Abstract
Heart failure (HF) with preserved ejection fraction (HFpEF) is a common condition in clinical practice, affecting more than half of patients with HF. HFpEF is associated with morbidity and mortality and with considerable healthcare resource utilization and costs. Therefore, early diagnosis is crucial to facilitate prompt management, particularly initiation of sodium-glucose co-transporter 2 inhibitors. Although European guidelines define HFpEF as the presence of symptoms with or without signs of HF, left ventricular EF ≥ 50%, and objective evidence of cardiac structural and/or functional abnormalities, together with elevated natriuretic peptide levels, the diagnosis of HFpEF remains challenging. First, there is no clear consensus on how HFpEF should be defined. Furthermore, diagnostic tools, such as natriuretic peptide levels and resting echocardiogram findings, are significantly limited in the diagnosis of HFpEF. As a result, some patients are overdiagnosed (i.e., elderly people with comorbidities that mimic HF), although in other cases, HFpEF is overlooked. In this manuscript, we perform a systematic narrative review of the diagnostic approach to patients with HFpEF. We also propose a comprehensible algorithm that can be easily applied in daily clinical practice and could prove useful for confirming or ruling out a diagnosis of HFpEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a common condition, affecting more than half of patients with HF [1, 2]. Moreover, with aging of the population, the prevalence of HFpEF is expected to increase in the coming years [2]. HFpEF is associated with high morbidity and mortality. A recent study showed that among patients with HFpEF, event rates for hospitalization with HF reach 198 per 1000 person-years [1]. In addition, healthcare resource utilization and costs among patients with HFpEF are huge [3]. Therefore, early diagnosis of HFpEF can play a key role in facilitating prompt initiation of drugs that reduce the burden of HF in this population [4, 5].
European guidelines define HFpEF as the presence of symptoms with or without signs of HF, left ventricular ejection fraction (LVEF) ≥ 50%, and objective evidence of cardiac structural and/or functional abnormalities (presence of left ventricular diastolic dysfunction/raised left ventricular filling pressure), including elevated natriuretic peptide levels [6]. However, the diagnosis of HFpEF remains challenging. First, there is no clear consensus on how HFpEF should be defined, including the LVEF cut-off to use [7]. In addition, whereas some authors consider that in many patients, particularly elderly people, HF is unrecognized [7], others state that HFpEF is overdiagnosed, as various conditions share symptoms and signs, mainly in patients with many comorbidities [8]. Furthermore, diagnostic tools, such as natriuretic peptide testing and resting echocardiogram, are subject to limitations in the diagnosis of HFpEF [9] or are not applied in many patients [10].
Therefore, with the aim of ensuring a more correct diagnosis of this syndrome, a group of multidisciplinary experts met to provide a simple and practical approach to the diagnosis of HFpEF based on a systematic narrative review of currently available evidence.
Search strategy
A bibliographic search on the diagnostic approach to HFpEF was performed using MEDLINE and Embase. The strategy was carried out using the OVID meta-search engine in the case of Embase. The search was performed on December 27, 2022, and included references from 2016 to that date. References in English and Spanish were included.
Two strategies were applied for the different databases with the keywords of interest, namely both the MeSH/Emtree terms from the PubMed/Embase thesaurus and the free text terminological variants in the title or in the abstract. Standard date filters from 2016 and a language filter (English and Spanish) were applied. As the most recent articles in the database did not have MeSH/Emtree terms assigned, respectively, in the PubMed/Embase databases, which were consulted through the OVID metasearch engine, specific strategies were created using the full-text search in the title and/or summary and included HF (heart failure), LVEF (left ventricular ejection fraction), and diagnostic tools. The initial search strategies recorded 134 references in Embase (OVID) and 280 references in MEDLINE (PubMed) (Supplementary material). After eliminating duplicate references with the reference management software (Zotero 6.0), a total of 377 references were recovered. These were subsequently reduced to 185 after manual selection (PRISMA flow chart is shown in supplementary Fig. 2).
Diagnosis of HF
HF is a clinical syndrome with symptoms and/or signs caused by structural and/or functional cardiac abnormalities and confirmed by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion [11]. Therefore, these aspects should be taken into consideration when attempting to confirm a diagnosis of HFpEF.
Clinical suspicion
Diagnosis of HF requires the presence of symptoms with or without signs of HF. Typical symptoms include breathlessness, orthopnea, paroxysmal nocturnal dyspnea, reduced exercise tolerance, fatigue, and ankle swelling; typical signs include peripheral edema, lung rales, elevated jugular venous pressure, or third heart sound [6]. However, since symptoms and signs alone are not sufficiently accurate to confirm a diagnosis of HF, additional diagnostic tools are required to correctly diagnose HFpEF [12, 13].
Dyspnea is the cardinal symptom of HFpEF. However, patients with HFpEF are usually elderly and may have many comorbidities, some of which can mimic HF, such as coronary artery disease, lung disease, obesity, diabetes, atrial fibrillation, and anemia. Therefore, these comorbidities should be ruled out, or, at least, their contribution to symptomatology should be determined [14, 15]. Importantly, it is mandatory to investigate whether dyspnea has a respiratory or a cardiac origin. Table 1 shows key aspects that may be helpful to differentiate between them [16].
Another key point is that no symptoms or signs by themselves can help us to determine whether a patient has HFpEF or HF with reduced EF. For example, the CHARM program included three clinical trials, two of which enrolled patients with HF with reduced EF and one with patients with HFpEF. Although some symptoms or signs could be more common in one type of HF than the other, they are frequent in both HFpEF and HF with reduced EF (Table 2) [17].
Electrocardiogram, chest X-ray, and lung ultrasound
The evaluation of patients with suspected HF should include an electrocardiogram, as a normal electrocardiogram is unusual in patients with HF. In addition, it may be helpful to consider the etiology of HF (arterial hypertension [left ventricular hypertrophy, systolic overload]; ischemic heart disease [ST-T alterations, Q waves]). Electrocardiogram abnormalities, such as atrial fibrillation/flutter, conduction disorders, left ventricular hypertrophy, pathologic Q waves, ST-T segment alterations, and left bundle branch block, are common in patients with HF [6, 18].
A chest X-ray may provide supportive evidence of HF, such as pulmonary congestion or cardiomegaly, although it can also be used to investigate other potential causes of dyspnea, particularly pulmonary diseases [6]. Moreover, the use of lung ultrasound can help in the diagnosis of HF and may have prognostic value, as the number of B-lines is associated with adverse outcomes [19].
Natriuretic peptides
European guidelines recommend determination of natriuretic peptide levels to rule out the diagnosis in patients with symptoms suggestive of HF [6]. However, natriuretic peptide levels are increased not only in HF but also in other clinical conditions (acute setting [acute coronary syndrome, atrial or ventricular arrhythmias, pulmonary embolism, acute kidney disease, sepsis]; chronic setting [increasing age, chronic kidney disease, left ventricular hypertrophy, chronic obstructive pulmonary disease, atrial fibrillation]) [6, 20]. By contrast, natriuretic peptide levels may be disproportionately low in obese patients. In fact, low NT-proBNP levels in overweight and obese patients do not rule out the diagnosis of HFpEF [21]. In this context, European guidelines recommend an upper limit of normal in the non-acute setting of 35 pg/mL for BNP and 125 pg/mL for NT-proBNP, as these values have a very high negative predictive value (from 0.94 to 0.98) and values under these levels make a diagnosis of HF very unlikely [6]. However, it should be noted that, for the same NYHA functional class, natriuretic peptide levels are higher in patients with HF with reduced EF than in patients with HFpEF and that in patients with HFpEF in NYHA functional class I or II, natriuretic peptide levels are not markedly increased [22]. In addition, many conditions that may modify natriuretic peptide levels are also common in patients with HFpEF [6]. Therefore, higher natriuretic peptide levels should be considered to rule out a diagnosis of HFpEF in this population. In fact, recent clinical trials enrolling patients with HFpEF (i.e., EMPEROR-Preserved [NT-proBNP: sinus rhythm: > 300 pg/mL; atrial fibrillation; > 900 pg/mL], DELIVER [NT-proBNP: sinus rhythm: ≥ 300 pg/mL; atrial fibrillation; ≥ 600 pg/mL], and PARAGON-HF [NT-proBNP: sinus rhythm: > 300 pg/mL; atrial fibrillation; > 900 pg/mL]) have defined higher cut-off levels of NT-proBNP as inclusion criteria (Table 3) [4, 5, 23]. As a result, we recommend as cut-off levels for NT-proBNP ≥ 300 pg/mL if sinus rhythm and ≥ 600 pg/mL if atrial fibrillation. In patients with low natriuretic peptide levels in whom HFpEF is suspected, the risk of adverse outcomes is much lower [24]. In this context, the HFA-PEFF score proposes higher levels of natriuretic peptides for a diagnosis of HFpEF (Table 4) [25,26,27,28]. On the other hand, as BNP seems a worse marker than NT-proBNP for the diagnosis of HFpEF, the latter would be better in this clinical setting [29]. Finally, other biomarkers tested in HFpEF include high-sensitivity troponins and novel biomarkers, particularly soluble suppression of tumorigenesis-2, galectin-3 (Gal-3), growth differentiation factor 15 (GDF-15), and carbohydrate antigen 125 (CA125), which have been shown to predict adverse outcomes independently from natriuretic peptide levels, as well as EF [30,31,32]. Biomarkers such as soluble glycoprotein 130 and heat shock protein 27 (hsp27) have also been proposed as biomarkers of chronic HFpEF [33].
Echocardiography
Echocardiography is the key diagnostic tool in HFpEF. It provides relevant information about functional and morphological aspects of the heart [6, 34]. Thus, the echocardiogram enables us to determine the left ventricular and right ventricular ejection fraction, chamber size, and valvular function, as well as the presence of regional wall motion abnormalities, eccentric and concentric left ventricular hypertrophy, pulmonary hypertension, and markers of diastolic function [6, 26].
The echocardiographic workflow should be standardized [34]. The first step in identifying HFpEF is determination of LVEF, which should be measured, rather than estimated, ideally from biplane or three-dimensional images. Left ventricular diameters and volumes should then be recorded, with a special focus on assessing the presence of concentric remodeling or left ventricular hypertrophy and non-dilated left ventricle and left atrial enlargement [26, 34]. Although the presence of concentric left ventricular remodeling or hypertrophy renders a diagnosis of HFpEF more likely, its absence does not necessarily exclude the diagnosis of HFpEF. On the other hand, after excluding valvular heart disease, left atrial enlargement reflects chronically elevated left ventricular filling pressure (with or without atrial fibrillation) [6, 26].
The next stage should involve estimation of left ventricular filling pressure or pulmonary capillary wedge pressure using transthoracic echocardiography. These parameters include early (E) and late diastolic mitral inflow velocity (mitral E/A ratio), septal and lateral mitral annular early diastolic velocity (e′), ratio of early diastolic mitral inflow and annular velocity (E/e′ ratio), maximal left atrial volume index, and tricuspid regurgitation peak velocity, which enables measurement of pulmonary artery systolic pressure [35, 36]. The E/e′ ratio is usually considered the first step when assessing diastolic function. A mean E/e′ index ≥ 15 at rest identifies patients with high mean pulmonary capillary wedge pressure, thus making a diagnosis of HFpEF more likely. However, a value of 9–14 is less sensitive and should be considered a minor criterion. As E/e′ is subject to limitations [37, 38], this parameter should not be considered alone and should be included within a comprehensive echocardiographic approach for the diagnosis of HFpEF. A recent study showed that a multivariable-based approach including different parameters assessed using echocardiography increases accuracy in the diagnosis of HFpEF. In other words, the greater the number of echocardiographic abnormalities, the higher the likelihood of HFpEF [39]. The structural and functional alterations for the diagnosis of HFpEF using echocardiography are summarized in Table 5 [6, 26, 40, 41].
It should be noted that, in some cases, access to a rapid echocardiography examination is difficult. Better coordination between healthcare levels is mandatory if we are to improve the diagnostic approach to patients with suspicion of HFpEF [26, 35]. In this context, the development of artificial intelligence–assisted echocardiography of HFpEF has been shown to be an accurate prescreening method capable of automatically generating quantitative metrics that could prove very valuable for clinicians [42].
Additional imaging techniques, such as cardiac magnetic resonance, can prove useful in cases of a doubtful diagnosis of HFpEF or when a particular etiology is suspected. In fact, cardiac magnetic resonance imaging provides relevant measurements for cardiac structure and function, enables tissue characterization, and could facilitate the early diagnosis of HFpEF. The main problem is its availability in daily clinical practice [43, 44].
Scores
In this context, two scoring systems have been proposed to simplify the diagnostic approach to patients with HFpEF, namely H2FPEF and HFA-PEFF. While the H2FPEF score relies mostly on comorbidities, the HFA-PEFF scoring system is based on echocardiographic structural and functional parameters and natriuretic peptide levels (Table 4) [25, 26]. Different studies have analyzed the validity of these scores for the diagnosis of HFpEF in real-world practice. Although most have shown that they are reliable diagnostic tools in HFpEF, with high diagnostic accuracy, and are associated with diastolic dysfunction, lower cardiac output, and exercise intolerance, they are barely used in clinical practice and their results may be discordant in patients affected by unexplained dyspnea, with relevant differences in sensitivity and specificity according to the clinical setting [45,46,47,48,49,50].
Additional diagnostic tools
Although the diagnosis of HFpEF can be reasonably performed in most patients after a clinical history, physical examination, measurement of biological parameters, and echocardiography, additional confirmatory tests may be needed when the diagnosis is not clear. In these cases, further investigation is required.
Diastolic stress test
Diastolic stress tests, mainly exercise echocardiography, can unmask left ventricular diastolic and systolic dysfunction and should be the next step when attempting to confirm a diagnosis of HFpEF. Of note, this is a mainly submaximal exercise stress test, whereas the maximal exercise stress test is generally used to exclude ischemia [6, 26, 51,52,53,54].
The parameters most commonly analyzed to rule out HFpEF are mitral E/e′ ratio and tricuspid regurgitation peak velocity, which are closely associated with mean pulmonary capillary wedge pressure and pulmonary artery systolic pressure, respectively. These parameters should be measured during standardized exercise. In addition, stroke volume and its change during exercise should also be determined. An average E/e′ ratio at peak stress ≥ 15 and a tricuspid regurgitation velocity > 3.4 m/s increase the probability of a diagnosis of HFpEF. In fact, an average E/e′ ratio at peak stress ≥ 15 adds two points to the HFA–PEFF score and three points when the two conditions are present. Additionally, the absence of increased cardiac output during exercise also favors HFpEF as the etiology of dyspnea [6, 26, 51,52,53,54].
Right heart catheterization
If exercise echocardiography cannot be performed or data are inconclusive, an invasive hemodynamic test is recommended. If the patient has an invasively measured pulmonary capillary wedge pressure of ≥ 15 mmHg or left ventricular end-diastolic pressure ≥ 16 mmHg at rest, then a diagnosis of HFpEF can be considered. If not, an invasive hemodynamic measurement of pulmonary capillary wedge pressure should be taken during exercise. In the case of pulmonary capillary wedge pressure ≥ 25 mmHg, the patient has HFpEF; if not, HFpEF can be ruled out [6, 26, 55]. It is important to note that this diagnostic procedure is subject to risks and may not always be available. In addition, invasive exercise hemodynamics is limited for the diagnosis of HFpEF, for example, it is subject to respiratory pressure swings that may impact on the results in up to 30% of patients [55]. Therefore, it should be limited to specific cases, particularly when therapy depends on the results [6, 26, 56].
Additionally, although further studies are required, the use of specific microRNA panels could add value to current biomarkers in the diagnosis of HFpEF [57].
Etiology of HFpEF
Once the diagnosis of HFpEF has been confirmed, the underlying cause should be determined in order to initiate specific treatment. In most cases, HFpEF is associated with risk factors and comorbidities, particularly with long-term poorly controlled arterial hypertension. However, conditions that mimic HFpEF should be ruled out, for example, hypertrophic cardiomyopathy, inflammatory or infiltrative cardiomyopathy, and storage disease [6, 26]. Additionally, myocardial ischemia, an abnormal blood pressure response to exercise, chronotropic incompetence, and supraventricular and ventricular arrhythmias should also be investigated if the patient has clinical findings that suggest a history of any of these conditions. Therefore, specific diagnostic tools should be indicated according to the clinical suspicion (Table 6) [6, 26].
In this clinical setting, it is important to exclude cardiac amyloidosis, which should be suspected in patients aged > 65 years with HF and left ventricular hypertrophy (septum ≥ 12 mm). Other parameters that increase the probability of cardiac amyloidosis include hypotension, which is more common in affected patients. Pseudo-infarct electrocardiographic pattern, low QRS voltage, and conduction abnormalities are typical findings on the electrocardiogram. Moreover, affected patients also have disproportionally elevated natriuretic peptide levels. In addition, granular sparkling of the myocardium, increased right ventricular wall thickness, pericardial effusion, and altered longitudinal strain can also be observed in echocardiography [6, 26, 58,59,60,61].
Diagnostic algorithm
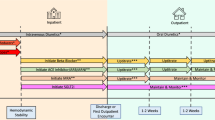
A diagnostic algorithm that can be translated into clinical practice is proposed in Fig. 1 [6, 26, 40, 41, 62,63,64,65,66,67,68]. The first step is clinical suspicion of HF. Not only should the symptoms and signs of HF be considered but also the presence of other comorbidities should also be taken into account when attempting to identify other causes of dyspnea or the contribution of these conditions to the patient’s clinical status. An electrocardiogram should then be performed, and natriuretic peptide levels (NT-proBNP) should be determined. We used the cut-off levels of clinical trials rather than ESC guidelines, to increase specificity. If any of the values are altered, echocardiography is mandatory (see criteria for HFpEF in Table 5). If all the data indicate a high probability, the diagnosis of HFpEF can be confirmed and further investigations can be considered if a specific etiology is suspected. Personalized treatment of HFpEF should be started early based on comorbidities and congestion status, with priority accorded to drugs showing an established clinical benefit, such as sodium-glucose co-transporter 2 inhibitors [4, 5]. If the probability of HFpEF is low, other cardiac and extracardiac causes of dyspnea should be considered. In the case of intermediate probability, invasive measurements can be performed to unmask left ventricular diastolic dysfunction [6, 26, 40, 41, 62,63,64,65,66,67,68]. On the other hand, there are some conditions (i.e., cardiac amyloidosis, hypertrophic cardiomyopathy, cardiac sarcoidosis, hemochromatosis, Fabry disease, high-output HF, myocarditis, pericardial disease) that in some cases can be considered as HFpEF mimics. As a result, these conditions should be taken into account and ruled out when clinical suspicion exists [68].
Diagnostic algorithm for HFpEF*. AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; HFpEF, heart failure with preserved ejection fraction; LVH, left ventricular hypertrophy; PCWP, pulmonary capillary wedge pressure; SR, sinus rhythm; TR, tricuspid regurgitation. *The presence of HFpEF mimics (i.e., cardiac amyloidosis, hypertrophic cardiomyopathy, cardiac sarcoidosis, hemochromatosis, Fabry disease, high-output HF, myocarditis, pericardial disease) should be considered and ruled out when clinical suspicion exists. Figure based on data from references #6, 26, 41, 62–68
In recent years, several algorithms have been published regarding the diagnosis of HFpEF. Some of them are too complex, with a lot of information, which decreases their applicability in clinical practice. Others, however, are too simple and not all the necessary information to perform an accurate diagnosis is included or is not updated. That is why we think that our algorithm provides all the necessary information, without being too complex, and thus may be helpful to make an appropriate diagnostic approach for patients with suspected HFpEF. As a result, this is a comprehensible algorithm that should be implemented in clinical practice at different healthcare levels, including cardiology, internal medicine, and primary care. Such an approach will most likely increase awareness of the need for early identification of this entity and facilitate early diagnosis and the initiation of drugs with proven efficacy in the affected population.
Conclusions
HFpEF is a very common condition that is associated with high morbidity and mortality. However, confirming a diagnosis of HFpEF is challenging, as affected patients have many comorbidities that can mimic the condition. Additionally, HFpEF is not defined based on a single criterion but on a cluster of parameters, mainly increased natriuretic peptide levels and specific echocardiographic alterations. We present a comprehensible algorithm that can easily be applied to real-world patients and prove useful when confirming or ruling out a diagnosis of HFpEF.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Change history
13 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10741-023-10365-8
References
Escobar C, Palacios B, Varela L et al (2022) Prevalence, characteristics, management and outcomes of patients with heart failure with preserved, mildly reduced, and reduced ejection fraction in Spain. J Clin Med 11(17):5199
Iyngkaran P, Thomas MC, Neil C et al (2020) The heart failure with preserved ejection fraction conundrum-redefining the problem and finding common ground? Curr Heart Fail Rep 17(2):34–42
Escobar C, Palacios B, Varela L et al (2022) Healthcare resource utilization and costs among patients with heart failure with preserved, mildly reduced, and reduced ejection fraction in Spain. BMC Health Serv Res 22(1):1241
Anker SD, Butler J, Filippatos G et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385(16):1451–1461
Solomon SD, McMurray JJV, Claggett B et al (2022) Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 387(12):1089–1098
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726
Ho JE, Redfield MM, Lewis GD, Paulus WJ, Lam CSP (2020) Deliberating the diagnostic dilemma of heart failure with preserved ejection fraction. Circulation 142(18):1770–1780
van Riet EE, Hoes AW, Limburg A, Landman MA, van der Hoeven H, Rutten FH (2014) Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 16(7):772–777
Thompson A, Crilley J, Wilson D, Hungin APS, Fuat A, Murphy J (2016) An epidemic of HFPEF? Heart 102(Supplement 6):A15–A16
Kapłon-Cieślicka A, Laroche C, Crespo-Leiro MG et al (2020) Is heart failure misdiagnosed in hospitalized patients with preserved ejection fraction? From the European Society of Cardiology - Heart Failure Association EURObservational Research Programme Heart Failure Long-Term Registry. ESC Heart Fail 7(5):2098–2112
Bozkurt B, Coats AJS, Tsutsui H et al (2021) Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 23(3):352–380
Oudejans I, Mosterd A, Bloemen JA et al (2011) Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail 13:518–527
Kelder JC, Cramer MJ, van Wijngaarden J et al (2011) The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 124:2865–2873
Abramov D, Parwani P (2021) Diving into the diagnostic score algorithms of heart failure with preserved ejection fraction. Front Cardiovasc Med 8:665424
Anderson T, Hummel SL, Konerman MC (2022) Epidemiology, Diagnosis, Pathophysiology, and Initial Approach to Heart Failure with Preserved Ejection Fraction. Cardiol Clin 40(4):397–413
Berliner D, Schneider N, Welte T, Bauersachs J (2016) The differential diagnosis of dyspnea. Dtsch Arztebl Int 113(49):834–845
McMurray J, Ostergren J, Pfeffer M et al (2003) Clinical features and contemporary management of patients with low and preserved ejection fraction heart failure: baseline characteristics of patients in the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur J Heart Fail 5(3):261–270
Bjerkén LV, Rønborg SN, Jensen MT, ørting SN, Nielsen OW (2023) Artificial intelligence enabled ECG screening for left ventricular systolic dysfunction: a systematic review. Heart Fail Rev 28(2):419–430
Morvai-Illés B, Polestyuk-Németh N, Szabó IA et al (2021) The prognostic value of lung ultrasound in patients with newly diagnosed heart failure with preserved ejection fraction in the ambulatory setting. Front Cardiovasc Med 8:758147
Mueller C, McDonald K, de Boer RA et al (2019) Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 21:715–731
Buckley LF, Canada JM, Del Buono MG et al (2018) Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail 5(2):372–378
Wei T, Zeng C, Chen L et al (2005) Systolic and diastolic heart failure are associated with different plasma levels of B-type natriuretic peptide. Int J Clin Pract 59(8):891–894
Solomon SD, McMurray JJV, Is A et al (2019) Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 381(17):1609–1620
Pabón MA, Cunningham JW, Claggett BL et al (2022) Natriuretic peptide-based inclusion criteria in heart failure with preserved ejection fraction clinical trials: insights from PARAGON-HF. Eur J Heart Fail 24(4):672–677
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138(9):861–870
Pieske B, Tschöpe C, de Boer RA et al (2019) How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 40(40):3297–3317
Adamczak DM, Oduah M-T, Kiebalo T et al (2020) Heart failure with preserved ejection fraction-a concise review. Curr Cardiol Rep 22(9):82
Marta Szabo M, Muk B, Majoros ZS et al (2018) The importance of the NTproBNP rule-in criteria in the early diagnosis of HFmrEF and HFpEF. Eur J Heart Fail 20(Supplement 1):433
Shah SJ (2022) BNP: Biomarker Not Perfect in heart failure with preserved ejection fraction. Eur Heart J 43(20):1952–1954
Morfino P, Aimo A, Castiglione V, Vergaro G, Emdin M, Clerico A (2022) Biomarkers of HFpEF: natriuretic peptides, high-sensitivity troponins and beyond. J Cardiovasc Dev Dis 9(8)
Trippel TD, Mende M, Düngen H-D et al (2021) The diagnostic and prognostic value of galectin-3 in patients at risk for heart failure with preserved ejection fraction: results from the DIAST-CHF study. ESC Heart Fail 8(2):829–841
Miñana G, de la Espriella R, Palau P et al (2022) (2022) Carbohydrate antigen 125 and risk of heart failure readmissions in patients with heart failure and preserved ejection fraction. Sci Rep 12(1):1344
Liu S, Iskandar R, Chen W et al (2016) Soluble glycoprotein 130 and heat shock protein 27 as novel candidate biomarkers of chronic heart failure with preserved ejection fraction. Heart Lung Circ 25(10):1000–1006
Hagendorff A, Helfen A, Brandt R et al (2023) Expert proposal to characterize cardiac diseases with normal or preserved left ventricular ejection fraction and symptoms of heart failure by comprehensive echocardiography. Clin Res Cardiol 112(1):1–38
Hagendorff A, Stöbe S, Kandels J, de Boer R, Tschöpe C (2022) Diagnostic role of echocardiography for patients with heart failure symptoms and preserved left ventricular ejection fraction. Herz 47(4):293–300
Johansson MC, Rosengren A, Fu M (2022) Echocardiographic diagnosis of heart failure with preserved ejection fraction in elderly patients with hypertension. Scand Cardiovasc J 56(1):368–377
Donal E, Galli E, Fraser AG (2017) Non-invasive estimation of left heart filling pressures: another nail in the coffin for E/e’? Eur J Heart Fail 19(12):1661–1663
Mitter SS, Shah SJ, Thomas JD (2017) A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 69(11):1451–1464
Dal Canto E, Remmelzwaal S, van Ballegooijen AJ et al (2022) Diagnostic value of echocardiographic markers for diastolic dysfunction and heart failure with preserved ejection fraction. Heart Fail Rev 27(1):207–218
Choi KH, Yang JH, Seo JH et al (2023) Discriminative role of invasive left heart catheterization in patients suspected of heart failure with preserved ejection fraction. J Am Heart Assoc 12(6):e027581
Oh JK, Miranda WR, Kane GC (2023) Diagnosis of heart failure with preserved ejection fraction relies on detection of increased diastolic filling pressure, but how? J Am Heart Assoc 12(6):e028867
Chiou Y-A, Hung C-L, Lin S-F (2021) AI-assisted echocardiographic prescreening of heart failure with preserved ejection fraction on the basis of intrabeat dynamics. JACC Cardiovasc Imaging 14(11):2091–2104
Hassan OKA, Higgins AR (2022) The role of multimodality imaging in patients with heart failure with reduced and preserved ejection fraction. Curr Opin Cardiol 37(3):285–293
He J, Yang W, Jiang Y et al (2023) Heart failure with preserved ejection fraction assessed by cardiac magnetic resonance: from clinical uses to emerging techniques. Trends Cardiovasc Med 33(3):141–147
Amanai S, Harada T, Kagami K et al (2022) The H(2)FPEF and HFA-PEFF algorithms for predicting exercise intolerance and abnormal hemodynamics in heart failure with preserved ejection fraction. Sci Rep 12(1):13
Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca H-P et al (2020) Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 22(3):413–421
Hwang I-C, Cho G-Y, Choi H-M et al (2021) H2FPEF score reflects the left atrial strain and predicts prognosis in patients with heart failure with preserved ejection fraction. J Card Fail 27(2):198–207
Nikorowitsch J, Bei der Kellen R, Kirchhof P et al (2021) Applying the ESC 2016, H(2) FPEF, and HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail 8(5):3603–3612
Parcha V, Malla G, Kalra R et al (2021) Diagnostic and prognostic implications of heart failure with preserved ejection fraction scoring systems. ESC Heart Fail 8(3):2089–2102
Przewlocka-Kosmala M, Butler J, Donal E, Ponikowski P, Kosmala W (2022) Prognostic value of the MAGGIC Score, H(2)FPEF Score, and HFA-PEFF Algorithm in patients with exertional dyspnea and the incremental value of exercise echocardiography. J Am Soc Echocardiogr 35(9):966–975
Baratto C, Caravita S, Sorropago A et al (2019) Exercise echocardiography or cardiopulmonary exercise test to detect heart failure with preserved ejection fraction? J Hypertens 37(Supplement 1):e113–e114
Caravita S, Baratto C, Sorropago A et al (2018) Exercise echocardiography or cardiopulmonary exercise test to detect pre-clinical heart failure with preserved ejection fraction? Eur Heart J 39(Supplement 1):977–978
Donal E (2019) The value of exercise echocardiography in heart failure with preserved ejection fraction. J Ultrason 19(76):43–44
Guazzi M, Wilhelm M, Halle M et al (2022) Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy - a clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail 24(8):1327–1345
Baratto C, Caravita S, Soranna D et al (2021) Current limitations of invasive exercise hemodynamics for the diagnosis of heart failure with preserved ejection fraction. Circ Heart Fail 14(5):e007555
Finet JE, Van Iterson EH, Wilson Tang WH (2021) Invasive hemodynamic and metabolic evaluation of HFpEF. Curr Treat Options Cardiovasc Med 23(5):32
Parvan R, Hosseinpour M, Moradi Y, Devaux Y, Cataliotti A, da Silva GJJ (2022) Diagnostic performance of microRNAs in the detection of heart failure with reduced or preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 24(12):2212–2225
Tomasoni D, Aimo A, Merlo M et al (2022) Value of the HFA-PEFF and H2 FPEF scores in patients with heart failure and preserved ejection fraction caused by cardiac amyloidosis. Eur J Heart Fail 24(12):2374–2386
Aimo A, Merlo M, Porcari A et al (2022) Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies. Eur J Heart Fail 24(12):2342–2351
de Marneffe N, Dulgheru R, Ancion A, Moonen M, Lancellotti P (2022) Cardiac amyloidosis: a review of the literature. Acta Cardiol 77(8):683–692
Sennott J, Ananthasubramaniam K (2022) Multimodality imaging approach to cardiac amyloidosis: part 2. Heart Fail Rev 27(5):1515–1530
Çavuşoğlu Y, Çelik A, Altay H et al (2022) Heart failure with non-reduced ejection fraction: epidemiology, pathophysiology, phenotypes, diagnosis and treatment approaches. Turk Kardiyol Dern Ars 50(Supp1):S1–S34
Gevaert AB, Kataria R, Zannad F et al (2022) Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart 108(17):1342–1350
Nagueh SF (2020) Diagnostic algorithms for heart failure with preserved ejection fraction. JACC Heart Fail 8(8):654–656
Sundaram V, Zakeri R, Witte KK, Quint JK (2022) Development of algorithms for determining heart failure with reduced and preserved ejection fraction using nationwide electronic healthcare records in the UK. Open Heart 9(2)
Tadic M, Cuspidi C, Calicchio F, Grassi G, Mancia G (2021) Diagnostic algorithm for HFpEF: how much is the recent consensus applicable in clinical practice? Heart Fail Rev 26(6):1485–1493
Vaishnav J, Sharma K (2022) A stepwise guide to the diagnosis and treatment of heart failure with preserved ejection fraction. J Card Fail 28(6):1016–1030
Kittleson MM, Panjrath GS, Amancherla K et al (2023) 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 81(18):1835–1878
Funding
The Alliance Boehringer Ingelheim (BI)—Lilly has supported and funded the publication of this article. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author information
Authors and Affiliations
Contributions
JN, MJCM, MIE, JMFR, FF, MCM, CFG-P, AT-S and AMG have contributed to the conception, design or acquisition of data, or analysis and interpretation of data. All authors have participated in drafting, reviewing, and/or revising the manuscript and have approved its submission. The authors meet the authorship criteria recommended by the International Committee of Medical Journal Editors (ICMJE).
Corresponding author
Ethics declarations
Ethics approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Competing interests
The authors did not receive payment related to the development of this manuscript. FF has declared no conflict of interest related to this manuscript. JN reports personal fees or advisory boards from Alleviant, AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, NovoNordisk, Rovi, and Vifor Pharma (outside of the submitted work). MJCM has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events for Astra Zeneca, Vifor Pharma, Novartis, Boehringer-Ingelheim, Bayer, Novonordisk. MCM has declared no conflict of interest related to this manuscript. MIE has declared no conflict of interest related to this manuscript. CFG-P and AT-S are full-time employees and minority shareholders of Eli Lilly and Company España. AMG is full employee of Boehringer Ingelheim España. JMFR has declared no conflict of interest related to this manuscript.
Medical writing/editorial assistance
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain), which was contracted and funded by Boehringer Ingelheim.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Formiga, F., Nuñez, J., Castillo Moraga, M.J. et al. Diagnosis of heart failure with preserved ejection fraction: a systematic narrative review of the evidence. Heart Fail Rev 29, 179–189 (2024). https://doi.org/10.1007/s10741-023-10360-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10360-z