Abstract
TiO2 white pigments were used in various applications due to their magnificent optical and electronic properties. In this paper TiO2 was recovered from red gypsum which is a waste generated at TiO2 production industry. The effect of time and temperature in sulphuric acid leaching processes was optimized. TiO2 powders were synthesized through thermal precipitation process with the presence of ethylenediaminetetraacetic acid as a complexing and purification agent. The obtained powders were characterized by X-ray diffraction, transmission electron microscope, UV–Vis spectroscopy and inductively coupled plasma optical emission spectroscopy. Experimental results show that powders in anatase form have a 3.2 eV band gap with a crystallite size 30–50 nm.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide, an important intermediate material, is widely used as a pigment in the manufacture of paints, paper, printing inks, rubber, floor covering, ceramics, pharmaceuticals and other areas of chemical industry paints, plastics, rubber, and paper industries. In recent application, TiO2 has been used in photocatalytic application mainly in environmental purification such as reduction NO x gases [1] and water purification by treating various chemicals [2]. TiO2 has attracted to be used due to its chemical inertness, high reflectivity—brightness and brilliance, high refractive index, non-toxicity and thermal stability. There are three crystal forms of TiO2 namely brookite, anatase and rutile. Among these three, anatase has shown higher photocatalytic activity due to its strong absorption capacity towards organic molecules and low recombination rate of photo-generated electron–hole pair in the anatase. Rutile is used in producing printing inks, cosmetics, plastics and paints.
There are two routes to produce titanium dioxide commercially which are through chlorination and sulphate process. Although the chlorination process is regarded as a clean process to yield titanium white, it is not suitable to be used for recovering titanium dioxide from titanium ores which contain low-titanium content. The chlorination process requires high-purity chlorine as a raw material. The processing of ore with a low-titanium content not only requires a large quantity of chlorine, but also produces chloride impurities that clog the transportation pipes [3]. The sulfuric acid process, which can digest many kinds of low-titanium-content resources, will continue to be used if its drawback of acid and iron disposal and environmental pollution is overcome.
Red gypsum is synthetic gypsum produced during titanium dioxide production to neutralize weak acid before release to the environment. Ilmenite as the source of titanium was exposed to high concentrated sulphuric acid to leach out titanium from ilmenite. After several clarification and purification steps, titanyl sulphate was hydrolyzed to produce titanium dioxide pigment which generated weak acid which will be further treated with lime generating red gypsum [4]. Red gypsum was composed of SO3 and CaO while CaSO4·2H2O as the main crystalline phase. Gypsum also has higher TiO2 content around 5 % originated from weak acid. The reddish colour was explained by the presence of iron hydroxides, about 8 % [5].
In the hydrolysis process of TiO2, the presence of Fe3+ in titanyl sulphate solution will commence to precipitate together with TiO2+ due to their small pH differences according to the different solubility product constants of H2TiO3 (1 × 10−29) and Fe(OH)3 (4 × 10−38). Thus, the removal of Fe3+ in the titanyl solution is a biggest challenge. It is also important to remove Fe2O3 because at low content as low 100 ppm (10−6 wt%), it still gives TiO2 product yellow in colour which limited its photocatalytic application [3].
In the present work, we utilize gypsum as a source of titanium via sulphate process to leach out titanium to the solution by optimizing two parameters which are temperature and reaction time. Different ratio EDTA/Fe was also studied to determine the purity of TiO2 product.
Methodology
Material
Red gypsum (RG) used in the present study was supplied by the Tioxide (Malaysia) Sdn Bhd. RG was dried in 100 °C oven for 24 h and ground passed 500-µm sieves. The chemical composition is listed in Table 1: hydrochloric acid (37 %), sulphuric acid (95–97 %) and ammonium hydroxide (30 %) EDTA.
Experimental procedure
Summary of the production of TiO2 nanopowders was shown in flow chart in Fig. 1.
HCl leaching
HCl leaching was carried out in 1-L flat bottom flask connected to a reflux condenser on a hot plate. Hydrochloric acid (20 wt%) was heated to 100 °C and definite amount of gypsum was added. The mixture was left under vigorous stirring for 2 h. After that the mixture was filtered and washed with 5 % HCl three times. The grey-colour residue (high-titanium residue) was collected and dried in 100 °C overnight.
H2SO4 decomposition
High-titanium residue (HTR) was decomposed on digital hotplate stirrer in 1-L flat bottom flask connected with condenser. Sulphuric acid was heated to definite temperature and an amount of HTR was added. Parameters that affected the titanium extraction were studied. Titanium extraction was calculated by the following formula
where X % is the titanium fractional conversion, MTi is the titanium concentration in the water solution measured by ICP after dissolution of the product (g L−1), Gt is the total mass of the titania slag (g), CTi % is the titanium mass fraction in the slag, and V is the volume of water solution. After digestion, water with a ratio 1:1 to a paste was added and stirred for 1.5 h at 90 °C for water leaching process.
Hydrolysis and synthetic TiO2
Titanyl sulphate solution (Ti: 2.54 g/L, Fe: 1.76 g/L) obtained from best leaching condition was used in hydrolysis process. Hydrolysis process was carried out in 1-L beaker complete with thermometer and stirrer. 100 ml of titanyl sulphate was mixed with 300 ml of distilled water (1:4) and left stir for 1 h. An EDTA solution was added into the mixture and subjected to the precipitation by slow addition of ammonium hydroxide (30 %) under constant stirring at 80 °C. The hydrolysis was controlled until mixture reached pH 8–9. White precipitate was separated by filtration and washed with distilled water for 3 times. The precipitate was collected and left for drying in room temperature for 24 h. The powder was calcined at 400 °C to obtain anatase crystal structure.
Analysis
Elemental analysis of solution was analyzed using inductively coupled plasma–optical emission spectroscopy (ICP–OES). Crystal structure was analyzed using XRD (Phillips PW1729, CuKα); particles’ size and surface morphology were analyzed using FESEM (FESEM: JEOL-JSM-7600F) and HRTEM (Technai G2 20S Twin TEM) and elemental mapping of the particles was observed with EDAX. The UV–Visible spectrum of the sample was recorded in the spectral range of 200–800 nm using a double-beam CARY 5000 UV–Vis–NIR spectrophotometer. The band gap calculation was based on method [6, 7].
Results and discussions
Hydrochloric acid leaching
The gypsum was initially treated with hydrochloric acid leaching since it is advantageous in removing impurities such as MgO and CaO [8]. The slag produced by hydrochloric acid leaching was proven to contain reduced iron content from 16.85 to 1.16 %, with a little amount of titanium being leached during HCl leaching as shown in Table 2. This shows that HCl leaching is a good process to initially remove iron from gypsum.
Factors affecting decomposition with sulphuric acid
Effect of time
To study effect of leaching time on red gypsum by 80 % H2SO4 several experiments were performed at different reaction times ranging from 2 to 10 h. The other parameters were fixed at L/S mass ratio of 3/1 and temperature 110 °C. The results tabulated in Fig. 2 show that the dissolution of Ti decreases as reaction time increases. The higher recovery of Ti was at 2 h of reaction time with 90 % recovery while recovery values decrease tremendously after 4 h and are constant at 6, 8 and 10 h of reaction. This trend was matched with previous works that reported the decreases of recovery value as time increases [9–11]. During long exposure to temperature near boiling point, some of the polymeric species (including TiOSO4, [TiO(SO4)2]2−) were destroyed which encourages the formation of multinuclear complexes of types [Ti2O2(OH)3]+ and [Ti4O6(OH)3]+ [12]. This will lead to reduction of Ti at longer leaching time, therefore 2 h represents an optimum leaching time of Ti by sulfuric acid [9].
Effect of temperature
The effect of temperature on leaching of titanium was studied in the range 100–250 °C under constants of others parameters: S/L = 1:3, time = 2 h, acid concentration = 80 %. The results obtained are shown in Fig. 3. Amount of titanium recovered is highest at 100 °C and starts to decrease at 150 °C. This result is same as reported by other researchers where at near 100 °C the recovery of titanium is above 90 % and starts to decrease extremely at higher temperature [12]. This is due to instability of titanium-contained solution, therefore it greatly enhanced polymerization and hydrolysis which led to decrease in the amount of titanium extracted [13]. Thus, temperature 100 °C was chosen as optimized temperature for Titanium extraction in this study.
Hydrolysis and synthetic TiO2
Purity
The relationship between TiO2 product purity and EDTA/Fe3+ molar ratio is shown in Fig. 4. The purity of TiO2 rose from 90.1 to 96.9 % as the EDTA/Fe3+ molar ratio increased from 1.5 to 3.0. High purity of TiO2 was obtained when EDTA/Fe molar ratio is 3. High amount of EDTA is needed to chelate out Fe since EDTA has six forms in solution, which are H6Y2+, H5Y+, H4Y, H3Y−, H2Y2−, HY3− and Y4−. Among six forms, only HY3− anion was capable to chelate Fe3+ since it carries same amount of charge as that of Fe3+. Chelating effect of EDTA decreased Fe3+ free ions and prevents Fe3+ from precipitating together with TiO2+ therefore helping improve the purity of TiO2 [3]. From EDAX analysis at Fig. 5 and Table 3 only titanium, carbon and copper appeared in the result. Carbon element comes from the reaction of laser and sample while copper element appeared since analysis used copper grid as a holder. From this result, it shows that EDAX analysis was correlated with ICP–OES analysis since there are no foreign elements existing in the powder.
Morphology
Figure 6 shows the FESEM micrographs of calcined TiO2. The secondary particles comprised agglomerated irregular primary particles (Fig. 6a). The size of secondary particles is varied from 100 to 200 nm where the primary particles varied from 30 to 50 nm (Fig. 6b). This evolution of primary size to secondary size was caused by the agglomeration of primary particles during drying process. Removal of water will reduce the distance between individual particles and lead to the agglomeration due to the van der Waals force [14]. In this analysis we also found that primary particles are in nanosize due to the presence of EDTA which controls the precipitation rate by adsorbing the primary particles and preventing them from growing. It is well known that in preparing TiO2 nanopowder, bidentate ligands are always used to control the reactivity of metal alkoxides [15]. Ligand functionality served as a versatile tool to control the assembly behaviour of titania nanocrystals [16]. Ligand functioned as assemblers that usually are adsorbed on the surface of particles resulting nanostructure particles. In this study, EDTA acts as a control for the agglomeration and also improves the purity of TiO2 nanoparticles [16, 17].
XRD
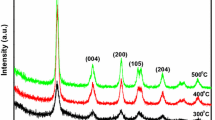
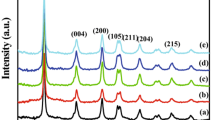
Figure 7 presents the powder XRD pattern of TiO2 synthesized via Ti-EDTA chelated complex. The diffraction peaks (101), (004), (200), (105) and (211) are analogous to anatase crystal structure and the peaks of other polymorphs; rutile or brookite is not detected.
UV–Vis diffuse reflectance spectra of TiO2
According to Fig. 8, the uncalcined and calcined TiO2 had the absorption edges at 376 and 386 nm, respectively. The band gap values of uncalcined and calcined TiO2 are 3.30 and 3.22 eV, respectively. These values are normal for anatase phase and support the XRD analysis for uncalcined TiO2 having an anatase phase. Even though there is no significant value of band gap energies, the absorption values between these two samples are different whereas calcined TiO2 shows higher absorption values of UV light which is useful to improve the photocatalytic performance.
Conclusion
Titanium ions were extracted from gypsum using sulphate method by studying effect or reaction temperature and leaching time which gave a 90 % extraction recovery at mild condition compared to the extraction at low and extreme condition. TiO2 nanoparticles were produced from control precipitation of extracted titanyl sulphate with the presence of EDTA as a complexing agent. The presence of EDTA will help to inhibit Fe(OH)3 from precipitate and subsequently help improve purity of TiO2 as a final product. Effect of EDTA/Fe ratio has been studied and as results 96 % of purity level was achieved at EDTA/Fe ratio 3. Using EDTA as complexing agent also produces a TiO2 nanoparticle with size ranging from 30 to 50 nm.
References
Guo, M.-Z., Poon, C.-S.: Photocatalytic NO removal of concrete surface layers intermixed with TiO2. Build. Environ. 70, 102–109 (2013)
Lee, S.-Y., Park, S.-J.: TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 19, 1761–1769 (2013)
Wang, M., Woo, K.D., Kim, I.Y., Woong, K., Sui, Z.: Separation of Fe3+ during hydrolysis of TiO2+ by addition of EDTA. Hydrometallurgy 89, 319–322 (2007)
Gazquez, M.J., Bolivar, J.P., Vaca, F., García-Tenorio, R., Caparros, A.: Evaluation of the use of TiO2 industry red gypsum waste in cement production. Cement Concr. Compos. 37, 76–81 (2013)
Fauziah, I., Zauyah, S., Jamal, T.: Characterization and land application of red gypsum: a waste product from the titanium dioxide industry. Sci. Total Environ. 188, 243–251 (1996)
Dharma, J., Pisal, A.: Simple method of measuring the band gap energy value of TiO2 in the powder form using a UV/Vis/NIR spectrometer. Perkin elmer, Application Note 935, pp. 1–4 (2009)
Christy, P.D., Melikechi, N., Nirmala Jothi, N.S., Baby Suganthi, A.R., Sagayaraj, P.: Synthesis of TiO2 nanorods by oriented attachment using EDTA modifier: a novel approach towards 1D nanostructure development. J. Nanopart. Res. 12, 2875–2882 (2010)
Walpole, E.A., Winter, J.D.: Chloride metallurgy. In International Conference on the Practice and Theory of Chloride/Metal Interaction, Montreal (2002)
Nayl, A.A., Aly, H.F.: Acid leaching of ilmenite decomposed by KOH. Hydrometallurgy 97, 86–93 (2009)
Wu, F., Li, X., Wang, Z., Wu, L., Guo, H., Xiong, X., Zhang, X., Wang, X.: Hydrogen peroxide leaching of hydrolyzed titania residue prepared from mechanically activated Panzhihua ilmenite leached by hydrochloric acid. Int. J. Miner. Process. 98, 106–112 (2011)
Mehdilo, A., Irannajad, M.: Iron removing from titanium slag for synthetic rutile production. Physicochem. Probl. Miner. Process. 48, 425–439 (2012)
Xiong, X., Wang, Z., Wu, F., Li, X., Guo, H.: Preparation of TiO2 from ilmenite using sulfuric acid decomposition of the titania residue combined with separation of Fe3+ with EDTA during hydrolysis. Adv. Powder Technol. 24, 60–67 (2013)
Li, C., Liang, B., Guo, L.-H.: Dissolution of mechanically activated Panzhihua ilmenites in dilute solutions of sulphuric acid. Hydrometallurgy 89, 1–10 (2007)
Borhan, M.Z., Ahmad, R., Rusop, M., Abdullah, S.: Green extraction: enhanced extraction yield of asiatic acid from Centella asiatica (L.) nanopowders. J. Appl. Chem. 2013, 7 (2013)
Schubert, U., Arpac, E., Glaubitt, W., Helmerich, A., Chau, C.: Primary hydrolysis products of methacrylate-modified titanium and zirconium alkoxides. Chem. Mater. 4, 291–295 (1992)
Polleux, J., Pinna, N., Antonietti, M., Niederberger, M.: Ligand-directed assembly of preformed titania nanocrystals into highly anisotropic nanostructures. Adv. Mater. 16, 436–439 (2004)
Liu, Y., Li, Y., Guo, Y.: Synthesis of high purity TiO2 nanoparticles from Ti(SO4)2 in presence of EDTA as complexing agent. China Particuol. 03, 240–242 (2005)
Acknowledgments
The project was sponsored by Science Fund from Ministry of Science and Technology, Malaysia (03-03-02-SF0258).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Borhan, M.Z., Nee, T.Y. Synthesis of TiO2 nanopowders from red gypsum using EDTA as complexing agent. J Nanostruct Chem 5, 71–76 (2015). https://doi.org/10.1007/s40097-014-0137-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0137-7