver potentially lifesaving cancer therapies whilst mitigating cardiovascular disease risk and the provision of cardioprotective agents [70].
The scope of cardio-oncology services is wide ranging, including the prevention and early identification of cardiotoxicity, timely cardiovascular risk factor modification, serial monitoring with imaging and/or biomarkers, and the provision of evidence-based medical therapy for existing or emerging cardiovascular disease [4]. Lancellotti et al. outlined that the central tenets of cardio-oncology service are expert specialized multidisciplinary teams (including medical and radiation oncologists, haematologists, cardiologists, and specialized nurses) collaborating within a partnership network using established referral pathways, care protocols, effective communication tools and administrative resources [71]. This is described visually in Fig. 2. Unfortunately, the availability and structure of current cardio-oncology services remain globally diverse [71, 72], which can be attributed to limited organizational structures and competence of professionals to manage cardiovascular issues that arise in cancer patients. This can ultimately lead to poorer health outcomes for patients [73,74,75].
Nevertheless, the benefits of a dedicated cardio-oncology service have been reported by studies conducted in Italy and the United Kingdom [76, 77]. Collaboration among cardiology and oncology specialists is integral prior to the delivery of any cancer therapy to enable early recognition, management, support and optimal care of cardiac toxicity [78,79,80]. Patients emphasized the need for more personalized care and multi-disciplinary collaboration to ensure more tailored and holistic care [74, 80, 81].
An interpretative qualitative study conducted by White et al. [82] involved 15 patients who attended a newly established cardio-oncology clinic in a large regional city in Australia. The aim of the study was to explore the patients’ perceptions of cardio-oncology services and the impact of such a service on an integrated approach to care. The study found that access to a cardio-oncology service promoted feelings of personalized patient-centred care and improved patients’ understanding of the association between cancer treatment and cardiotoxicity. In contrast, some patients reported difficulty prioritizing cardiovascular risk factor modification (weight management, diet, alcohol, engaging in physical activity) during their cancer treatment as limited education and support were received from healthcare professionals. The findings from this study underline the need for the development of dedicated cardio-oncology rehabilitation programmes [4].
Optimizing Patient-Reported Outcomes
Several recent publications have focused on the importance of health-related quality of life (HR-QoL) for patients living with both a cancer and HF diagnosis [80, 83]. In general, perceived HR-QoL can vary according to the time the assessment was carried out (prior to diagnosis, patient undergoing treatment or as a cancer survivor), the unique symptoms (functional, psychological, or social) as well as the priorities of each patient. However, a variety of instruments have been used to assess quality of life in this cohort of patients, ranging from the EQ-5D to the SF-36 and the European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire C30 (QLQ-C30 or QLQ) [84]. Harrison et al. carried out a population study in America, recruiting females aged > 65 years with a history of breast cancer. The authors reported that those who developed HF showed an impairment in all HR-QOL domains (SF-36 instrument) and a resultant negative impact on daily activities. Additional analysis found that those females who had a HF and cancer diagnosis experienced more physical HR-QOL deficits across all cancer stages and mental HR-QOL deficits in females specifically with stage I/II cancer. Of particular interest was that females at an earlier stage of the cancer journey experienced the worst impact on HR-QOL associated with a diagnosis of HF [85].
Regular patient self-assessment and reporting of HR- QoL status can significantly improve physical and mental well-being, reduce emergency room visits, and extend mean survival in patients with solid tumours [83]. Notably, barriers such as a lack of knowledge by health professionals and misconceptions that cardiac monitoring is not a necessity in oncology patients delayed cancer treatment, adversely affecting patients’ cardiac surveillance and HR-QoL [74]. The development and validation of a specific patient-reported outcome tool to assess quality of life is urgently required. Furthermore, a multidisciplinary team of physicians and nurse practitioners working across cardiology and oncology specialisms should aim to integrate short- and long-term follow-up appointments, enabling a holistic care approach that enhances patients’ physical, spiritual, and psychosocial well-being [81].
Conclusion
The increasing global prevalence of cancer and likelihood of HF make the early identification and risk stratification of patients a clinical priority. Tools such as the HFA-ICOS tool have been developed to prompt tailored cancer therapies and early initiation of cardioprotective agents. Patient information and support is required to promote self-management and improve health-related quality of life. This would best be facilitated within a cardio-oncology clinic, enabling short- and long-term follow-up of this vulnerable cohort of patients.
References
de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21(12):1515–25.
Geisberg CA, Sawyer DB. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr Hypertens Rep. 2010;12(6):404–10.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361.
Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801.
Pan J, Garza F, Lyon AR. Cardio-oncology: rationale, aims and future directions. Curr Opin Support Palliat Care. 2021;15(2):134–40.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–8.
Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–99.
Minami M, Matsumoto S, Horiuchi H. Cardiovascular side-effects of modern cancer therapy. Circ J. 2010;74(9):1779–86.
Albagoush SA, Limaiem F. HER2. StatPearls. Treasure Island: StatPearls Publishing LLC; 2022.
Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–305.
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–90.
Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur J Heart Fail. 2020;22(11):1966–83.
Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36(2):517–22.
Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13(5):710–5.
Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24(6):774–82.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80.
Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101(23):1874–80.
Newby LK, Rodriguez I, Finkle J, Becker RC, Hicks KA, Hausner E, et al. Troponin measurements during drug development—considerations for monitoring and management of potential cardiotoxicity: an educational collaboration among the Cardiac Safety Research Consortium, the Duke Clinical Research Institute, and the US Food and Drug Administration. Am Heart J. 2011;162(1):64–73.
Zardavas D, Suter TM, Van Veldhuisen DJ, Steinseifer J, Noe J, Lauer S, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2017;35(8):878–84.
Leerink JM, Verkleij SJ, Feijen EAM, Mavinkurve-Groothuis AMC, Pourier MS, Ylänen K, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart. 2019;105(3):210–6.
Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al-Rashid F, Rassaf T, et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22(2):350–61.
Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS ONE. 2014;9(5): e96736.
Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, et al. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem. 2005;51(8):1405–10.
Daugaard G, Lassen U, Bie P, Pedersen EB, Jensen KT, Abildgaard U, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7(1):87–93.
López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41(18):1720–9.
Tonry C, Russel-Hallinan A, McCune C, Collier P, Harbinson M, Dixon L, et al. Circulating biomarkers for management of cancer therapeutics related cardiac dysfunction. Cardiovasc Res. 2022. https://doi.org/10.1093/cvr/cvac087.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61(1):77–84.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25):2751–68.
Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. 2018;11(8):1173–86.
Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54(7):618–24.
Stanton T, Ingul CB, Hare JL, Leano R, Marwick TH. Association of myocardial deformation with mortality independent of myocardial ischemia and left ventricular hypertrophy. JACC Cardiovasc Imaging. 2009;2(7):793–801.
Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–36.
Dobson R. BSE and BCOS guideline for transthoracic echocardiographic assessment of adult cancer patients receiving anthracyclines and/or trastuzumab. J Am Coll Cardiol CardioOnc. 2021;3(1):1–16.
Jordan JH, Vasu S, Morgan TM, D’Agostino RB Jr, Meléndez GC, Hamilton CA, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016. https://doi.org/10.1161/CIRCIMAGING.115.004325.
Muehlberg F, Funk S, Zange L, von Knobelsdorff-Brenkenhoff F, Blaszczyk E, Schulz A, et al. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail. 2018;5(4):620–9.
Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111(5):717–22.
Chen Y, Chow EJ, Oeffinger KC, Border WL, Leisenring WM, Meacham LR, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112(3):256–65.
Melendez G. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. 2020;7:26.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–20.
Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48(11):2258–62.
Taylor RS, Long L, Mordi IR, Madsen MT, Davies EJ, Dalal H, et al. Exercise-based rehabilitation for heart failure: cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019;7(8):691–705.
Nair N, Gongora E. Heart failure in chemotherapy-related cardiomyopathy: can exercise make a difference? BBA Clin. 2016;6:69–75.
Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26(3):305–15.
Tsai E, Mouhayar E, Lenihan D, Song J, Durand JB, Fadol A, et al. Feasibility and outcomes of an exercise intervention for chemotherapy-induced heart failure. J Cardiopulm Rehabil Prev. 2019;39(3):199–203.
Yang HL, Hsieh PL, Hung CH, Cheng HC, Chou WC, Chu PM, et al. Early moderate intensity aerobic exercise intervention prevents doxorubicin-caused cardiac dysfunction through inhibition of cardiac fibrosis and inflammation. Cancers (Basel). 2020;12(5):1102.
Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137(11):1176–91.
Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev. 2019;6(6):cd009955.
Hughes DC, Lenihan DJ, Harrison CA, Basen-Engquist KM. Exercise intervention for cancer survivors with heart failure: two case reports. J Exerc Sci Fit. 2011;9(1):65–73.
Hojan K, Procyk D, Horyńska-Kęstowicz D, Leporowska E, Litwiniuk M. The preventive role of regular physical training in ventricular remodeling, serum cardiac markers, and exercise performance changes in breast cancer in women undergoing trastuzumab therapy—an REH-HER study. J Clin Med. 2020;9(5):1379.
Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol. 2014;64(13):1310–9.
Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, et al. Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the Breast Cancer Randomized Exercise Intervention (BREXIT) study. Circulation. 2022. https://doi.org/10.1161/CIRCULATIONAHA.122.062814.
Williamson T, Moran C, Chirico D, Arena R, Ozemek C, Aggarwal S, et al. Cancer and cardiovascular disease: the impact of cardiac rehabilitation and cardiorespiratory fitness on survival. Int J Cardiol. 2021;343:139–45.
Okwuosa TM, Ray RM, Palomo A, Foraker RE, Johnson L, Paskett ED, et al. Pre-diagnosis exercise and cardiovascular events in primary breast cancer: women’s health initiative. JACC CardioOncol. 2019;1(1):41–50.
Westphal JG, Schulze PC. Exercise training in cancer related cardiomyopathy. J Thorac Dis. 2018;10(Suppl 35):S4391–9.
Kerrigan DJ, Reddy M, Walker EM, Cook B, McCord J, Loutfi R, et al. Cardiac rehabilitation improves fitness in patients with subclinical markers of cardiotoxicity while receiving chemotherapy: a randomized controlled study. J Cardiopulm Rehabil Prev. 2022. https://doi.org/10.1097/HCR.0000000000000719.
Alves CR, da Cunha TF, da Paixão NA, Brum PC. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015;125:9–14.
Rausch V, Sala V, Penna F, Porporato PE, Ghigo A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis. 2021;10(1):1.
Antunes JMM, Ferreira RMP, Moreira-Gonçalves D. Exercise training as therapy for cancer-induced cardiac cachexia. Trends Mol Med. 2018;24(8):709–27.
Lira FS, Antunes Bde M, Seelaender M, Rosa Neto JC. The therapeutic potential of exercise to treat cachexia. Curr Opin Support Palliat Care. 2015;9(4):317–24.
Chung WP, Yang HL, Hsu YT, Hung CH, Liu PY, Liu YW, et al. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65(2): 101485.
Kubota Y, Evenson KR, Maclehose RF, Roetker NS, Joshu CE, Folsom AR. Physical activity and lifetime risk of cardiovascular disease and cancer. Med Sci Sports Exerc. 2017;49(8):1599–605.
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, et al. Feasibility of high intensity interval training in patients with breast cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC Cancer. 2019;19(1):653.
Chen YW, Hunt MA, Campbell KL, Peill K, Reid WD. The effect of Tai Chi on four chronic conditions-cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sports Med. 2016;50(7):397–407.
Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63.
Andres MS, Pan J, Lyon AR. What does a cardio-oncology service offer to the oncologist and the haematologist? Clin Oncol (R Coll Radiol). 2021;33(8):483–93.
Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, Van der Meer P, et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J. 2019;40(22):1756–63.
Sadler D, Arnold A, Herrmann J, Daniele A, Silva C, Ghosh AK, et al. Reaching across the aisle: cardio-oncology advocacy and program building. Curr Oncol Rep. 2021;23(6):64.
Lenihan DJ, Fradley MG, Dent S, Brezden-Masley C, Carver J, Filho RK, et al. Proceedings from the Global Cardio-Oncology Summit: the top 10 priorities to actualize for cardiooncology. JACC CardioOncol. 2019;1(2):256–72.
Peng J, Rushton M, Johnson C, Brezden-Masley C, Sulpher J, Chiu MG, et al. An international survey of healthcare providers’ knowledge of cardiac complications of cancer treatments. Cardiooncology. 2019;5:12.
Asteggiano R, Aboyans V, Lee G, Salinger S, Richter D. Cardiology care delivered to cancer patients. Eur Heart J. 2020;41(2):205–6.
Cuomo A, Mercurio V, Varricchi G, Galdiero MR, Rossi FW, Carannante A, et al. Impact of a cardio-oncology unit on prevention of cardiovascular events in cancer patients. ESC Heart Fail. 2022;9(3):1666–76.
Pareek N, Cevallos J, Moliner P, Shah M, Tan LL, Chambers V, et al. Activity and outcomes of a cardio-oncology service in the United Kingdom—a five-year experience. Eur J Heart Fail. 2018;20(12):1721–31.
Domercant J, Polin N, Jahangir E. Cardio-oncology: a focused review of anthracycline-, human epidermal growth factor receptor 2 inhibitor-, and radiation-induced cardiotoxicity and management. Ochsner J. 2016;16(3):250–6.
Díaz-Gavela AA, Figueiras-Graillet L, Luis ÁM, Salas Segura J, Ciérvide R, Del Cerro PE, et al. Breast radiotherapy-related cardiotoxicity. When, how, why. Risk prevention and control strategies. Cancers (Basel). 2021;13(7):1712.
Lambrinou E, Decourcey J, Hill L. Personalizing heart failure care to the patient with cancer. Curr Heart Fail Rep. 2022;19(1):1–6.
Koop Y, van Zadelhof N, Maas A, Atsma F, El Messaoudi S, Vermeulen H. Quality of life in breast cancer patients with cancer treatment-related cardiac dysfunction: a qualitative study. Eur J Cardiovasc Nurs. 2022;21(3):235–42.
White J, Byles J, Williams T, Untaru R, Ngo DTM, Sverdlov AL. Early access to a cardio-oncology clinic in an Australian context: a qualitative exploration of patient experiences. Cardiooncology. 2022;8(1):14.
Geršak BM, Kukec A, Steen H, Montenbruck M, Šoštarič M, Schwarz AK, et al. Relationship between quality of life indicators and cardiac status indicators in chemotherapy patients. Zdr Varst. 2021;60(4):199–209.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Harrison JM, Davis MA, Barton DL, Janz NK, Pressler SJ, Friese CR. Functional status and quality of life among breast cancer survivors with heart failure: results of the Medicare Health Outcomes Survey. Support Care Cancer. 2017;25(8):2463–73.
Koop Y, Dobbe L, Maas A, van Spronsen DJ, Atsma F, El Messaoudi S, et al. Oncology professionals’ perspectives towards cardiac surveillance in breast cancer patients with high cardiotoxicity risk: a qualitative study. PLoS ONE. 2021;16(3): e0249067.
Acknowledgements
Funding
No funding or sponsorship was received for this study or the publication of this article.
Author Contributions
Loreena Hill: design, development, sourcing evidence, analysis, drafting and revision of the manuscript. Bruno Delgado: sourcing evidence, analysis, drafting and revision of the manuscript. Ekaterini Lambrinou: sourcing evidence, analysis, drafting and revision of the manuscript. Tara Mannion: sourcing evidence, analysis, drafting and revision of the manuscript. Mark Harbinson: drafting and revision of the manuscript. Claire McCune: development, sourcing evidence, analysis, drafting and revision of the manuscript.
Disclosures
Loreena Hill: honorarium from Vifor Pharma. Claire McCune: received funding from The Heart Trust Fund Registered Charity Number: NIC100399 (“Late Anthracycline Induced Cardiotoxicity—Childhood Cancer Survivors”; see https://clinicaltrials.gov/ct2/show/NCT04852965). Bruno Delgado, Ekaterini Lambrinou, Tara Mannion and Mark Harbinson have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hill, L., Delgado, B., Lambrinou, E. et al. Risk and Management of Patients with Cancer and Heart Disease. Cardiol Ther 12, 227–241 (2023). https://doi.org/10.1007/s40119-023-00305-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00305-w