Abstract
All heart muscle diseases that cause chronic heart failure finally converge into one dreaded pathological process that is myocardial fibrosis. Myocardial fibrosis predicts major adverse cardiovascular events and death, yet we are still missing the targeted therapies capable of halting and/or reversing its progression. Fundamentally it is a problem of disproportionate extracellular collagen accumulation that is part of normal myocardial ageing and accentuated in certain disease states. In this article we discuss the role of cardiovascular magnetic resonance (CMR) imaging biomarkers to track fibrosis and collate results from the most promising animal and human trials of anti-fibrotic therapies to date. We underscore the ever-growing role of CMR in determining the efficacy of such drugs and encourage future trialists to turn to CMR when designing their surrogate study endpoints.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Myocardial fibrosis is the shared final common pathological pathway in the development of chronic heart failure and it predicts poor prognosis. |
Myocardial fibrosis results from the accumulation of collagen in the extracellular matrix, mediated by the complex interplay between pro-fibrotic cells, growth factors and inflammatory cytokines. |
Various proposed anti-fibrotic drugs have been tested but their direct beneficial effects on human myocardial fibrosis are yet to be proven. |
CMR techniques like native T1 mapping and extracellular volume have become the gold standard non-invasive imaging biomarkers of myocardial fibrosis. |
Newer technologies in CMR are paving the way for large-scale, multicentre, randomised trials to determine the efficacy of newer anti-fibrotic therapies. |
Introduction
Disruption of the interstitial extracellular matrix (ECM) has an important role in the adverse myocardial remodelling that leads to systolic and/or diastolic dysfunction and the eventual development of chronic heart failure [1, 2]. Myocardial fibrosis is characterised by the disproportionate accumulation of collagen in the ECM and it can be focal (following an acute ischaemic event) or diffuse (in non-ischaemic cardiomyopathy) [3]. Its presence is an important prognostic marker in the development of adverse cardiovascular events such as acute heart failure, malignant arrhythmia and death.
There are three types of myocardial fibrosis: (1) replacement fibrosis induced by cardiomyocyte injury and death, as seen post myocardial infarction; (2) reactive fibrosis which is the gradual and reversible diffuse distribution of excess collagen in the ECM like that observed in non-ischaemic cardiomyopathy (Fig. 1a), valvular heart disease and normal ageing [4]; (3) infiltrative fibrosis that is secondary to the accumulation (in ECM or in myocytes) of non-collagenous materials such as amyloid (in cardiac amyloidosis), iron (in haemochromatosis) or glycosphingolipids (in Fabry disease, Fig. 1b) [5].
The value of CMR tissue characterisation in distinguishing the underlying cause for LVH phenotypes. Panel a shows a patient with familial HCM caused by a pathogenic MYBPC3 mutation. There is asymmetric septal hypertrophy and extensive diffuse and patchy LGE in the hypertrophied segments with corresponding high native myocardial T1 in these areas of fibrosis. Panel b shows a male patient with LVH secondary to myocardial glycosphingolipid accumulation in Fabry disease as a result of which myocardial T1 times are low and there is the classic patch of subepicardial fibrosis in the inferolateral wall. b/m/aSAX basal/mid/apical short axis, 4/2C 4/2-chamber, HCM hypertrophic cardiomyopathy, LGE late gadolinium enhancement, LVH left ventricular hypertrophy, MOCO motion-corrected, MOLLI modified Look-Locker inversion recovery, MYBPC3 myosin-binding protein C3, PSIR phase-sensitive inversion recovery, ShMOLLI shortened MOLLI, SSFP steady-state free precession
A number of mediators have emerged as potential targets for anti-fibrotic therapies but most studies to determine their benefit are based on animal models with mixed results from human studies.
Our understanding of the mechanisms of cardiac fibrosis has improved greatly in recent years allowing us to refine non-invasive imaging techniques to better track its development and CMR is at the forefront of these innovations [6].
This review explains how myocardial fibrosis develops and how it can be non-invasively detected and measured using CMR imaging. We summarise findings from selected animal and human trials of some of the more promising targeted anti-fibrotic therapies and re-examine the potential future role of CMR in such trials. This review is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Pathophysiology of Myocardial Fibrosis
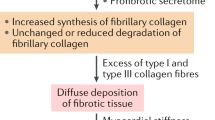
The pathophysiological mechanisms of myocardial fibrosis have been discussed elsewhere [1]. Broadly speaking, the fibrotic response involves a complex interplay between circulating pro-fibrotic cell types, growth factors, hormones and pro-inflammatory cytokines, which is summarised in Fig. 2. Cardiomyocyte call death (apoptosis) can result from either a primary cardiac insult (e.g. ischaemia) or in the context of systemic disease (e.g. hypertension or chronic kidney disease) [2]. Apoptosis can result in fibroblast proliferation through direct or indirect activation. Direct activation is facilitated by cell signalling proteins e.g. microRNAs (miRNAs) [7] and matrix metalloproteinases (MMPs) [8]. Indirect activation is via circulating pro-fibrotic mediators such as endothelial, epithelial and inflammatory cells. Cardiac fibroblasts are the cellular precursors of myofibroblasts which are formed through a process of differentiation that is driven by multiple growth factors and pro-inflammatory cytokines, such as connective tissue growth factor (CTGF) [9], tissue growth factor-β (TGFβ) and tumour necrosis factor-α (TNFα) [1]. Renin–angiotensin–aldosterone system (RAAS) activation is also thought to play an important role [10] as well as newer mediators such as galectin-3 (Gal-3) [11], endothelin [12] and interleukin-11 (IL-11) [13]. The final common pathway of cardiac fibrosis is the deposition of collagen (from myofibroblasts) in the ECM and histologically it can be quantified as the collagen volume fraction (CVF), which is the proportion of collagen in the myocardium compared to the volume of the myocardial interstitium [14]. Our understanding of these mechanisms has allowed us to develop potential therapeutic options which target the various components of the pathway, from basic science to animal and translational human trials.
Schema representing the development of myocardial fibrosis and sites of action of potential therapeutic targets. RAAS renin–angiotensin–aldosterone system, TGFβ tissue growth factor-β, TNFα tumour necrosis factor-α, CTGF connective tissue growth factor, IL-11 interleukin-11, miRNA microRNA, MMP matrix metalloproteinases, Gal-3 galectin-3
CMR Biomarkers of Myocardial Fibrosis
Native T 1
T1 mapping in CMR [15] measures the T1 relaxation time of the myocardium. The native T1 time, before the administration of gadolinium-based contrast agents (GBCA), lengthens because of the expansion of the interstitial space arising from infarction, oedema, fibrosis or infiltration and shortens with accumulation of fat or iron [15]. The late gadolinium enhancement (LGE) and extracellular volume (ECV) techniques also measure related readouts of the myocardial T1 but after the administration of GBCA.
Native T1 mapping can be used to detect areas of diffuse or focal myocardial fibrosis without the need for any GBCA administration, which can be particularly useful in patients in whom the use of gadolinium is contraindicated. The native myocardial T1 time predicts chronic heart failure, arrhythmia and death [16] and lengthens in the presence of frank myocardial scarring (areas of LGE [10]). However, it can also lengthen in the absence of (resolvable) LGE, for example in dilated cardiomyopathy [17], hypertrophic cardiomyopathy (HCM) [18] or severe aortic stenosis [19] indicating more diffuse myocardial fibrosis. The ability to detect this so-called preclinical fibrosis (i.e. before visible LGE appears) is attractive because it means that T1 can serve as a surrogate endpoint in trials of novel anti-fibrotic therapies including the effects on early phenotypes to prevent chronic, irreversible heart failure.
Research into T1 mapping is ongoing with the aim of improving accuracy and overcoming challenges in acquisition and processing times [15]. For example, motion artefacts are being addressed with free-breathing and single breath-hold sequences, and with whole heart T1 mapping techniques [20, 21]. Similar innovations are also being used in patients with arrhythmia, particularly atrial fibrillation [22]. Dark-blood T1 mapping has been developed to clean the myocardial signal from the partial volume effects of the blood pool at the blood–myocardial interface which enables parametric mapping of thin-walled structures and improves data quality towards the apex [23, 24]. Post-processing innovations and computer reconstructions continue to improve the accuracy of T1 measurements [25]. Dedicated T1 mapping phantoms have been developed by our group to aid in quality assurance (QA) and bring into line the CMR data across multiple sites each operating different software and mapping sequences [26]. Such QA infrastructure opens up the possibility for collaborative multicentre trials in rare heart muscle diseases that are urgently needed to upscale the currently limited studies of anti-fibrotic therapies.
Late Gadolinium Enhancement
LGE corresponds to an area of focal scar (focal fibrosis) in the myocardium and its presence is associated with poor long-term cardiovascular outcomes, including sudden cardiac death [27]. In previous studies myocardial biopsy of LGE areas in patients with dilated cardiomyopathy [28], myocarditis [29] and hypertrophic cardiomyopathy [27] revealed both inflammation and fibrosis. One caveat of relying on the sole LGE biomarker as a drug-trial surrogate endpoint is its inability to detect diffuse fibrosis, meaning that subtle improvements with therapy will be missed. Another caveat is that the evolution of new LGE foci, even in advanced disease states, is an indolent process which requires a certain threshold of conglomerated myocardial cell death to be detectable using modern-day CMR technologies. It means that study follow-up to detect significant serial change in LGE mass would have to be exceedingly long (and costly).
Extracellular Volume
The redistribution of water and collagen into the extracellular space that occurs with myocardial fibrosis can be quantified by measuring the myocardial T1 time before and after GBCA administration and adjusting for blood haematocrit, yielding the ECV [30]. ECV has been shown to correlate with the amount of collagen deposition on myocardial biopsy in patients with various forms of cardiomyopathy [31, 32]. ECV measurements are technically limited in that they are most accurate in the midwall and may therefore miss myocardial fibrosis in the subendocardial and subepicardial layers [1]. ECV’s correlation with fibrosis is dependent on the absence of oedema or infiltration [30] and can be overestimated in the presence of capillary vasodilation [15]. Despite these potential drawbacks, a high myocardial ECV has been consistently demonstrated in patients with heart muscle disease [19, 33] and shown to predict poor outcome [30], sometimes better than LGE [30]. Interestingly in HCM, myocardial ECV was also shown to be increased in asymptomatic, mutation-positive relatives of HCM probands [18] in whom there was no detectable LGE. Similar to native T1 therefore, ECV is lucrative biomarker of ‘subclinical’ fibrosis and another ideal therapeutic target for anti-fibrotic therapies.
T 2 Mapping
T2 mapping, though not directly measuring fibrosis, does provide insight into closely affiliated biological processes, namely myocardial inflammation and oedema [34]. It does so without the disadvantages of conventional T2-weighted techniques [6]. Magnetic resonance fingerprinting is an emerging technique which allows for simultaneous T1 and T2 pixelwise maps to be acquired in the same breathhold [35]. This has the advantage of reducing scan time whilst improving the reproducibility because it allows for precise spatial correlation of T1 and T2 mapping values per voxel, for a more complete understanding of the interaction between myocardial inflammation and fibrosis [36]. It could prove to be a powerful tool in future trials testing the usefulness of anti-fibrotic therapies.
Targeted Anti-Fibrotic Therapies
In this next part of the review we collate results from the most promising animal and human trials of anti-fibrotic therapies to date and highlight some of the opportunities but also the challenges of future clinical roll-out to patient care.
Connective Tissue Growth Factor Antagonists
CTGF is a matricellular protein which modulates the cell signalling pathways responsible for myofibroblast activation which underpins the pathogenesis of fibrosis [9]. CTGF antagonists may represent a novel strategy to limit and reverse cardiac fibrosis. In one study, mice with pressure overload-induced heart failure treated with CTGF monoclonal antibodies (FG-3019) showed a significant improvement in left ventricular function when compared to controls [37].
Galectin-3 Inhibitors
Gal-3 is a soluble beta-galactoside-binding lectin and has recently emerged as a promising biological target in heart failure as it mediates cardiac fibroblast proliferation resulting in fibrosis [38]. Multiple studies have shown that Gal-3 is upregulated in hypertrophied hearts [39,40,41], interstitial pneumonitis [42] and in the plasma of patients with heart failure [11]. In one study it was found that left ventricular hypertrophy was prevented in Gal-3 knockout mice and that left ventricular function was ameliorated [43]. Gal-3 knockout in hypertensive mice exposed to aldosterone led to reduced vascular inflammation and hypertrophy compared to controls [40]. Thus Gal-3 may serve not only as an important clinical biomarker of heart failure but also as a therapeutic target capable of slowing the progression of cardiac fibrosis [11].
Anti-MicroRNAs
MiRNAs are members of a class of non-coding RNAs expressed by cardiac myocytes which regulate signalling pathways in fibroblasts. Silencing miRNas in vivo has been shown to prevent interstitial fibrosis and inhibit cardiac function in pressure-overloaded mouse hearts [7]. In another animal model, silencing the long noncoding RNA (IncRNA) maternally expressed gene 3 (Meg3) following aortic constriction resulted in reduced myocyte fibrosis and improved diastolic function [44]. Proteomics comparison studies have also shown that rat cardiac fibroblasts transfected with anti-miRNA (pre-miR-29b) lost their ability to stick to myocytes. It is this modulatory effect on the ECM that has made miRNAs a tractable target for therapeutic intervention but further work is needed to improve our understanding of their complex role in facilitating cardiac fibrosis [45].
Renin–Angiotensin–Aldosterone System Inhibitors
RAAS inhibitors were amongst the first drugs used to target cardiac fibrosis and many studies proved their benefit independent of their antihypertensive effects. In a study of 34 patients with hypertension, endomyocardial biopsies showed a reduction in left ventricular chamber stiffness and fibrosis in those patients treated with losartan [46]. Another biopsy study found that lisinopril reduced CVF in cardiomyocytes when compared to hydrochlorothiazide [47]. Similar results were obtained when comparing losartan with amlodipine in patients with end-stage renal disease [10] and hypertension [48]. The anti-fibrotic effect of RAAS inhibitors has been verified by CMR in a cohort of losartan-treated patients with HCM where a significant reduction in LGE was observed [49]. Mineralocorticoid receptor antagonists (MRAs) share a similar anti-fibrotic effect. Circulating levels of fibrosis biomarkers procollagen type 1 carboxy-terminal propeptide (PICP) and procollagen-N-peptide (PINP) have been used to study the effects of MRAs on cardiac fibrosis in humans. Spironolactone reduced PICP and PINP levels in 80 patients with metabolic syndrome whilst improving left ventricular diastolic function [50]. Similar data emerged for eplerenone in patients with heart failure with preserved ejection fraction [51] and diastolic dysfunction [52].
Nevertheless, in clinical practice patients with heart failure on optimal medical therapy that includes RAAS inhibitors continue to have poor outcomes attributable to cardiac fibrosis, suggesting that more work is needed to elucidate their anti-fibrotic benefits independent of any combined antihypertensive and haemodynamic effects.
Tissue Growth Factor-β Inhibitors
TGFβ is a powerful profibrotic cytokine with an active role in cardiac fibrosis. TGFβ inhibitors have been shown to reduce ECM protein synthesis and fibrosis in pressure-overloaded mouse hearts but the downside was increased mortality and inflammation [53] which has limited their clinical translation.
Pirfenidone (PFD) is an anti-fibrotic drug which reduces the synthesis and release of TGFβ [54], thus reducing collagen deposition in both lungs and kidneys [55]. In one murine study it was also found to slow the decline in left ventricular dysfunction, resulting in less ventricular arrhythmia [56]. Similar effects were reported for cardiac stiffness [57], left ventricular hypertrophy [58] and cardiac fibrosis [59] in pressure-overloaded animal models. PFD can, however, cause gastrointestinal upset and liver dysfunction which may hamper its widespread use as a targeted anti-fibrotic drug.
Tranilast (N-[3,4-dimethoxycinnamoyl]anthranilic acid), previously used to treat keloid scars and dermopathies characterised by excessive fibrosis, has been shown to reduce collagen deposition and improve left ventricular systolic and diastolic function [60] in a diabetic rat model. Yet results from the Prevention of Restenosis With Tranilast and Its Outcomes (PRESTO) trial were negative for tranilast as the drug failed to improve quantitative measures of restenosis using intravascular ultrasound and angiography whilst causing a range of adverse effects [61]. Other anti-TGFβ drugs are on the horizon and they include compound FT011 which is showing promising results and improved tolerability when compared to tranilast [62].
Endothelin Inhibitors
Endothelin is thought to play an important role in the pathophysiology of fibrosis, so endothelin receptor blockade could potentially prevent the fibrosis of heart failure. Indeed in animal models, endothelin antagonists reduced cardiac fibrosis and left ventricular hypertrophy, and improved survival [12, 63] whilst in 36 patients with heart failure, bosentan improved left and right heart haemodynamics including vascular resistance when compared to placebo [64]. However, a more recent trial using CMR to measure left ventricular end-diastolic volumes found that administration of enrasentan resulted in adverse cardiac remodelling compared to controls [63]. Similar disappointing results were reported in a large double-blind, placebo-controlled trial of darusentan based on CMR endpoints of remodelling in chronic heart failure [65]. Taken together these data suggest that endothelin blockers may not be the ideal anti-fibrotic molecules but more work is needed to demonstrate this conclusively.
Matricellular Protein Antagonists
Non-structural MMPs are overexpressed following myocardial damage and contribute to the functioning of cardiac fibroblasts [66]. MMP knockout in animal models improved left ventricular end-diastolic pressure following aortic constriction [8] and reduced collagen deposition following myocardial infarction [67]. It has also been postulated that angiotensin converting enzyme inhibitors (ACEi) exhibit direct MMP antagonism [68, 69] which could be an important indicator of the targeted effects of ACEi in fibrosis. Less promising results have been reported in humans where MMP inhibition failed to resolve left ventricular remodelling in an echocardiographic study [70]. Similarly, MMP deletion in mice exacerbated cardiac dysfunction following scar formation in myocardial infarction which continues to raise the question of the usefulness of MMP inhibitors in clinical practice [71].
Relaxin
Relaxin is a vasodilator hormone which reduces the synthesis of collagen in the ECM through a variety of processes including the inhibition of profibrotic factors such as TGFβ [72]. In numerous animal studies it has been shown to reduce cardiac fibrosis in the setting of acute myocardial infarction [73], diabetes [74] and hypertension [75]. It also reduced the incidence of atrial fibrillation by reducing collagen deposition and reversing atrial fibrosis in rat hearts [76]. In another animal model, however, endogenous relaxin had no effect on cardiac fibrosis or hypertrophy following an 8-week period of simulated pressure overload [77]. Clinical trials have replicated these negative findings with the Relaxin for the Treatment of Acute Heart Failure (RELAX-AHF) international double-blind, placebo-controlled trial showing that treatment with serelaxin did not affect hospital readmission rates in acute heart failure [78]. A phase II randomised trial using recombinant human relaxin in patients with scleroderma also showed no improvement in patient symptoms as reflected by skin thickness scores [79]. This disparity of benefit between animal and human trials highlights the need for further research into the use of relaxin in real-world cardiac settings, in particular chronic heart failure.
Loop Diuretics
The loop diuretic torsemide was superior to furosemide in improving plasma brain natriuretic peptide levels, left ventricular function and mortality in heart failure [80]. A randomised study of patients with chronic heart failure found reduced levels of PICP and collagen deposition in septal myocardial biopsies in those receiving torsemide compared to furosemide [81]. These findings were echoed in another study showing a reduction in markers of fibrosis and improved left ventricular stiffness [82, 83]. The large multicentre randomised Torasemide Prolonged Release Versus Furosemide in Patients With Chronic Heart Failure (TORAFIC) trial, however, found no significant difference between torsemide and furosemide in terms of circulating PICP levels [84], although patients recruited to this study had milder heart failure compared to those from previous studies [80]. There have been no CMR trials to date directly testing the myocardial impacts of torsemide in terms of fibrosis burden.
Anti-Inflammatory Drugs
Inflammation has a role to play in the development of myocardial fibrosis so drugs with direct and indirect inflammatory modulatory effects have long been trialled for their use in this setting. Rosuvastatin reduced myocardial fibrosis and had a positive effect on left ventricular remodelling in a hypertensive rate model [85] but the results of recent human trials have been disappointing. The drug failed to improve clinical outcomes in patients with heart failure in a large (n = 5011) placebo-controlled trial [86] and these findings were echoed in subsequent trials showing an overall neutral benefit [87, 88]. TNFα has also been studied as a potential fibrotic target in heart failure but trials have been surprisingly negative. The targeted anticytokine drug etanercept failed to show any clinical benefit in chronic heart failure in terms of mortality and hospital admissions [89]. A randomised study using infliximab, a selective TNFα antagonist, failed to show any benefit and actually increased hospitalization in patients with moderate to severe heart failure [90]. Overall, inflammatory modulating drugs have not yet been shown to have a promising effect as targeted anti-fibrotic medication in heart failure. There have been some promising data emerging from a study that used peroxisome proliferator-activated receptor (PPAR) in hypertensive rats which showed a reduction in fibrosis and diastolic dysfunction [91]. However, the widespread use of PPAR agonists in cardiovascular disease continues to be limited by their unfavourable safety profile [91].
Anti-Fibrotic Therapies on the Horizon
IL-11 is believed to play an important role in the pro-fibrotic pathway, downstream of TGFβ. It has been shown to be positively correlated with cardiac fibroblast number and ECM deposition ultimately leading to the development of cardiac dysfunction [92]. In mouse models, deletion of the IL-11 receptor was protective against the development of myocardial fibrosis and anti-IL-11 antibodies negated the effects of TGFβ stimulation on atrial myofibroblasts. This promising work is inspiring the development of a new class of anti-fibrotic therapies without the side effects of TGFβ inhibition discussed previously [13].
Role of CMR in Trialling Anti-Fibrotic Therapies
The problem with determining the efficacy of anti-fibrotic therapies lies in proving causality between fibrosis regression and improved outcomes. The majority of the aforementioned treatments effect multiple generic disease pathways so conclusively proving their specific anti-fibrotic benefits is difficult. ECV may be especially helpful here: it has already been shown to be strongly and independently associated with poor outcomes in patients with systolic heart failure and in those with heart failure and preserved ejection fraction [93]; it is a well-established CMR technique available to most CMR units globally; and it is non-invasive and quick, making serial assessment affordable for drug companies, and tolerable for most patients. Importantly myocardial fibrosis burden as assessed by ECV has also been shown to have a dose–response relationship with hospitalisation and death in heart failure independent of LGE [94], suggesting it is tracking additional biology. ECV may go some way to help establish the causal link that is needed to energise anti-fibrotic drug trials. This biomarker is already being exploited in randomised control trials as a surrogate endpoint [95, 96]. We call for more phase 2 and phase 3 drug trials to rely on the ever-growing armamentarium of CMR sequences for fibrosis, in an effort to improve the reliability and cost-effectiveness of studies, and maximise their translational impact.
Conclusions
Myocardial fibrosis is the final point of convergence for all heart muscle diseases, ushering in the development of heart failure and death. Effective anti-fibrotic therapies are urgently needed but clinical trials are proving expensive and results frankly disappointing. CMR imaging biomarkers, in particular ECV and native T1, are fascinating periscopes into the myocardial response to drug therapy yet they are underutilised by pharma. These CMR techniques are so sensitive that they can detect subclinical fibrosis, opening that all-important window of early drug intervention, before the irreversible devastation caused by fibrosis takes hold.
References
González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–706.
Fang L, Murphy AJ, Dart AM. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front Pharmacol. 2017;8:186.
Aherne E, Chow K, Carr J. Cardiac T1 mapping: techniques and applications. J Magn Reson Imaging. 2020;51:1336–56.
Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging. 2020;13:1221–344.
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903.
Messroghli DR, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75.
Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4.
Matsusaka H, et al. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension. 2006;47:711–7.
Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24.
Shibasaki Y, et al. Impact of the angiotensin II receptor antagonist, losartan, on myocardial fibrosis in patients with end-stage renal disease: assessment by ultrasonic integrated backscatter and biochemical markers. Hypertens Res. 2005;28:787–95.
de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–7.
Clozel M, Salloukh H. Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann Med. 2005;37:2–12.
Schafer S, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–5.
Hoyt RH, Ericksen E, Collins SM, Skorton DJ. Computer-assisted quantitation of myocardial fibrosis in histologic sections. Arch Pathol Lab Med. 1984;108:280–3.
Radenkovic D, Weingärtner S, Ricketts L, Moon JC, Captur G. T1 mapping in cardiac MRI. Heart Fail Rev. 2017;22:415–30.
Puntmann VO, et al. T1-mapping and outcome in nonischemic cardiomyopathy all-cause mortality and heart failure. JACC Cardiovasc Imaging. 2016;9:40–50.
Dass S, et al. Myocardial tissue characterization using magnetic resonance noncontrast T1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726–33.
Ho CY, et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–22.
Mahmod M, et al. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson. 2014;16:92.
Chow K, Yang Y, Shaw P, Kramer CM, Salerno M. Robust free-breathing SASHA T1 mapping with high-contrast image registration. J Cardiovasc Magn Reson. 2016;18:47.
Mehta BB, Chen X, Bilchick KC, Salerno M, Epstein FH. Accelerated and navigator-gated look-locker imaging for cardiac T1 estimation (ANGIE): development and application to T1 mapping of the right ventricle. Magn Reson Med. 2015;73:150–60.
Zhao L, et al. Systolic MOLLI T1 mapping with heart-rate-dependent pulse sequence sampling scheme is feasible in patients with atrial fibrillation. J Cardiovasc Magn Reson. 2016;18:13.
Weingärtner S, Meßner NM, Zöllner FG, Akçakaya M, Schad LR. Black-blood native T1 mapping: blood signal suppression for reduced partial voluming in the myocardium. Magn Reson Med. 2017;78:484–93.
Ferreira VM, et al. Systolic ShMOLLI myocardial T1-mapping for improved robustness to partial-volume effects and applications in tachyarrhythmias. J Cardiovasc Magn Reson. 2015;17:77.
Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2.
Captur G, et al. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance - the T1 mapping and ECV standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18:1–20.
Weng Z, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging. 2016;9:1392–402.
Assomull RG, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85.
Mahrholdt H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8.
Schelbert EB, Sabbah HN, Butler J, Gheorghiade M. Employing extracellular volume cardiovascular magnetic resonance measures of myocardial fibrosis to foster novel therapeutics. Circ Cardiovasc Imaging. 2017;10:e005619.
Ravenstein CDM, et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48.
Kammerlander AA, et al. T1 mapping by CMR imaging from histological validation to clinical implication. JACC Cardiovasc Imaging. 2016;9:14–23.
Su MYM, et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991–7.
Mavrogeni S, et al. T1 and T2 mapping in cardiology: ‘mapping the obscure object of desire’. Cardiology. 2017;138:207–17.
Liu Y, Hamilton J, Rajagopalan S, Seiberlich N. Cardiac magnetic resonance fingerprinting: technical overview and initial results. JACC Cardiovasc Imaging. 2018;11:1837–53.
Kvernby S, et al. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson. 2014;16:102.
Szabó Z, et al. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–40.
Li X, Tang X, Lu J, Yuan S. Therapeutic inhibition of galectin-3 improves cardiomyocyte apoptosis and survival during heart failure. Mol Med Rep. 2018;17:4106–12.
Sharma UC, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8.
Calvier L, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75.
Suthahar N, et al. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: an update. Theranostics. 2018;8:593–609.
MacKinnon AC, et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185:537–46.
Yu L, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107–17.
Piccoli MT, et al. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res. 2017;121:575–83.
Abonnenc M, et al. Extracellular matrix secretion by cardiac fibroblasts: role of MicroRNA-29b and MicroRNA-30c. Circ Res. 2013;113:1138–47.
Díez J, et al. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7.
Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93.
López B, et al. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104:286–91.
Shimada YJ, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1:480–7.
Kosmala W, et al. A randomized study of the beneficial effects of aldosterone antagonism on lv function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging. 2011;4:1239–49.
Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM-PEF). J Card Fail. 2011;17:634–42.
Mak GJ, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–82.
Engebretsen KVT, et al. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. Curr Ther Res Clin Exp. 2014;76:148–57.
Lopez-de la Mora DA, et al. Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci. 2015;12:840–7.
Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: a role for transforming growth factor-β. Cardiovasc Ther. 2012;30:e30–e40.
Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7:1438–45.
Mirkovic S, et al. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. Br J Pharmacol. 2002;135:961–8.
Yamazaki T, et al. The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res. 2012;35:34–40.
Yamagami K, et al. Pirfenidone exhibits cardioprotective effects by regulating myocardial fibrosis and vascular permeability in pressure-overloaded hearts. Am J Physiol Heart Circ Physiol. 2015;309:H512–H522522.
Kelly DJ, et al. Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H2860–H2869.
Holmes DR, et al. Results of prevention of REStenosis with tranilast and its outcomes (PRESTO) trial. Circulation. 2002;106:1243–50.
Zammit SC, et al. Evaluation and optimization of antifibrotic activity of cinnamoyl anthranilates. Bioorganic Med Chem Lett. 2009;19:7003–6.
Prasad SK, et al. Comparison of the dual receptor endothelin antagonist enrasentan with enalapril in asymptomatic left ventricular systolic dysfunction: a cardiovascular magnetic resonance study. Heart. 2006;92:798–803.
Sütsch G, et al. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–8.
Anand I, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin A Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–54.
Heymans S, et al. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail. 2015;17:764–71.
Ducharme A, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Investig. 2000;106:55–62.
Yamamoto D, Takai S. Pharmacological implications of MMP-9 inhibition by ACE inhibitors. Curr Med Chem. 2009;16:1349–54.
Yamamoto D, et al. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007;43:670–6.
Hudson MP, et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction. Results of the PREMIER (prevention of myocardial infarction early remodeling) trial. J Am Coll Cardiol. 2006;48:15–20.
Ma Y, et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res. 2013;112:675–88.
Wang D, Zhu H, Yang Q, Sun Y. Effects of relaxin on cardiac fibrosis, apoptosis, and tachyarrhythmia in rats with myocardial infarction. Biomed Pharmacother. 2016;84:348–55.
Samuel CS, et al. Relaxin remodels fibrotic healing following myocardial infarction. Lab Investig. 2011;91:675–90.
Samuel CS, Hewitson TD, Zhang Y, Kelly DJ. Relaxin ameliorates fibrosis in experimental diabetic cardiomyopathy. Endocrinology. 2008;149:3286–93.
Lekgabe ED, et al. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension. 2005;46:412–8.
Henry BL, et al. Relaxin suppresses atrial fibrillation in aged rats by reversing fibrosis and upregulating Na+ channels. Heart Rhythm. 2016;13:983–91.
Xu Q, et al. Endogenous relaxin does not affect chronic pressure overload-induced cardiac hypertrophy and fibrosis. Endocrinology. 2008;149:476–82.
Teerlink JR, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised placebo-controlled trial. Lancet. 2013;381:29–39.
Khanna D, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60:1102–11.
Buggey J, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323–33.
López B, et al. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–35.
López B, et al. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–67.
López B, et al. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009;53:236–42.
Díez J, et al. TORAFIC study protocol: torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev Cardiovasc Ther. 2009;7:897–904.
Chang SA, et al. Effect of rosuvastatin on cardiac remodeling, function, and progression to heart failure in hypertensive heart with established left ventricular hypertrophy. Hypertension. 2009;54:591–7.
Kjekshus J, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–61.
GISSI-HF investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–9.
Krum H, et al. Double-blind, randomized, placebo-controlled study of high-dose HMG CoA reductase inhibitor therapy on ventricular remodeling, pro-inflammatory cytokines and neurohormonal parameters in patients with chronic systolic heart failure. J Card Fail. 2007;13:1–7.
Mann DL, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized etanercept worldwide evaluation (RENEWAL). Circulation. 2004;109:1594–602.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133–40.
Sarma S. Use of clinically available PPAR agonists for heart failure; do the risks outweigh the potential benefits? Curr Mol Pharmacol. 2012;5:255–63.
Corden B, Adami E, Sweeney M, Schafer S, Cook SA. IL-11 in cardiac and renal fibrosis: late to the party but a central player. Br J Pharmacol. 2020;177:1695–708.
Schelbert EB, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017;2:995–1006.
Schelbert EB, et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc. 2015;4:e002613.
Heydari B, et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction. Circulation. 2016;134:378–91.
Lewis GA, et al. Pirfenidone in heart failure with preserved ejection fraction—rationale and design of the PIROUETTE trial. Cardiovasc Drugs Ther. 2019;33:461–70.
Acknowledgements
Funding
No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Matthew Webber and Gabriella Captur are both supported by British Heart Foundation Special Programme Grant MyoFit46 (SP/20/2/34841). Gabriella Captur and James Charles Moon are funded by the Heartome1000 Barts Charity grant #1107/2356/MRC0140. Gabriella Captur is supported by the Josephine Lansdell British Medical Association research grant. James Charles Moon is directly and indirectly supported by the UCL Hospitals NIHR BRC and Biomedical Research Unit at Barts Hospital respectively. Stephen P Jackson has no disclosures for this article.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12791828.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Webber, M., Jackson, S.P., Moon, J.C. et al. Myocardial Fibrosis in Heart Failure: Anti-Fibrotic Therapies and the Role of Cardiovascular Magnetic Resonance in Drug Trials. Cardiol Ther 9, 363–376 (2020). https://doi.org/10.1007/s40119-020-00199-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-020-00199-y