Abstract
Purpose
Stevia rebaudiana residues composting is studied and optimized using a central composite experimental design
Methods
The influence of controllable composting variables [moisture (40–70%), aeration (0.05–0.30 Lair min−1 kg−1), and time (0–50 days)] on the temperature history and the properties of the compost (pH, organic matter) produced to determine suitable composting conditions. Mass balance and emitted gases of all the composting reactors have been done.
Results
Compost with high degradation entails operating at 50 days of composting under high moisture content (70%) and aeration level with values close to 0.05 Lair min−1 kg−1. Moreover, the composting process kinetic for Stevia rebaudiana residues has been studied. The magnitude of the kinetic parameters on the studied conditions varies among 1/42 and 1/46 for 1/K1 and 0.15 and 1.6 for K2. Where 1/K1 is a value that measures the affinity between microorganisms and substrate and K2 depends on the composting variable optimization.
Conclusions
Both moisture and aeration affects positively and negatively the composting process. Moreover, low effect of aeration has been found. The values of 1/K1 and K2 obtained showed higher values (higher degradation kinetic) under 55% moisture content and 0.30 Lair min−1 kg−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the most important, with worldwide medicinal and commercial uses, Stevia rebaudiana is found (Ahmad et al., 2016), because is increasingly being used as a sugar substitute (Magnuson et al. 2016). In that form, the steviol glycosides (active compounds in Stevia which is found mainly in leaves) are estimated to be 200–300 times sweeter than saccharose and have no influence on the glycemic index (Geuns 2010). In this sense, both, harvesting, to get its leaves, and pruning, which includes the elimination of all dead, dying, or diseased wood and branch to obtain a suitable regrowth of plants are necessary. This fact leads to Stevia rebaudiana residues (SRR), essentially, trunks and fine branches (0.5–5 cm thickness). The lack of markets for these wastes is a serious problem for the economic sustainability of this process. To enhance the added value of these wastes, composting could be an appropriate solution, because it is a natural way low cost of recycling organic matter and nutrients.
For extensive use of this technology, an adequate optimization is necessary. According to Haug (1993) and Peláez et al. (2004), composting process kinetic could be modelling using enzyme kinetic concepts and, in this sense, could be described through a first-order reaction (catalysed by enzymes).
Composting optimization entails finding the best values of the main involved variables to obtain a greater and faster degradation of the residues studied. The main factors controlling composting are: operating parameters (moisture content and aeration), pH, and temperature are not industrially altered, and nature of substrate (C/N ratio and particle size). Among them, temperature is a key factor to judge the process efficiency in composting (Ekinci et al. 2004; Lei and Vandergheynst 2000; Kianirad et al. 2009). Moreover, in accordance with Wang and Ai (2016) and Ponsá et al. (2009), a balance among moisture content, diffusion transport, and oxygen supply is necessary to obtain a suitable composting process; hence, an adequate size, as physical pretreatment, is necessary in these residues.
On the other hand, knowledge of the degradation kinetics, to find the most suitable conditions for proper degradation, is necessary. Several kinetic data, by inductive and deductive models, have been obtained by Sánchez (2007), Bueno et al. (2008), and Wang and Ai (2016). In these models, references about the influence of several composting factors such (C/N ratio, moisture content, temperature, particle size, pH, mixing or turning frequency, and aeration rate) have been reported. However, to my knowledge, no previous studies on Stevia process composting have been found.
The main objective of this study is to measure the relative influence of the main controllable composting variables (moisture and aeration) and time on this process to obtain suitable industrial composting conditions for S. rebaudiana residues.
Materials and methods
Stevia residues (branches and twigs) with 0.5–5 cm in diameter were used. Plants were cut at ~ 5 cm above the soil. The material was collected, using closest individual sampling method, from mature plants growing at industrial scale plantation in Moguer (Huelva, Spain).
Composting process
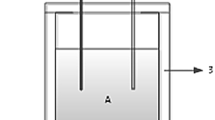
Stevia rebaudiana residues (SRR) were mixed, to get uniform initial material, and chipped, to ensure an adequate particle distribution during the process and an adequate initial porosity. About 20 kg mixture was placed in each reactor (Fig. 1). Relevant characteristics of the raw materials are reported in Table 1.
Cylindrical composting reactors were formed from an acrylic column (0.5 m in diameter and 1 m in depth). These reactors were insulated with polyurethane foam to minimize the conductive heat loss. Compressed air was introduced to the bottom of each reactor, through a perforated plate, and evenly distributed to the waste mixture. Two temperature sensors were fixed at the centre and on top of each reactor (K thermocouples, TMC6-HA). To obtain environmental temperature (Protimeter-MMS-Plus), an environmental temperature sensor was placed outside the reactors. Temperatures were recorded every 12 h in each reactor by two data loggers (HOBO U12-006).
Moisture (M), aeration (A), and time (t) have been used as independent and controllable composting variables. Each parameter has been established following a central composite experimental design (three levels for each variable). In that form, the values have been fixed in: 40%, 55 and 70% for moisture (Hong et al. 2013; Kim et al. 2015), 0.05, 0.175, and 0.3 Lair kg−1 min−1 for aeration (Gao et al. 2010) and from 1 to 55 days for composting time with except to temperature (fixed in 90 days). During active composting phase, water losses were compensated to ensure and sustain initial conditions, due to they have been established as constants in the experimental design.
Experimental design for the incubation process and statistical analysis
A 2n central composite factor design (Montgomery 1991) has been used to relate the dependent and independent (A, M, and t) variables. The central combination for the experimental design was: A = 0.175 Lair kg−1 min−1, M = 55%, and t = 27 days. The obtained results were implemented in SPSS system package to obtain multiple linear regressions (one for each dependent variable).
Independent variables were normalized using the following equation:
where X is the absolute value of the independent variable concerned, \(\bar{X}\) is the average value of the variable, and Xmax and Xmin are its maximum and minimum values, respectively.
The number of tests required was calculated as N = 2n + 2n + nc. In this form, 2n being the number of points constituting the factor design, 2n axial points, and nc central point. Under this study conditions, N = 10.
The experimental results were fitted as second-order polynomial equation (Eq. 2):
In this sense, only independent variables were those having a statistically significant coefficient (viz. those not exceeding a significance level of 0.05 in Student’s t test and having a 95% confidence interval excluding zero) has been used in the obtained equations relating to both types of variables.
Mass balance and kinetic model
According to Haug (1993), composting process could be expressed as biodegradable solid oxidation (Eq. 3). In this balance, initial non-biodegradable solid mass is equal to the final non-biodegradable solid mass is assumed (Haug 1993):
The stoichiometric oxygen required for decomposition can be determined from the organic feed decomposition. Carbon dioxide (CO2), ammonium (NH3), and water (H2O) produced for organic decomposition were determined throughout Eqs. 3, 4, and 5. Nitrogen input is equal to nitrogen output is assumed and there have been no leachates. Moreover, the water added to maintain the moisture of each of the reactors has not been taken into account to obtain an adequate reactor comparison.
The kinetic model of Whang and Meenaghan (1980) and described by Haug (1993) was used. In essence, this model assumes the formation of an intermediate complex (CX*) in which the hydrolytic enzyme (or free microorganism, X) is absorbed to an active site of the surface solid substrate (C, kg) yielding the product (P, kg):
Being k1 the specific reaction (I) rate constant, k− 1 the specific desorption rate constant, k2 the specific reaction (II) rate constant
Assuming a quasi-equilibrium state for the intermediate complex:
where K1 is the dissociation constant of the microorganism–substrate complex. This constant is characteristic for the biological system considered. Its inverse, 1/K1 is a measure of the affinity between microorganisms and substrate (Whang and Meenaghan, 1980).
If R (kg day−1) is the consumption rate of the substrate C (kg):
where K2 = k2 (Xt) and Xt is the total microorganism concentration.
K2 is a kinetic variable of the system and depends on the optimization parameters of the process such as temperature, moisture, C/N, pH, aeration, etc. It varies considerably depending on the experimental conditions. Its contribution to the reaction rate is directly proportional to its magnitude.
To determine the values of K1 and K2 from the experimental data, Eq. 8 is transformed as follows:
where C is the carbon content expressed on ash-free basis (kg) and R is the carbon loss per day (kg day−1) and can be obtained each day from the tangent of the curve C (ash-free basis) vs. t (days).
The plot 1/R vs. 1/C permits the graphical estimation of the constants, K1 and K2.
Analytical methods
Samples (150 g approx.) were collected every 3 days in the early composting and every week in the mesophilic and maturation stages. All samples have been dried (60 °C) and ground to pass in a sieve of 0.5 mm diameter. On a significant sample of the original material, moisture was determined by drying at 105 °C to constant weight. The samples were analysed for: pH (1:5 w/v) using a pH electrode, total organic matter (OM) by loss on ignition (550 °C for 5 h) (Klute et al., 1986), and carbon was estimated as OM/1.8 (Haug 1993). Carbon was also expressed on ash-free basis, total P (HCl acid digest) using the ascorbic acid method (Jones 2001). Total K, Ca, Mg, Fe, Cu, Mn, and Zn are determined by total digestion of the compost in strong acid (concentrated HCl), with subsequent analysis by atomic absorption spectrometry (Variant SpectrA 220FS, Jones 2001) and the total Kjeldahl-N was determined by Kjeldahl digestion (Faithfull 2002).
Bulk density has been measured by following Schaub-Szabo and Leonard (1999) method. Particle density is calculated as an indirect estimation using Martinez (1992) method for high organic matter percentage materials. Under this method, density is calculated as an indirect estimation from organic matter and ash values obtained in calcination process. According to Boodt and Verdonck (1972), particle density of organic materials (OM, %) is 1.45 g cm−3 and that of ashes, as mineral matter (MM, %), is 2.65 g cm−3:
The final compost characterizations are also shown in Table 1.
Results and discussion
To assess the relative influence of the selected independent variables (moisture, aeration, and time) on each dependent variable (temperature, pH, OM, and emitted gases) by substituting the values of the measured independent variables for each dependent variable, and applying a polynomial model analysis, the polynomial mathematical models have been obtained (Table 2). The average of three measurements has been used to obtain the equations. Suitable fits with values of R2 greater than 0.94 in all cases have been obtained. The differences between the experimental and estimated (using the obtained equations) values have been lower than 5%.
To better envisage the influence of operational variables on measured parameters of compost, and to compare different conditions, the surface responses in for each dependent variable have been plotted.
Temperature and pH profiles
The temperature profiles (values expressed as mean of the two temperatures in each reactor) of the reactors are presented in Fig. 2. As expected, after the establishment of composting conditions, the temperature of the reactors began to rise to thermophilic temperatures in 4 days. A general tendency, during the first 15 days, of temperature elevation in all the reactors, has been observed. After that, as the transformation process takes place, a subsequent decrease. Because winter temperatures, in which the study was conducted, low-temperature peaks have been found. Moreover, the several ups and downs in the graphs of each reactor correspond to the water additions to compensate water losses during the process to maintain the initial experimental design moisture conditions. This fact is observed in all the reactors, a little drop during thermophilic stage is showed and high drop during mesophilic stage (with low-temperature values during 2 days) when it began to rise again.
For all experiments (with exception to reactors 8, 9, and 10), thermophilic temperatures (> 45 °C) were observed. The temperature profiles of reactors with high moisture content (70%) are, in general, higher than those found for medium (55%) and low (40%) moisture content. No significant aeration effect among reactor is observed. After these periods, composting temperatures began to drop to levels below 40 °C for all the reactors.
On the other hand, pH could be used as a suitable bio-oxidative phase evolution indicator (Nogueira et al. 1999). Furthermore, the pH levels will vary throughout the decomposition process and pH also significantly affects the composting process. In this sense, similar to that found for temperature, in Fig. 3, the evolution of pH with respect to time and aeration at three moisture contents is shown. In that figure, among the different studied aeration levels, a low pH variation during composting is observed. Nonetheless, under high moisture content (70%), pH increases gradually from 7.0 to 7.6. According to Nakasaki et al. (1993), it could be due to the metabolic degradation and proteolysis liberating ammonia compounds. However, under medium moisture content (55%), the pH maintains the same values during the whole composting process, understood to be between 6.9 and 7.1. As observed in Fig. 3, the pH, in reactors under low moisture content (40%), remains slightly below 7 during the composting process. Therefore, low sign of organic acid breakdown is found under these conditions.
Emitted gases profiles
The O2 evolution with respect to time and aeration at three moisture contents is shown in Fig. 4. The mean O2 concentration during the composting process was in the range 14–21% (Fig. 4), which indicated that the process for all the reactors was aerobic. The minimum suitable O2 concentration in interstitial gas for optimal composting is 5% (Haug 1993). In general, a linear increase over time for all reactors is observed. In addition, although initially, differences in O2 levels among the different aerations and moistures, these values have a high tendency to converge (near to 21%) as the process stabilizes (45–90 days).
The interstitial CO2 evolution with respect to time and aeration at three moisture contents is shown in Fig. 5. Under lower operating times (0–15 days), a higher content of CO2 is observed, mainly in higher moisture content (55–70%) reactors. This fact is also observed in the temperature evolution (Fig. 2) and pH (Fig. 3). Few statistical variations in the CO2 concentration with respect to the aeration levels have been observed. Overall, the range of CO2 concentrations within the reactor was higher during the initial monitoring period (thermophilic phase 1–15 days). After that, a progressive decrease to negligible values for all the reactors at 40 days is observed.
The partial depletion in O2 and the increase in CO2 were indicative of significant biological activity in the medium-to-high moisture (55–70%) reactors, and all studied aeration levels are considered suitable for Stevia residues composting process.
The volatile organic compounds’ (VOCs’) evolution with respect to time and aeration at three moisture contents is shown in Fig. 6. Emitted VOCs represent volatile breakdown products of aerobic and anaerobic processes (Maulini-Duran et al. 2013). A similar behaviour to that shown for CO2 is observed in Fig. 6 for VOCs. On this occasion, high initial contents, within the thermophilic phase, are found. Moreover, low aeration influence and medium influence of moisture content are found. In this case, the lowest VOCs concentrations are observed in reactors with high moisture contents (70%). After that, a progressive decrease in the VOCs emission has been observed for all reactors and no significant statistical differences were found under the selected experimental composting conditions at medium-to-high (25–55 days).
Organic matter mineralization
The organic matter progression with respect to time and aeration at three moisture contents is shown in Fig. 7. As expected, during the composting process, organic matter content decreased for all reactors (Fig. 7). According to Kim et al. (2015), the composting process could be described by a rapid initial degradation followed by a longer slow degradation phase. However, a quasi-linear decrease has been observed for all reactors, and as described for previous parameters, low aeration influence and medium influence of moisture content is found. Loss of organic matter of 6.5, 6.8, and 7.2% for low (40%), medium (55%), and high (70%) moistures, respectively, have been recorded after 55 days of composting. In addition, low differences the organic matter evolution under low and medium moistures with respect to high moisture content have been calculated.
The organic matter balances for the studied reactors are shown in Table 3. The main gas losses, according to the proposed stoichiometry, consisted of carbon dioxide produced and water evaporated from the compost. Variations in organic matter losses between 3.1 and 13% have been found depending on the process conditions.
At the beginning and after the maturation phase, the reactors were weighed. The highest loss has been found for R2 (70.96% of the initial dry weight under 70% moisture and 0.05 Lair min−1 kg−1 aeration) followed by R7 (70.07% under, 70% moisture and 0.15 Lair min−1 kg−1 aeration).
Lower weight losses have been found for R10 (29.67% of the initial dry weight under 40% moisture and 0.30 Lair min−1 kg−1 aeration) followed by R9 (33.66% of the initial dry weight under 40% moisture and 0.15 Lair min−1 kg−1 aeration).
Calculated ammonium losses were higher for R7 (55% moisture and 0.30 Lair min−1 kg−1 aeration) followed by R3 (70% moisture and 0.30 Lair min−1 kg−1 aeration). Lower ammonium losses have been found for R8 (40% moisture and 0.05 Lair min−1 kg−1 aeration) followed by R9 (40% moisture and 0.15 Lair min−1 kg−1 aeration).
In that form, aeration is a determining factor in nitrogen losses as ammonia. Therefore, the use of high moisture and low aeration levels to reduce nitrogen losses is advisable to obtain suitable final compost.
In general, the total weight losses were higher under high moisture levels. This fact can be explained by the low water retention capacity of the Stevia residues, so it is necessary to maintain high levels of moisture for effective water absorption within the mass.
Particle and bulk density evolution
Particle and bulk density evolution of each reactor is also calculated. Similar values in the initial particle density have been calculated (1520–1550 kg m−3). After 55 days of composting, R10 (40% moisture and 0.3 Lair min−1 kg−1 aeration) show the highest particle density value (1650 kg m−3), followed by R2 (70% moisture and 0.175 Lair min−1 kg−1 aeration, 1670 kg m−3). The lowest value was recorded in R7 (55% moisture and 0.175 Lair min−1 kg−1 aeration, 1570 kg m−3), followed by R5 (55% moisture and 0.3 Lair min−1 kg−1 aeration, 1570 kg m−3). As expected, based on the results obtained by Van Ginkel et al. (1999) a direct relation between degradation and particle density is found, owing to increases in ash concentrations (with higher density) during the composting process.
On the other hand, a progressive increase in bulk density in all of the studied reactors is found, mainly due to a settlement effect. In accordance with the results showed by López-Real (1990), when the compost mix is constituted, a low bulk density is observed. However, during the process, the combined effects of biological degradation processes and change of water content due to transport processes, a marked influence on bulk density is noted. This effect is observed mostly in R2 (137.49 kg m−3 under 70% moisture and 0.175 Lair min−1 kg−1 aeration), followed by R4 (94.93 kg m−3 under 55% moisture and 0.175 Lair min−1 kg−1 aeration), have been observed. Low increase in R5 (3.38 kg m−3 under 55% moisture and 0.3 Lair min−1 kg−1 aeration), followed by R7 (5.08 kg m−3 under 55% moisture and 0.175 Lair min−1 kg−1 aeration), have been found. Similar data and evolution has been found by Mohee and Mudhoo (2005).
Kinetic constants’ modelling
The first 30 day data (thermophilic temperatures and maximum degradation rate) have been used for the estimation of the kinetic constants.
Following the model proposed by Whang and Meenaghan (1980), to obtain the kinetic values, for each reactor, Lineweaver–Burke plots (1/R and 1/R) (figure not shown) were linearly correlated with high regression coefficients (Table 3). In this sense, due to the suitable regression coefficients obtained, the used kinetic model seems to be adequate to describe the thermophilic phase for Stevia residues composting process.
The polynomials’ mathematical models for K1 and K2, similar to that used for the previous dependent parameters, have been obtained by substituting independent variables values of the for each dependent variable and applying a multiple linear regression analysis. Moreover, to identify the relative influence among independent variables on the dependent variables and to determine the values of the independent variables giving the K1 and K2 evolutions, the response surfaces for each variable were plotted three levels of the most strongly independent variable (Figs. 8 and 9).
Figure 8 show that the variable most strongly influencing the 1/K1 evolution is the moisture content, whereas aeration has the lowest effect on that evolution. In that form, higher 1/K1 values when the composting moisture is 70% are found. Furthermore, according to Whang and Meenaghan (1980), the stability of the substrate–microorganism complex is increased under optimum moisture (probably due to optimal balance among aeration–moisture) in that form medium aeration (0.175 Lair min−1 kg−1) could be used to obtain maximum 1/K1 value.
According to that found for 1/K1 and as can be seen from Fig. 9, the K2 values were less influenced by aeration than by the moisture. K2 shows a high influence in aeration under medium moisture value (55%). Maximum K2 value under medium moisture content and high aeration level was found. This value depends on the operational controllable variables of the process such as temperature, moisture, aeration and chemical conditions.
Among the studied variables, a 1/K1–K2 balance shows that under 55–70% moisture, and 0.175 Lair min−1 kg−1 aeration could be a suitable option.
Conclusions
The composting process of Stevia rebaudiana residues is technically reachable and could be considered as a natural way to recycle these wastes. Among the studied composting conditions, [time (0–50 days), aeration (0.05–0.30 Lair min−1 kg−1), and moisture (40–70%)] suitable temperature progression have been found for the reactors with high moisture content.
The kinetic model proposed is useful to describe the Stevia rebaudiana residues composting.
The values of 1/K1 (related to affinity between microorganisms and substrate) and K2 (depends on the optimization parameters of the composting process) obtained using this model showed a optimal balance under 55% of moisture content, and 0.03 Lair min−1 kg−1 of aeration.
References
Ahmad N, Rab A, Ahmad N (2016) Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J Photochem Photobiol B Biol 154:51–56. https://doi.org/10.1016/j.jphotobiol.2015.11.015
Boodt MD, Rab A, Verdonck O (1972) The physical properties of the substrates in horticulture. Acta Hortic 3:37–44. https://doi.org/10.17660/actahortic.1972.26.5
Bueno P, Yáñez R, Ariza J, Díaz M (2008) Influence of environmental parameters on the composting kinetic of lignocellulosic residues. Compost Sci Util 16:132–138. https://doi.org/10.1080/1065657x.2008.10702368
Ekinci K, Keener HM, Akbolat D (2004) Effect of thermocouple location on the optimum composting rate. Biosyst Eng 83(3):345–353. https://doi.org/10.1016/j.biosystemseng.2004.07.004
Faithfull NT (2002) Methods in agricultural chemical analysis: a practical handbook. Cabi Pub, Oxon
Gao M, Li B, Yu A, Liang F, Yang L, Sun Y (2010) The effect of aeration rate on forced-aeration composting of chicken manure and sawdust. Bioresour Technol 101:1899–1903. https://doi.org/10.1016/j.biortech.2009.10.027
Geuns JMC (2010) Stevia and steviol glycosides: properties, techniques, uses, exposure, toxicology, pharmacological effects. Euprint, Heverlee
Haug RT (1993) The practical handbook of compost engineering. Lewis Publishers, Boca Raton
Hong N, Chen J, Zhang XH, Chen TB (2013) Effect of turning on moisture content in sewage sludge composting. Adv Mat Res. 777:457–460. https://doi.org/10.4028/www.scientific.net/amr.777.457
Jones JB (2001) Laboratory guide for conducting soil tests and plant analysis. CRC Press, Boca Raton
Kianirad M, Muazardalan M, Savaghebi G, Farahbakhsh M, Mirdamadi S (2009) Effects of temperature treatment on corn cob composting and reducing of composting time: a comparative study. Waste Manag Res 28:882–887. https://doi.org/10.1177/0734242x09342359
Kim E, Lee DH, Won S, Ahn H (2015) Evaluation of optimum moisture content for composting of beef manure and bedding material mixtures using oxygen uptake measurement. Asian-Australas J Anim Sci 29:753–758. https://doi.org/10.5713/ajas.15.0875
Klute A, Weaver RW, Sparks DL, Dane JH, Topp GC (1986) Methods of soil analysis. Soil Science Society of America, Madison
Lei F, Vandergheynst J (2000) The effect of microbial inoculation and pH on microbial community structure changes during composting. Process Biochem 35:923–929. https://doi.org/10.1016/s0032-9592(99)00155-7
López-Real J (1990) Agro-industrial waste composting and its agricultural significance. Proc Fertil Soc 293:1–26
Magnuson BA, Carakostas MC, Moore NH, Poulos SP, Renwick AG (2016) Biological fate of low-calorie sweeteners. Nutr Rev 74:670–689. https://doi.org/10.1093/nutrit/nuw032
Martínez FX (1992) Propuesta de metodología para la determinación de las propiedades físicas de los sustratos. In: Proceedings I substrate workshop. Spanish Society for Horticultural Science (SECH) Sept 30–Oct 2. Villaviciosa (Asturias-Spain), pp 55–65
Maulini-Duran C, Artola A, Font X, Sánchez A (2013) A systematic study of the gaseous emissions from biosolids composting: raw sludge versus anaerobically digested sludge. Bioresour Technol 147:43–51. https://doi.org/10.1016/j.biortech.2013.07.118
Mohee R, Mudhoo A (2005) Analysis of the physical properties of an in-vessel composting matrix. Powder Technol 155(1):92–99. https://doi.org/10.1016/j.powtec.2005.05.051
Montgomery DC (1991) Design and analysis of experiments. Wiley, New York
Nakasaki K, Yaguchi H, Sasaki Y, Kubota H (1993) Effect of pH control composting of garbage. Waste Manag Res 11(2):117–125. https://doi.org/10.1006/wmre.1993.1013
Nogueira WA, Nogueira FN, Devens DC (1999) Temperature and pH control in composting of coffee and agricultural wastes. Water Sci Technol 40(1):113–119. https://doi.org/10.1016/S0273-1223(99)00371-6
Peláez C, Mejı́a A, Planas A (2004) Development of a solid phase kinetic assay for determination of enzyme activities during composting. Process Biochem 39:971–975. https://doi.org/10.1016/s0032-9592(03)00208-5
Ponsá C, Pagans E, Sánchez A (2009) Composting of dewatered wastewater sludge with various ratios of pruning waste used as a bulking agent and monitored by respirometer. Biosyst Eng 102(4):433–443. https://doi.org/10.1016/j.biosystemseng.2009.01.002
Sánchez A (2007) A kinetic analysis of solid waste composting at optimal conditions. Waste Manag 27:854–855. https://doi.org/10.1016/j.wasman.2006.07.003
Schaub-Szabo S, Leonard J (1999) Characterizing the bulk density of compost. Compost Sci Util 7:15–24. https://doi.org/10.1080/1065657x.1999.10701980
Van Ginkel JT, Raats PAC, Van Haneghem IA (1999) Bulk density and porosity distributions in a compost pile. Neth J Agrix Sci 47:105–121
Wang Y, Aai P (2016) Integrating particle physical geometry into composting degradation kinetics. Bioresour Technol 200:514–520. https://doi.org/10.1016/j.biortech.2015.10.073
Whang DS, Meenaghan GF (1980) Kinetic model of composting process. Compost Sci 21(3):44–46
Acknowledgements
The authors gratefully acknowledge the funding of this work from the Andalusian Regional Ministry of Economy, Innovation, Science, and Employment (Project number RNM 2323) and Ministry of Economy and Competitiveness (Spain National Programme for Research Amend at the Challenges of Society, CTQ2013-46804-C2-1-R and CTQ2017-85251-C2-1-R).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Castro-Fernández, J.J., Serrato-González, A., Ruiz-Montoya, M. et al. Influence of controllable variables on the composting process, kinetic, and maturity of Stevia rebaudiana residues. Int J Recycl Org Waste Agricult 7, 277–286 (2018). https://doi.org/10.1007/s40093-018-0214-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-018-0214-x