Abstract
This study presents the results obtained in compostability tests of organic fraction of municipal solid waste (OFMSW) digestate. The final aim was to obtain mature compost without phytotoxic effects. For the evaluation of the composting process, a novel parameter describing the performance of the composting process, the relative heat generation standardized with the initial volatile solid content (RHGVS0), was defined and evaluated at laboratory-scale. From these laboratory-scale test, the optimum operational conditions were obtained, a mixing ratio (v/v) of 1:1:0 (bulking agent:digestate:co-substrate) and with 15% of mature compost as inoculum. Subsequently, these optimum operational conditions were applied in the active phase of the composting pilot-scale reactor. The active composting stage took 7 days, subsequently a curing phase of 60 days was carried out at ambient conditions. After 30 days of curing, the mature compost showed a specific oxygen uptake rate (SOUR) of 0.14 mg O2/g VS·h, a germination index (GI) of 99.63% and a low volatile fatty acids (VFA) concentration (41.3 AcH mg/kgdm), being indicative of the good compost stability and maturity of the compost. The very good quality of the final compost obtained indicated that the RHGVS0 accurately describes the performance of the composting process.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In Europe, about 502 kg of municipal solid wastes (MSWs) was generated per capita in 2019 [1]. According to the European Environment Agency, the biowaste fraction of the MSWs represents more than 34% of the total amount, accounting the biomass waste production about 86 million tons in 2017 [2]. Therefore, the adequate treatment of these biowastes is an important component of any integrated solid waste management strategy, mainly because it could reduce the toxicity and volume of the MSWs requiring final disposal in a landfill.
Biological treatment of the organic fraction of municipal solid wastes (OFMSW) offers a sustainable waste treatment strategy combining waste stabilization with nutrient or energy recovery where the end-product could be used for soil applications [3, 4]. These biological treatments can be accomplished under anaerobic or aerobic conditions, mainly by anaerobic digestion/fermentation or composting, respectively [5, 6]. The applications of these technologies are widely used in the municipal waste management of European countries due to several reasons [7]. These include: the requirements of the EU Landfill directive (99/31/EC) [8]; Framework directive (2018/851/EC) [9] and the need for alternative and energetically more efficient routes than incineration for the recovery of value from wastes such as municipal waste (2009/28/EC) [10].
Anaerobic digestion can be used for the treatment of OFMSW with the additional benefit of energy recovery, producing a solid product for crops applications [11]. This end-product is called digestate: it is the by-product of anaerobic digestion or bio-hydrogen of organic wastes. Depending on the technology applied, the digestate could be a solid or liquid material. The liquid digestate contains less than 15% Dry Matter (DM) content, while the solid digestate contains a highest DM percentage. The digestate contains a high proportion of mineral nitrogen, especially in the form of ammonium which is available for plants. Moreover, it contains other macro and microelements necessary for plant growth. Therefore, the digestate could be a useful fertilizer for crop plants. In addition, the organic fractions of digestate can contribute to soil organic matter turnover, influencing the biological, chemical and physical soil characteristics [12]. Unfortunately, anaerobic digestate in its basic form may not be a suitable soil conditioning because its phytotoxicity [13], viscosity, odor, difficult handling and soil application techniques require complex and expensive machinery [14]. In the literature, the VFA have been refereed to be responsible for phytotoxicity and plant growth suppression when immature compost is used for growing plants [15]. Because of that, the use of digestate as a fertilizer is governed by regulations and standards that protect animal and human health as well as the quality of crops [14]. Furthermore, anaerobic digestate may pose health risks for animals and humans [16].
Because of these reasons, the anaerobic digestates require treatments to enhance their fertilization potential and applicability as a soil conditioner [17] being one of the best options to improve the digestate quality by means of the composting [18].
Composting treatment of OFMSW digestate presents low operational and investments costs, at the same time that offers material valorization as compost for soil application [16]. The composting process is an aerobic biological process which uses naturally occurring microorganisms to convert biodegradable organic matter into a humus-like product [16, 19]. At the same time, the composting process destroys pathogens, converts unstable ammonia nitrogen into stable organic nitrogen forms, reduces the volume of waste and improves the nature of the waste [20, 21]. Hence, the composting of the anaerobic digestate could be a solution to the problems caused by the direct application of the raw digestate in soils. The effectiveness of the composting process is influenced by factors such as oxygen supply (i.e., aeration), moisture content, pH (related to Volatile Fatty Acids), C:N ratio, temperature, particle size and degree of compaction [22, 23]. The addition of microorganisms, bioaugmentation, can accelerate the rate of the composting process and promote the biodegradation of the organic matter. In the literature, the benefits of bioaugmentation have been described by different researchers [24, 25]. In addition, the addition of bulking agents is another important factor for the optimum operation of the composting process, because it provides the optimum free air space and regulates the water content of the waste to be composted [26, 27]. In this sense, the size of the bulking agent is very important for the optimization of the process. In the literature, Gea et al. (2007) obtained the optimal values of bulking agent particle size: 0–5 mm [28, 29]. After a proper composting of the digestate, the maturity and stability of the final product can be reached [30,31,32,33]. To enhance the composting process performance, the digestate could also be co-composted with fresh and/or partially stabilized organic waste [32]. Unfortunately, co-composting of digestate has not been significantly investigated in the literature and there is much to learn about the process [31, 32]. The potential advantages of co-composting digestate and OFMSW could be the improvement of the physico-chemical characteristics of the final compost obtained [32, 33]. Another interesting variable to investigate is the amount of inoculum that should be added to obtain an optimum composting performance [32]. The inoculation with mature compost is more convenient when compared with direct microbial inoculation because it is more economically convenient and supply an adapted mixed culture avoiding acclimatization stages.
Composting processes can be developed in open or closed systems. In general, closed systems allow to a better control of composting conditions and a reduction of processing times. Anyway, most composting processes involve the development of an active phase inside the reactor, under thermophilic conditions (50–70 °C) [29], and a subsequent curing phase. The composition, stability and maturity of the final product depend largely on how the process is controlled, rather than in the technology used. In addition, the number of phytonutrients available depends on the original content in the starting raw materials, but also on the transformations taking place along the composting process. Usually, the concept of maturity is used as synonymous with stability. But this is not correct because maturity is defined as the degree of absence of phytotoxic compounds, whereas the biological stability can be defined as the state in which the organic matter contained in the substrate is kept under optimal conditions, showing a negligible microbiological activity [34, 35]. In the literature, different parameters have been used to evaluate the heat generation as indirect performance parameter of the composting process. The main parameters are described hereunder. The temperature method (TEM) monitored the temperature along the composting process without taking into account the time scales and the amount of composting mass. A modification of this method is the relative heat generation (RHG). In this method, the temperatures of the compost and the environment are monitored along the experiment [36]. The integral along the experiment of the temperatures difference, measured as °C day, is defined as the RHG. The RHG takes into account the time scale, but unfortunately does not take into account the composting mass. The Heating Value (HV) is defined as the amount of energy released per kg of mass and usually measured as MJ/kg. The HV is usually obtained in calorimeters [36, 37]. Unfortunately, the calorimeter is an expensive and sophisticated setup, and the procedure is time consuming [37]. The main drawbacks of this procedure are that the result does not inform about the time scale and that it is referred to all the composting mass, overlooking the biodegradability of the different fractions contained in the composting mass. Considering all the possible advantages and drawbacks of the previously defined parameters, a novel parameter, related to the heat generated, has been defined in this work. This parameter, named relatives heat generated to initial volatile solids (RHGVS0), deals with the heat generated taking into account not only the heat generated, but also the time scale and the initial biodegradable material. This parameter has been used to study the impact of the mixing ratios as well as the inoculum, in laboratory-scale composting processes.
In this context, the aim of this work was to study, at lab-scale, the optimal mixing ratios and amount of inoculum using the RHGVS0 as performance index, being the main aim to reduce the initial VFA concentration of the digestate to non-toxic levels. Subsequently, a pilot-scale composting process was carried out and the quality of the final compost obtained was evaluated for agricultural purposes according to the Spanish regulations.
Materials and methods
Experimental setup
Laboratory-scale tests
The laboratory-scale composting tests were performed in 1.5 L Dewar® vessels (KGW-Isotherm, Karlsruhe, Germany) conditioned for composting by placing a percolating plate in the bottom. The percolating plate allows to keep the solid material to be composted independent of the possible leachate generated. The Dewar® vessels were equipped with thermocouple sensors to measure the internal and ambient temperatures. In addition, the vessels present sampling gas port to evaluate the oxygen level in the solid mass to be composted. More information related to this setup can be found in the literature [28]. A scheme of the setup is presented in Fig. 1.
The vessel was filled up to 2/3 of its capacity with the mixture to be tested, being the remaining 1/3 of volume the headspace for the gas phase. The oxygen concentration in the gas phase was periodically determined by an oxygen probe (Model XP-204, Eijkelkamp Company, The Netherlands). The oxygen probe was connected to a controller with the aim to ensure oxygen concentrations higher than 10% in the gas phase throughout the composting tests, as recommended in the literature [28]. The temperature system consisted of a data logger (Testo 175-T2, Lenzkirch, Germany) with a dual-channel temperature probe inserted into the compost matrix. The data logger registered the temperature evolution in both channels every 30 min. To ensure the reproducibility, the different experiments were carried out by duplicate. The obtained results were used for the calculation of the RHG measured as °C·d, which was obtained using the Simpson integration method [38]. Then, the RHG was normalized in terms of the percentage of initial biodegradable volatile solids (RHGVS0) by dividing the obtained RHG by the initial volatile solids (VS) of the sample and measured as °C·d/kg VS0. This parameter has been used as performance index of the composting lab-scale tests.
Composting pilot-scale reactor and maturation process
The reactor used for the composting process was designed and built at the University of Cadiz.
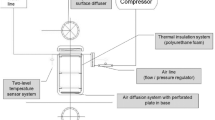
The pilot-scale setup consisted of a static bed reactor with a total volume of 50 L (70% working volume). The walls were thermally isolated with polyurethane foam to avoid heat losses. A percolating plate was fitted over the bottom of the reactor to support the solid material [39]. The reactor had two inlets at the bottom: an orifice to introduce air with an air compressor and another with a valve for leachate removal. Six more inlets were located at the top cover; four to insert temperature probes (Pt-100, SR-NOH-Desin, Spain) which were placed at different heights of the composting matrix and gas phase, and the other two to measure the oxygen concentration in the head space and for pressure relief and gas excess evacuation. Two additional temperature probes (Pt-100, SR-NOH-Desin, Spain) were installed. One of them was located on the wall, to evaluate the temperature gradients of the reactor, and the last one was used to register the ambient temperature. Supervisory control and data acquisition systems software (SCADA) was used to control the temperature and oxygen level during the active composting phase. A scheme of the composting pilot-scale reactor can be seen in Fig. 2.
Scheme of the 50 L composting reactor used: (1) Inlet airflow, (2) Percolated plate and leachates collection, (3) Leachate outlet valve, (4) Composting volume, (5) Reactor with thermal insulation, (6) Temperature probe at different heights, (7) Gas port for oxygen measurement, (8) Sampling point at different heights, and (9) Gas venting
The control strategy on the reactor was based on the air flow-rate introduced due to its direct effect on the oxygen availability for the bio-oxidative processes and, therefore, on the temperature of the composting matrix. An air flow-rate below the composting process requirements would lead to oxygen mass transfer limitations and anaerobic conditions, which could slow down and even stop the composting process. On the contrary, an excessive air flow-rate would cause the cooling of the mass and moisture loss which could lead to a slowdown of the composting process.
Therefore, the air flow-rate control is required to ensure optimum conditions for the composting process. The control system used in this work ranged from 0.3 to 3 L air/min·kg VS0. On the one hand, low flow-rates were used to provide the oxygen required to avoid anaerobic conditions or mass transfer limitations during low oxygen requirement stages in the reactor, i.e., start-up of the composting process and temperature drop period at the end of the active phase. On the other hand, high flow-rates were used to ensure an extra oxygen supply to allow the increase of the temperature and to fulfill the requirements due to an increasing metabolic demand by the microorganisms. These flow-rate levels were controlled by automatized valves activated/deactivated by the control system. In the literature, similar flow-rates have been used. Wiley and Pierce (1955) developed an airflow control system in function of the temperature for an agitated vessel in a very wide range of flow-rate values applied for municipal waste composting (0.34–1.10 L air/min·kg VS0) [40], this range was also ratified by the other authors [41]. Lau et al. (1992) determined, as optimum flow-rate for swine waste, a range between 1.0 and 2.0 L air/min·kg VS0 [42]. Other researcher, Hong et al. (1983), concluded that, for dairy cattle manure with crop and forest residues, the maximum decomposition rate occurred with an aeration flow-rate between 0.87 and 1.87 L air/min kg VS0 [43]. The temperature set point actuating over the air flow-rate level was 55 °C. This value was the average temperature of the composting mass sensors. In addition, this action can be activated by a low oxygen level alarm, below 11% of oxygen in the gas phase. Finally, a curing phase took place in a slotted box with manual mixing twice a week during the maturation stage, 60 days. The temperature profile was also monitored throughout the curing phase.
Substrates and mixtures used
Digestate from a OFMSW bio-hydrogen process for bio-hydrogen obtention was used as the main substrate and raw OFMSW was used as co-substrate. The inoculum was taken from a composted OFMSW. Finally, wood chips were used as bulking agent. The physico-chemical characteristics of these raw materials are shown in Table 1.
The bio-hydrogen digestate was obtained from an acidogenic laboratory-scale reactor fermenting OFMSW to produce bio-hydrogen at a solid retention time (SRT) of 6.6 days. The solid–liquid separation to obtain the digestate was carried out by means of gravity filtration through Teknofanghi® filter bags. The solid fraction, concentrated digestate, showed a moisture content slightly higher than the optimum conditions for the composting process. Therefore, the concentrated digestate was dried at room temperature until the optimum moisture value, between 40 and 50%, was reached. As can be seen on Table 1, the digestate presented a high VFA concentration. The VFA are known to be phytotoxic when they are directly applied on the ground [44].
As co-substrate, raw OFMSW was used. The role of the co-substrate is to enable the proper development of the composting process and allowing the degradation of the substrates contained. Co-substrates are usually required to avoid toxic or inhibitory initial concentrations as well as to compensate the carbon to inorganic elements ratio [32, 33, 45].
The bulking agent used was wood chips from a carpenter’s workshop. One factor to consider is the particle size of the bulking agent because it influences the porosity of the final mixture. In previous compostability studies carried out with the same bulking agent, it was observed that the best results were obtained when operating with a particle size fraction smaller than 5 mm [28]. Hence, this particle size was used in the present study.
As inoculum, OFMSW mature compost was used. The inoculation accelerated the starting phase because it contains adapted microbiota. Concerning the adapted microbiota, it must be highlighted that it is important to use as inoculum mature compost with similar characteristics to the main substrate to avoid adaptation issues [32, 33, 45]. For this reason, OFMSW compost from an industrial facility (“Las Calandrias” located in Jerez de la Frontera, Spain) was used. A screen fraction (< 5 mm mesh) was used to obtain a homogeneous fraction. The stability degree of the mature compost used as inoculum was assessed through a self-heating test following the procedure described in the literature [46].
To evaluate the best combinations of bulking agent, digestate, co-substrate and inoculum, nine different composting lab-scale experiments were carried out. Composting tests without and with inoculum (percentage of 15 or 20%), with different bulking agents, digestate and co-substrate ratios (v/v) of 1:2:0, 2:1:0, 1:1:0, and 2:1:1 were studied. Table 2 presents the mixtures used in the lab-scale composting tests carried out. The different mixture ratios were selected to study its influence on the overall development of the composting process. Once finished the study, the optimum lab-scale operating conditions were selected. Subsequently, these optimum operating conditions were implemented in a pilot-scale composting process.
Analytical methods
Measurements of moisture, organic matter, carbon and total Kjeldahl nitrogen (TKN) for the characterization of the substrates were carried out according to “standard methods” (APHA, 1989) [47]. Individual VFA levels were determined by gas chromatography (SHIMADZU GC-17A, Shimadzu Corporation, Kyoto, Japan) with a flame ionization detector and a capillary column filled with Nukol (polyethylene glycol modified by nitro-terephthalic acid) [48].
Along the maturation stage of the compost, the biogermination and respirometric tests were performed to assess the degree of maturation and stabilization. The biogermination and the respirometric tests were performed according to the methodology previously described in the literature [49,50,51]. The stability degree of the obtained compost was evaluated in the curing phase by determining the specific oxygen uptake rate (SOUR). These respirometric tests were carried out using an OxiTop® (WTW, Weilheim, Germany). The OxiTop® system uses a pressure sensor that allows for the determination of the pressure drop in the gaseous phase. To perform these measurements, representative samples were taken along curing process. The SOUR (mg O2/g VS·h) was determined using the following equation:
where Smax is the maximum oxygen uptake (mg O2/L·h), V is the volume (L), m is the sample mass (g), TS is the decimal fraction of total solids, and VS is the decimal fraction of volatile solids.
In addition, heavy metals concentration was evaluated to check the possible agronomic application of the final compost according to the Spanish legislation (R.D. 506/2013 and R.D. 865/2010) [52, 53]. To do that, the compost was dried at 60 °C and subjected to acid digestion with a microwave digester to determine the different metals’ concentration through inductively coupled plasma mass spectrometry (Model X7 Thermo Elemental; Thermo Electron Co., Beverly, USA).
Results and discussion
The main innovation proposed in this work is the definition of a novel performance index of the composting process. This novel parameter is based on the normalization of the RHG to the percentage of initial volatile solids (VS0) in the sample and named RHGVS0. This parameter relates the composting rate, indirectly measured as heat generation, with VS0 of the composting mass. In this case, the VS0 come from easily biodegradable substrate contained in the bio-hydrogen digestate and the co-substrate. The VS from the chips, used as a bulking agent, were not taken into account since it is considered as a hardly biodegradable material in the active composting period [54].
Bio-hydrogen digestate characterization
In the first stage, the bio-hydrogen digestate was characterized with the aim to evaluate its potential agronomic valorization. The results obtained from the characterization are presented on Table 1. In this table, it can be seen that the direct agronomic use of the bio-hydrogen digestate is not viable due to its high VFA concentration. The VFA concentration of the raw bio-hydrogen digestate, 7200 mg/kgdm expressed as AcH, was much higher than the concentration considered as phytotoxic by some authors [15, 44, 54,55,56] and within the highest range (4000–10,000) as described in the literature [57]. In the literature, it has been described that VFA levels as low as 500 mg/kgdm present phytotoxic effects on plant seedlings [55], a value 14 times lower than that found in the bio-hydrogen digestate used in this work. De Vleeschauwer et al. (1981) observed that acetic acid, at 300 mg/kgdm, inhibited the growth of cress seed (Lepidium sativum L.), a value 24 times lower than that found in the digestate of this study [44]. Similar results have been described in the literature by Keeling et al. (1994) [56].
Laboratory-scale composting tests
Different compostability experiments were performed using different mixing ratios and amount of inoculum. From these experiments, the optimum mixing ratios and amount of inoculum were selected. The parameter RHGVS0 was used as performance index of the tests. To determine the RHGVS0, the Simpson method for numeric integration was applied to integrate the vessel and the ambient temperature profiles during the length of the experiments. The difference between both temperature areas was considered to be the net value of RHG. Then, the RHG was divided by the initial VS concentration to obtain the RHGVS0.
As example, Fig. 3 shows the evolution of both temperatures for the two assays performed with mixing ratio 1:1 (chip:digestate) with inoculum (a) and without inoculum (b). As can be seen in this figure, the higher temperature evolution was obtained by the tests carried out with inoculum, reaching a maximum value of 35 °C, whereas the non-inoculated test reached a maximum temperature of only 29 °C. The temperature peak of the inoculated test was maintained during a longer period of time, leading to a significantly higher heat generation. This heat generation can be explained by the microbial metabolisms taking place in the composting pile [58]. In addition, the steady temperature maintained after the temperature peak was also higher in the inoculated pile, 29 °C, whereas in the non-inoculated this temperature was 26 °C due to the lower metabolic activity observed during the active phase [59]. Hence, the application of a 15% v/v inoculum presented a better performance in the composting process which only could be explained by the faster conversion rate due to the higher inoculum concentration in the composting pile [59].
The laboratory-scale composting study was carried out in 2 stages. In the first stage, the influence of the co-substrate and the inoculum mixing ratios was studied. The experiments were carried out at different inoculation percentages and at mixing ratios (chip:digestate:co-substrate) of 2:1:0 and 2:1:1. The results of the RHGVS0 obtained in these experiments are shown in Fig. 4.
Regarding the contribution of the co-substrate, it can be observed in Fig. 4 that the RHGVS0 obtained when adding co-substrate was lower in all the cases. These results indicate that the addition of the co-substrate, in the range studied in this work, reduced the performance of the composting process. Regarding the inoculum, in principle, a higher inoculum addition should lead to a faster conversion rate due to the higher concentration of active microorganisms. However, in this study, the best results were obtained when operating with a 15% inoculum, presenting a maximum value of 19.09(± 1.48) for RHGVS0 when operating with the mixture 2:1:0 and an inoculum of 15%. On the one hand, the possible explanation for the higher performance index observed when operating without co-substrate could be the reduction of the C/N ratio observed. As can be seen in Table 1, the lower C/N ratio of the co-substrate could lead to C/N ratios far from the optimum values, around 30, reducing the performance of the process [60]. These results are in accordance with results previously described in the literature [54]. On the other hand, the lower performance when increasing the inoculum over 15% could be caused composting by-products inhibition or the reduction of the moisture [61,62,63].
Then, in a second stage, the effect of an increase of digestate proportion was evaluated performing additional tests with mixtures 1:1:0 (bulking agent:digestate:co-substrate) and different inoculum percentages (0, 15 and 20%). In addition, a mixture 1:2:0 with a 15% inoculum was studied for the sake of clearness. As can be seen in Fig. 5, the best results were obtained for the mixture 1:1:0 with 15% of inoculum, obtaining a value of 19.75(± 1.41) for RHGVS0.
The ratio bulking agent:digestate of 1:1 ensured an adequate relative density of the mass to be composted, allowing for an effective oxygen transference. In addition, a percentage of 15% of inoculum provides microbiota and nutrients in the composting mass, sufficient to accelerate and increase the effectiveness of the process [58, 59] without experiencing inhibitory effects described in the literature [61,62,63]. In accordance with the obtained results described above, the mixture 1:1:0 + 15% inoculum was selected as the optimum operating conditions for the development of the composting process at pilot-scale.
Pilot-scale composting tests
To assess the potential agronomic use of the final product generated once finished the composting process of the bio-hydrogen digestate, a pilot-scale experiment with 20 kg of mixture was carried out at the optimum operational conditions previously determined 1:1:0 (+ 15%). The aim of this experiment was to obtain a significant amount of compost to be used in the agronomic tests. To determine the oxygen requirements of the composting process, the VS were determined being its percentage a 55.40%. The different VS fractions were characterized, being easily biodegradable 3.09 kgdm. The C/N ratio of the mixture presented an initial value of 32.7, a value within the optimum range (from 25 to 35) described in the literature for the correct development of a composting process [20, 64]. The whole composting process can be divided into two general stages, the active or thermophilic stage, which occurs within the composting reactor, and the curing or mesophilic stage which takes place outside the reactor.
Active composting stage
The active composting stage was carried out inside the pilot-scale reactor. The overall performance of the fermentation stage was completed in 7 days. During this period, temperature and oxygen levels in the reactor were monitored and controlled, respectively. The data obtained are presented in Fig. 6a and b.
In this work, based on the literature [40, 41], the minimum air flow-rate was defined at 1.0 L/min, which correspond to 0.33 L air/min·kg VS0, and the highest air flow-rate was fixed at 3 L/min (1.0 L air/min·kg VS0). Due to the initial evolution of the experiment (see Fig. 4b), with an oxygen consumption rate higher than expected, the initial air flow-rate level was increased from 0.66 to 0.98 L air/ min·kg VS0 to reduce the temperature of the process which was above 60 °C (Fig. 6a). In the literature, it has been described that temperatures in the range between 50 and 60 °C could inhibit microbial activity or generate endospores from some pathogens [65]. From the third day, when the temperature started to decrease, see Fig. 6a, and the outlet oxygen level increased up to 17%, see Fig. 4b, the air flow-rate was changed again to 0.66 L air/ min·kg VS0 to avoid excessive cooling and moisture loss in the reactor. At the end of the third day, the control system of the reactor changed to the lowest air flow-rate, 0.33 L air/min·kg VS0, this flow-rate was kept until the end of the process because the temperature was always below 55 °C (set point of high temperature). In the literature, it has been described that the active composting process is finished when temperature difference between reactor and environment are under 10 °C [54]. Other researchers indicate that the bio-oxidative stage, active phase, of composting is finished when the temperature of the pile is stable and near to that of the atmosphere [66]. In this work, these conditions were reached after 7 days of composting at the pilot-scale reactor.
Curing phase: evaluation of the maturity and stability of the compost
Once finished the active composting stage, the curing stage was developed. In the literature, it has been stated that the degree of maturity of the compost is related to the properties that would enable their use as an agricultural amendment with adequate stability and maturity. Different parameters have been analyzed to evaluate the maturity, such as GI as well as the presence of N-NH4 and VFA in the compost [49]. Furthermore, respirometric tests measuring the SOUR were used to evaluate the compost stability. In addition, these characterization procedures were complemented with analysis of heavy metal contents, which is one of the main criteria set by the different legislations to establish its quality level and to protect the environment and public health. In this work, stability and maturity parameters were determined on a monthly base. The results obtained are presented in Table 3.
Regarding the compost maturation, the GI evolution over time was used to assess the maturity of the compost due to its relationship with the presence of phytotoxic substances. According to Zucconi et al. (1981) [49] and Emino and Warman (2004) [67], there are different ranges of GI values, depending on the effects of the compost on the plants. A value of the GI lower than 80% indicates that the material presents phytotoxic effects. A GI value between 80 and 100% indicates the disappearance of phytotoxins in composts. Finally, a value above 100% indicates that the substrate presents a phytostimulant effect. As can be seen in Table 3, a GI value of 75.8% was obtained at the beginning of the maturation period. This value indicates the presence of phytotoxic compounds in the compost obtained. However, the levels of ammonia (200 mg/kgdm) and VFA (108.27 mg AcH /kgdm) at the beginning of the curing stage were below the phytotoxic levels described in the literature. In the literature, Zucconi and De Bertoldi (1987) established that an ammonium nitrogen value below 400 mg/kgdm indicates mature compost [68]. Regarding to the acetic acids’ concentration, it has been described that acetic acid concentrations over the range 300–500 mg/kgdm showed phytotoxic effect on the plants, and thus could inhibit the plant growth [56, 57]. In this work, during the previous composting stage, a significant reduction of the VFA concentration was observed in the digestate: decreasing from 3,818.1 mg AcH/kgdm at the beginning of the composting process to 108.3 mg AcH/kgdm the last day of the composting process, accounting a 97% of VFA removal efficiency. Because of the low levels of ammonia and VFA, a possible synergistic effect of both parameters could be the cause the phytotoxic effect experienced as previously described in the literature [69]. The GI value at day 30 of the curing stage was 99.63%, indicating the absence of phytotoxic effect and coinciding with the drastic reduction of the initial levels of ammonia. At that moment, the VFA concentration was 41.3 mg AcH/kgdm, a value below the level considered as phytotoxic (< 400 mg AcH/kgdm according to the literature [68]). Therefore, after 30 days of curing phase, the compost obtained can be considered as mature compost. Subsequently, after 60 days of maturation process, GI showed a value of 112%, indicating a certain phytostimulant character, coinciding with the absence of ammonium and a very low VFA concentration, 9.1 mg AcH/kgdm.
Regarding the compost stability, two requirements were taken into consideration. On the one hand, the maximum respiration activity value according to the Working Document on “Biological treatment of Biowaste” [70], an EU-initiative to improve the present situation for biodegradable waste (biowaste) management and help meeting the targets of the Landfill Directive 1999/31/EC. This document defines a compost as stable when either the Respiration Activity after 4 days (AT4) is below 10 mg O2/gdm or when the oxygen uptake rate is below 1 mg O2/gVS·h, values in accordance with results described in the literature [71,72,73]. On the other hand, the EPA (Environmental Protection Agency) takes into consideration a stricter normative of the Canada Composting Council [74]. According to this normative, EPA proposes that compost is deemed mature if it meets two of the following five requirements: (1) C/N ratio is lower than or equal to 25; (2) oxygen uptake rate is lower than or equal to 150 mg O2/kg VS·h; (3) germination of cress (Lepidium sativum) seeds and radish (Raphanus sativus) seeds in compost must be greater than 90% of the germination rate of the control sample, and the growth rate of plants grown in a mixture of compost and soil must not differ more than 50% in comparison with the control sample; (4) compost must be cured for at least 21 days; (5) compost will not reheat upon standing to greater than 20 °C above ambient temperature.
At the beginning of the maturation process, see Table 3, high oxygen consumption was observed, resulting in a value of 1.09 SOUR (mg O2/gVS·h). This value is slightly higher than the maximum value set by the Working Document of EU, and additionally, it would not meet the criteria required by the EPA and the Canada Composting Council listed above.
When the curing process reached the day 30, a SOUR of 0.14 mg O2/g VS·h was obtained. This value is lower than the value proposed by the EU Working Document, SOUR < 1 mg O2/gVS·h. In addition, three of the five requirements of EPA and the Canada Composting Council were accomplished. The requirements accomplished were the following: SOUR was 0.14 mg O2/g VS·h; GI of 99.63% and the compost was cured for 30 days. Hence, the compost obtained in this work meets targets of both regulations. Other researchers found analogous results with similar substrates; for example, Iannottil, (1993) studied the composting process for MSW and found a high level of stability (0.50 mg O2/gVS·h) on day 31 [75]. Xiao and Zeng, (2009) found that stabilized compost from MSW presented a SOUR value lower than 2 mg O2/gVS·h after 20 days [76].
Compost quality evaluation
The compost quality should be considered from those characteristics that result from a proper composting process, including a rational management of waste and the obtention of a high-quality product to be used in the agriculture [77,78,79]. Possible agronomic uses of compost include its use as fertilizer or as grown media. The European Regulation 2003/2003 regulates the use of the compost as fertilizer [80]. It was transposed into Spanish legislation by the “Royal Decree” RD 506/2013 [52]; this regulation sets the quality class according to the limits established for heavy metals concentration in compost and fertilizer products made from wastes. The Spanish legislation regulating the use of compost as grown media is, in the, the “Royal Decree” RD 865/2010 [53].
The evolution of the chemical characteristics of the compost during the maturation stage is presented on Table 4. As can be seen on this table, after 30 days, the compost presented an organic matter content of 34.65%, moisture of 17.37% and a C/N ratio of 27.9. The value of C/N ratio decreased from 32.7, at the beginning of the active composting stage, to 27.9 at the end of maturation process. In the literature, it has been proposed a maximum value of 0.75 for the quotient between C/N final and C/N initial as a stabilization parameter for the composting of municipal solid waste [68]. In this study, a quotient of 0.85 was obtained, a value slightly higher than the value proposed. This value is strongly affected by the characteristics of the raw substrates. In this work, the substrates were the solid effluents of a bio-hydrogen production process fermenting OFMSW, thus, the initial substrate was partially mineralized and, therefore, would present a different evolution in comparison with more biodegradable or non-partially mineralized substrates. Regarding to the organic matter content (OM), its value was 34.65%, a value significantly higher than the 20% required by the RD 865/2010 [53].
Usually, the major limitation of a mature compost for its agronomic uses is related to the presence of heavy metals. In Spain, the RD 506/2013 [52] defines strict limits, establishing three classes of quality according to the metal content in the final product, and imposes restrictions on the use of the lowest quality compost, named as Class C. On the other hand, the Spanish Royal Decree RD 865/2010 [53], for contained grown media, establishes only two classes of quality, with values identical to those established for classes A and B of the Spanish RD 506/2013 [52]. Table 4 shows the levels of heavy metals in the compost obtained in this work and, considering both Spanish regulations, it can be sorted as class B. In comparison with the levels established for the highest quality of composts, class A, the compost obtained in this work presents a concentration of nickel 3.26 times greater, 1.35 times greater of copper, 3.25 times greater of mercury, 1.33 times greater of zinc and 1.22 times of lead. The presence of these metals could be explained by the improper discard of batteries, paint, inks, or other household hazardous wastes in the MSW that could be avoided by proper sorting of the wastes and educating on waste management. Because of the presence of metals, the resulting compost would present limitations of use as a fertilizer (RD 506/2013), also following the Code of Good Agricultural Practice. However, according to the R.D. 865/2010, a Class B can be used for contained grown media but not for edible horticultural crops. These limitations in the application of the obtained compost could be limited by avoiding the presence of these pollutants on the initial composting mass.
Conclusions
In this work, a novel heat generation parameter for composting performance evaluation was defined, the RHGVS0. This parameter was used for evaluating the performance of lab-scale composting processes with successful results. Experimental results obtained in these lab-scale compostability assays showed that the optimum mixing ratio bulking agent:digestate:co-substrate (v/v) was 1:1:0, plus 15% compost as inoculum. The optimum conditions obtained at lab-scale, were applied in an active composting process developed in a pilot-scale reactor and monitored during 7 days. Once finished the active phase, a VFA removal of 97% was obtained. Finally, a curing phase was developed for 60 days. After 30 days of the curing process, the obtained compost showed an adequate degree of stabilization and maturation, presenting 99.6% of GI, absence of ammonium and low levels of VFA (total VFA: 41.3 mg AcH/kgdm). The respirometric index of the compost was 0.14 mg O2/gVS·h, a value below the reference levels of the EU (1 mg O2/gVS·h) and the Canadian Composting Council. The obtained compost, within 30 days of maturation, meets all the requirements to be used as a contained grown media. However, it cannot be used for edible horticultural crops because of their high heavy metals’ content.
Abbreviations
- DM:
-
Dry matter
- GI:
-
Germination index
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- MSWs:
-
Municipal solid wastes
- OFMSW:
-
Organic fraction of municipal solid waste
- OM:
-
Organic matter
- RHG:
-
Relative heat generation
- RHGVS0 :
-
Relative heat generation standardized with the initial volatile solid content
- SOUR:
-
Specific oxygen uptake rate
- TEM:
-
Temperature method
- TKN:
-
Total Kjeldahl nitrogen
- VS:
-
Volatile solid
- VFA:
-
Volatile fatty acids
References
EUROSTAT Statistical Office of the European Union (2021) Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics#Municipal_waste_generation (Accessed on 24th April 2021).
European Environment Agency (2020) Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe. (Accessed on 6th December 2020).
Hartmann H, Ahring BK (2006) Strategies for the anaerobic digestion of the organic fraction of municipal solid waste; an overview. Water Sci Technol 53:7–22
Oviedo-Ocaña ER, Dominguez I, Komilis D, Sanchez A (2019) Co-composting of green waste mixed with unprocessed and processed food waste: influence on the composting process and product quality. Waste Biomass Valor 10:63–74
Fernández FJ, Sánchez-Arias V, Villaseñor J, Rodríguez L (2008) Evaluation of carbon degradation during co-composting of exhausted grape marc with different biowastes. Chemosphere 73(5):670–677
Infantes D, González del Campo A, Villaseñor J, Fernández FJ (2012) Kinetic model and study of the influence of pH, temperature and undissociated acids on acidogenic fermentation. Biochem Eng J 66:66–72
Zhang Y, Banks CJ, Heaven S (2012) Anaerobic digestion of two biodegradable municipal waste streams. J Environ Manage 104:166–174
EC (1999) Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. Off. J. Eur. Commun.
EC (2018) Directive 2018/851/EC of the European parliament and of the council of 30 May 2013 on waste and repealing Directive 2008/98. Off. J. Eur. Union.
EC (2009) Directive 2009/28/EC of the European parliament and of the council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing directives 2001/77/EC and 2003/30/EC. Off J Eur Union 5:2009
Molner L, Bartha I (1988) High solids anaerobic fermentation for biogas and compost production. Biomass 16:173–182
Makádi M, Tomócsik A, Lengyel J, Márton Á (2008) Problems and successes of digestate utilization on crops. Proceedings of the international conference ORBIT, Wageningen. CD-ROM (ISBN 3–935974–19–1).
Poggi-Varaldo HM, Trejo-Espino J, Fernandez-Villagomez G, Esparza-Garcia F, Caffarel-Mendez S, Rinderknecht-Seijas N (1999) Quality of anaerobic compost from paper mill and municipal solid wastes for soil amendment. Water Sci Technol 40:179–186
Tchobanoglous G, Kreith F (2002) Handbook of Solid Waste Management. 2nd edition. New York.
McDougall F, White P, Frank M, Handle P (2001) Integrated Solid Waste Management; A Life Cycle Inventory, 2nd edn. Blackwell Publishing, Cornwall
Sims JT, Wolf DC (1994) Poultry waste management: agricultural and environmental issues. Adv Agron 52:1–83
Abdullahi YA, Akunna JC, White NA, Hallet PD, Wheatley R (2008) Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresour Technol 99:8631–8636
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278
Georgacakis D, Tsavdaris A, Bakouli J, Symeonidis S (1996) Composting solid swine manure and lignite mixtures with selected plant residues. Bioresour Technol 56:195–200
Haug RT (1993) The Practical Handbook of Composting Engineering. Lewis Publishers Boca Raton, FL
Sequi P (1996) The role of composting in sustainable agriculture. In: de Bertoldi M, Sequi P, Lemmes B, Papi T (eds) The science of composting, part 1. Blackie, Glasgow, pp 23–29
Lau AK, Liao PH, Lo KV (1993) Evaluation of swine waste composting in vertical reactors. J Environ Sci Health 4:761–777
Stentiford EI (1996) Composting control: principles and practice. In: de Bertoldi M, Sequi P, Lemmes B, Papi T (eds) The science of composting, part 1. Blackie, Glasgow, pp 29–59
Tiquia SM, Tam NF, Hodgkiss IJ (1997) Effects of bacterial inoculum and moisture adjustment on composting of pig manure. Environ Pollut 96:161–171
Wakase S, Sasaki H, Itoh K, Otawa K, Kitazume O, Nonaka J (2008) Investigation of the microbial community in a microbiological additive used in a manure composting process. Bioresour Technol 99:2687–2693
Miner FD, Koeing R, Miller BE (2001) The influence of bulking material type and volume on in-house composting in high-rise, caged layer, facilities. Compost Sci Util 9:50–59
Eftoda G, McCartney D (2004) Determining the critical bulking requirement for municipal solid biosolids composting. Compost Sci Util 12:208–218
Gea T, Barrena R, Artola A, Sánchez A (2007) Optimal bulking agent particle size and usage for heat retention and disinfection in domestic wastewater sludge composting. Waste Manag 27(9):1108–1116
Petiot C, de Guardia A (2004) Composting in a laboratory reactor: a review. Compost Sci Util 12:69–79
Askri A, Laville P, Trémier A, Houot S (2016) Influence of origin and post-treatment on greenhouse gas emissions after anaerobic digestate application to soil. Waste Biomass Valorization 7:293–306
Zeng Y, De Guardia A, Dabert P (2016) Improving composting as a post-treatment of anaerobic digestate. Biores Technol 201:293–303
Arab G, McCartney D (2017) Benefits to decomposition rates when using digestate as compost co-feedstock: part I - focus on physicochemical parameters. Waste Manage 68:74–84
Partanen P, Hultman J, Paulin L, Auvinen P, Romantschuk M (2010) Bacterial diversity at different stages of the composting process. BMC Microbiol. https://doi.org/10.1186/1471-2180-10-94
Adani F (2000) Biostabilization of mechanically separated municipal solid waste fraction. Waste Manag res 18:471–477
Bargougui L, Guergueb Z, Chaieb M, Mekki A (2020) Co-composting of olive industry wastes with poultry manure and evaluation of the obtained compost maturity. Waste Biomass Valor 11:6235–6247
Demirbas A (2008) Relationships proximate analysis results and higher heating values of lignites. Energy Sources, Part A 30(20):1876–1883
Fan H, Liao J, Abass OK, Liu L, Huang X, Li J, Tian S, Liu X, Xu K, Liu C (2021) Concomitant management of solid and liquid swine manure via controlled co-composting: towards nutrients enrichment and wastewater recycling. Resour Conserv Recycl 168:10530
Velleman DJ (2005) The generalized Simpson’s rule. Am Math Mon 112(4):342–350. https://doi.org/10.2307/30037470
Sánchez Arias V, Fernández FJ, Rodríguez L, Villaseñor J (2012) Respiration indices and stability measurements of compost through electrolytic respirometry. J Environ Manage 95:S134–S138
Wiley JS, Pearce GW (1955) A preliminary study of high rate composting. Proc Am Soc Civil Engineering 846:1–28
Schulze KL (1962) Continuous thermophilic composting. Appl Microbiol 10(2):108–122
Lau AK, Lo KV, Liao PH, Yu JC (1992) Aeration experiments for swine waste composting. Bioresour Technol 41:145–152
Hong JH, Matsuda J, Ikeuchi Y (1983) High rapid composting of dairy cattle manure with crop and forest residues. Trans ASAE 26:533–545
De Vleeschauwer DO, Verdonock P, Van Assche P (1981) Phytotoxicity of refuse compost Biocycl 22:44–46
De Baere L (2008) Partial stream digestion of residual municipal solid waste. Water Sci Technol 57:1073–1077
Brinton WF (2001) An international look at compost standards: methods used for evaluating compost quality in Europe are summarized in a new report. Biocycl 42:74–76
APHA (1998) Standard methods for the examination of water and wastewater. 20th Edition, American Public Health Association, American Water Works Association and Water Environmental Federation, Washington DC.
Fernández-Morales FJ, Villaseñor J, Infantes D (2010) Modeling and monitoring of the acclimatization of conventional activated sludge to a biohydrogen producing culture by biokinetic control. Int J Hyd Energy 35(20):10927–10933
Zucconi F, Pera A, Forte M, De Bertoldi M (1981) Evaluating toxicity in immature compost. Biocycl 22:54–57
Katia E, Lasaridi E, Stentiford E (1998) A simple respirometric technique for assessing compost stability. Water Res 32(12):3717–3723
Flores-Solórzano SB, Huerta-Lwanga E, Cuevas-González R, Guillen-Navarro K (2022) Optimal conditions to produce extracts of compost and vermicompost from oil palm and coffee pulp wastes. J Mater Cycles Waste Manag 24:801–810
BOE (2013) Real decreto 506/2013, de 28 de junio, sobre productos fertilizantes. Boletín Oficial del Estado 164:51119–51207
BOE (2010) Real decreto 865/2010, de 2 de julio, sobre sustratos de cultivo. Boletín Oficial del Estado 170:61831–61859
Manios VI, Tsikalas PE, Siminis HI (1989) Phytotoxicity of olive tree compost in relation to organic acid concentration. Biol Wastes 27:307–317
Lynch JM (1977) Phytotoxicity of acetic acid produced in the anaerobic decomposition of wheat straw. J Appl Bacteriol 42:81–87
Keeling AA, Paton IK, Mullet JA (1994) Germination and growth of plants in media containing unstable refuse-derived compost. Soil Biol Biochem 26:767–772
Rynk R (1993) On-farm composting: guidelines for use of dairy and poultry manures in composting formulations. Woods End Research Laboratory, Maine.
Golueke CG, Card BJ, McGauhey PH (1954) A critical evaluation of inoculums in composting. Appl Microbiol 2:45–53
Barrena R, Pagans E, Faltys G, Sánchez A (2006) Effect of inoculation dosing on the composting of source-selected organic fraction of municipal solid wastes. J Chem Technol Biotechnol 81:420–425
Gea MT, Artola A, Sánchez A (2003) Application of experimental design technique to the optimization of bench-scale composting conditions of municipal raw sludge. Compost Sci Util 11:321–329
Greff B, Szigeti J, Varga Á, Lakatos E, Sáhó A, Varga L (2021) Effect of bacterial inoculation on co-composting of lavender (Lavandula angustifolia Mill) waste and cattle manure. Biotech 11:306
Jurado MM, Suárez-Estrella F, López MJ, Vargas-García MC, López-González JA, Moreno J (2015) Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour Technol 186:15–24
Alkoaik F, Ghaly AE (2005) Effect of inoculum size on the composting of greenhouse tomato plant trimmings. Compost Sci Util 13:262–273
Kumar M, Ou YL, Lin JG (2010) Co-composting of green waste and food waste at low C/N ratio. Waste Manage 30:602–609
Miyatake F, andIwabuchi. K., (2005) Effect of high compost temperature on enzymatic activity and species diversity of culturable bacteria in cattle manure compost. Bioresour Technol 96:1821–1825
Bernal MP, Paredes C, Sánchez-Monedero MA, Cegarra J (1998) Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresour Technol 63:91–99
Emino ER, Warman PR (2004) Biological assay for compost quality. Compost Sci Util 12:342–348
Zucconi F, De Bertoldo M (1987) Specifications for solid waste compost Biocycl 28:56–61
Iglesias E, Barral MT, Marhuenda FC (Ed) (2008) Indicadores de La estabilidad y madurez del compost. Moreno Casco, Joaquín. Compostaje (1ª ed). Mundi-prensa. 244–284.
EC (2001) Working Document on “Biological treatment of Biowaste”. 2nd Draft.
Binner E, Zahc A (1997) Laboratory test methods characterizing the biological reactivity of wastes. Ibidem. 485–494.
Adani F, Lozzi P, Genevini P (2001) Determination of biological stability by oxygen uptake on municipal solid waste and derived products. Compost Sci Util 9:163–178
Scaglia et al (2000) Respiration Index determination: dynamic and static approaches, Compost Sci. Util 2:90–98
CCME (2008) A summary of compost standards in Canada. Retrieved on 31st May, 2008.
Iannottil DA, Pang T, Toth BL, Elwell DL, Keener HM, Hoitink HAJ (1993) A quantitative respirometric method for monitoring compost stability. Compost Sci Util 1:52–65
Xiao Y, Zeng GM (2009) Continuous thermophilic composting (CTC) for rapid biodegradation and maturation of organic municipal solid waste. Bioresour Technol 100:4807–4813 (APHA, AWWA, WPCF)
Hase T, Kawamura K (2012) Evaluating compost maturity with a newly proposed index based on a germination test using Komatsuna (Brassica rapa var. peruviridis) seeds. J Mater Cycles Waste Manag 14:220–227
Fukushima M, Tu X, Aneksampant A, Tanaka A (2018) Analysis of branched-chain fatty acids in humic substances as indices for compost maturity by pyrolysis–gas chromatography/mass spectrometry with tetramethylammonium hydroxide (TMAH-py–GC/MS). J Mater Cycles Waste Manag 20(1):176–184
Pivato A, Raga R, Vanin S, Rossi M (2014) Assessment of compost quality for its environmentally safe use by means of an ecotoxicological test on a soil organism. J Mater Cycles Waste Manag 16(4):763–774
EU (2003) Regulation (EC) Nº 2003/2003 of the European parliament and of the council of 13 October 2003 relating to fertilizers.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was co-funded by Spanish Ministry of Science and Innovation (Project CTM2017-62164/TECNO).
Author information
Authors and Affiliations
Contributions
Conceptualization, JLG-M and MP; methodology, JLG-M; investigation, FN; data curation, FN, LFL-F and FJF-M; writing—original draft preparation, FN; writing—review and editing, FJF-M and LFL-F; supervision, JLG-M and MP; project administration, JLG-M and MP; funding acquisition, JLGM and MP. All the authors have read and agreed to publish the present version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Núñez, F., Pérez, M., Leon-Fernández, L.F. et al. Effect of the mixing ratio on the composting of OFMSW digestate: assessment of compost quality. J Mater Cycles Waste Manag 24, 1818–1831 (2022). https://doi.org/10.1007/s10163-022-01438-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01438-1