Abstract
Background
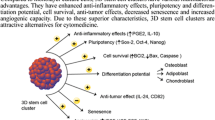
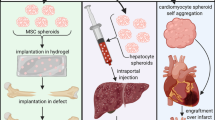
Mesenchymal stem cells (MSCs) are undifferentiated cells that can differentiate into specific cell lineages when exposed to the right conditions. The ability of MSCs to differentiate into particular cells is considered very important in biological research and clinical applications. MSC spheroids are clusters of MSCs cultured in three dimensions, which play an important role in enhancing the proliferation and differentiation of MSCs. MSCs can also participate in vascular formation by differentiating into endothelial cells and secreting paracrine factors. Vascularization ability is essential in impaired tissue repair and function recovery. Therefore, the vascularization ability of MSCs, which enhances angiogenesis and accelerates tissue healing has made MSCs a promising tool for tissue regeneration. However, MSC spheroids are a relatively new research field, and more research is needed to understand their full potential.
Methods
In this review, we highlight the importance of MSC spheroids’ vascularization ability in tissue engineering and regenerative medicine while providing the current status of studies on the MSC spheroids’ vascularization and suggesting potential future research directions for MSC spheroids.
Results
Studies both in vivo and in vitro have demonstrated MSC spheroids’ capacity to develop into endothelial cells and stimulate vasculogenesis.
Conclusion
MSC spheroids show potential to enhance vascularization ability in tissue regeneration. Yet, further research is required to comprehensively understand the relationship between MSC spheroids and vascularization mechanisms.






Similar content being viewed by others
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Henderson AR, Choi H, Lee E. Blood and lymphatic vasculatures on-chip platforms and their applications for organ-specific in vitro modeling. Micromachines. 2020;11:147.
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95.
Wang Y, Kankala RK, Ou C, Chen A, Yang Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact Mater. 2022;9:198–220.
Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone–starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–15.
Ruehle M, Eastburn E, LaBelle S, Krishnan L, Weiss J, Boerckel J, et al. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci Adv. 2020;6:eabb6351.
Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, et al. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359.
Jia P, Zhao X, Liu Y, Liu M, Zhang Q, Chen S, et al. The RGD-modified self-assembling D-form peptide hydrogel enhances the therapeutic effects of mesenchymal stem cells (MSC) for hindlimb ischemia by promoting angiogenesis. Chem Eng J. 2022;450:138004.
Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41–51.
Griffin KH, Fok SW, Kent LJ. Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling. NPJ Regen Med. 2022;7:70.
Daly AC, Davidson MD, Burdick JA. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nature Commun. 2021;12:753.
Ayan B, Heo DN, Zhang Z, Dey M, Povilianskas A, Drapaca C, et al. Aspiration-assisted bioprinting for precise positioning of biologics. Sci Adv. 2020;6:5111.
Ho SS, Hung BP, Heyrani N, Lee MA, Leach JK. Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects. Stem Cells. 2018;36:1393–403.
Ho SS, Murphy KC, Binder BY, Vissers CB, Leach JK. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med Stem Cells Transl Med. 2016;5:773–81.
Fang Y, Ji M, Wu B, Xu X, Wang G, Zhang Y, et al. Engineering highly vascularized bone tissues by 3D bioprinting of granular prevascularized spheroids. ACS Appl Mater Interfaces. 2023;15:43492–502.
Baer PC, Griesche N, Luttmann W, Schubert R, Luttmann A, Geiger H. Human adipose-derived mesenchymal stem cells in vitro: evaluation of an optimal expansion medium preserving stemness. Cytotherapy. 2010;12:96–106.
Lin RZ, Chou LF, Chien CCM, Chang HY. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and β1-integrin. Cell Tissue Res. 2006;324:411–22.
Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, De Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–15.
Tseng TC, Wong CW, Hsieh FY, Hsu SH. Biomaterial substrate-mediated multicellular spheroid formation and their applications in tissue engineering. Biotechnol J. 2017;12:1700064.
Yeh HY, Liu BH, Sieber M, Hsu SH. Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genom. 2014;15:10.
Ezquerra S, Zuleta A, Arancibia R, Estay J, Aulestia F, Carrion F. Functional properties of human-derived mesenchymal stem cell spheroids: a meta-analysis and systematic review. Stem Cells Int. 2021;2021:8825332.
Kaminska A, Wedzinska A, Kot M, Sarnowska A. Effect of long-term 3D spheroid culture on WJ-MSC. Cells. 2021;10:719.
Lee SY, Lee JW. 3D Spheroid cultures of stem cells and exosome applications for cartilage repair. Life. 2022;12:939.
Cheng NC, Chen SY, Li JR, Young TH. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med. 2013;2:584–94.
Ratushnyy A, Ezdakova M, Buravkova L. Secretome of senescent adipose-derived mesenchymal stem cells negatively regulates angiogenesis. Int J Mol Sci. 2020;21:1802.
Redondo-Castro E, Cunningham CJ, Miller J, Brown H, Allan SM, Pinteaux E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res Ther. 2018;9:11.
Hsu TW, Lu YJ, Lin YJ, Huang YT, Hsieh LH, Wu BH, et al. Transplantation of 3D MSC/HUVEC spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials. 2021;272:120765.
Gangadaran P, Oh EJ, Rajendran RL, Oh JM, Kim HM, Kwak S, et al. Three-dimensional culture conditioned bone marrow MSC secretome accelerates wound healing in a burn injury mouse model. Biochem Biophys Res Commun. 2023;673:87–95.
Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32.
Gao C, Zhang Y, Xie J, Wang X, Cao L, Chen G, et al. VE-cadherin functionalized injectable PAMAM/HA hydrogel promotes endothelial differentiation of hMSCs and vascularization. Appl Mater Today. 2020;20:100690.
Wang H, Li X, Lai S, Cao Q, Liu Y, Li J, et al. Construction of vascularized tissue engineered bone with nHA-coated BCP bioceramics loaded with peripheral blood-derived MSC and EPC to repair large segmental femoral bone defect. ACS Appl Mater Interfaces. 2023;15:249–64.
Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–13.
Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation. 2014;21:345–57.
Thomas HM, Cowin AJ, Mills SJ. The importance of pericytes in healing: wounds and other pathologies. Int J Mol Sci. 2017;18:1129.
Shah S, Kang KT. Two-cell spheroid angiogenesis assay system using both endothelial colony forming cells and mesenchymal stem cells. Biomol Ther. 2018;26:474–80.
Shologu N, Scully M, Laffey JG, O’Toole D. Human mesenchymal stem cell secretome from bone marrow or adipose-derived tissue sources for treatment of hypoxia-induced pulmonary epithelial injury. Int J Mol Sci. 2018;19:2996.
Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–47.
Rahimnejad M, Nasrollahi Boroujeni N, Jahangiri S, Rabiee N, Rabiee M, Makvandi P, et al. Prevascularized micro-/nano-sized spheroid/bead aggregates for vascular tissue engineering. Nanomicro Lett. 2021;13:182.
Piard C, Jeyaram A, Liu Y, Caccamese J, Jay SM, Chen Y, et al. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials. 2019;222:119423.
Summer S, Rossmanith E, Pasztorek M, Fiedler C, Gröger M, Rauscher S, et al. Mesenchymal stem cells support human vascular endothelial cells to form vascular sprouts in human platelet lysate-based matrices. PLoS One. 2022;17: e0278895.
Guo X, Zheng H, Guo Y, Heng BC, Yang Y, Yao W, et al. A three-dimensional actively spreading bone repair material based on cell spheroids can facilitate the preservation of tooth extraction sockets. Front Bioeng Biotechnol. 2023;11:1161192.
Yang C, Han B, Cao C, Yang D, Qu X, Wang X. An injectable double-network hydrogel for the co-culture of vascular endothelial cells and bone marrow mesenchymal stem cells for simultaneously enhancing vascularization and osteogenesis. J Mater Chem B. 2018;6:7811–21.
Thai VL, Candelas DO, Leach JK. Tuning the microenvironment to create functionally distinct mesenchymal stromal cell spheroids. Ann Biomed Eng. 2023;51:1558–73.
Lee J, Lee S, Kim SM, Shin H. Size-controlled human adipose-derived stem cell spheroids hybridized with single-segmented nanofibers and their effect on viability and stem cell differentiation. Biomater Res. 2021;25:14.
Li N, Dai X, Yang F, Sun Y, Wu X, Zhou Q, et al. Spontaneous spheroids from alveolar bone-derived mesenchymal stromal cells maintain pluripotency of stem cells by regulating hypoxia-inducible factors. Biol Res. 2023;56:17.
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8:45200–12.
Kim JY, Rhim W-K, Cha SG, Woo J, Lee JY, Park CG, et al. Bolstering the secretion and bioactivities of umbilical cord MSC-derived extracellular vesicles with 3D culture and priming in chemically defined media. Nano Converg. 2022;9:57.
Rovere M, Reverberi D, Arnaldi P, Palamà MEF, Gentili C. Spheroid size influences cellular senescence and angiogenic potential of mesenchymal stromal cell-derived soluble factors and extracellular vesicles. Front Bioeng Biotechnol. 2023;11:1297644.
Chiang CE, Fang YQ, Ho CT, Assunção M, Lin SJ, Wang YC, et al. Bioactive decellularized extracellular matrix derived from 3D stem cell spheroids under macromolecular crowding serves as a scaffold for tissue engineering. Adv Healthc Mater. 2021;10: e2100024.
Zeng J, Chen X, Zhang J, Qin Y, Zhang K, Li X, et al. Stem cell spheroids production for wound healing with a reversible porous hydrogel. Mater Today Adv. 2022;15:100269.
Gu Y, Pigeot S, Ahrens L, Tribukait-Riemenschneider F, Sarem M, Wolf F, et al. Toward 3D bioprinting of osseous tissue of predefined shape using single-matrix cell-bioink constructs. Adv Healthc Mater. 2023;12:e2202550.
Lee J, Huh SJ, Seok JM, Lee S, Byun H, Jang GN, et al. Surface engineering of 3D-printed scaffolds with minerals and a pro-angiogenic factor for vascularized bone regeneration. Acta Biomater. 2022;140:730–44.
Zhao N, Coyne J, Abune L, Shi P, Lian XL, Zhang G, et al. Exogenous signaling molecules released from aptamer-functionalized hydrogels promote the survival of mesenchymal stem cell spheroids. ACS Appl Mater Interfaces. 2020;12:24599–610.
Song YC, Park GT, Moon HJ, Choi EB, Lim MJ, Yoon JW, et al. Hybrid spheroids containing mesenchymal stem cells promote therapeutic angiogenesis by increasing engraftment of co-transplanted endothelial colony-forming cells in vivo. Stem Cell Res Ther. 2023;14:193.
Bayaraa O, Dashnyam K, Singh RK, Mandakhbayar N, Lee JH, Park JT, et al. Nanoceria-GO-intercalated multicellular spheroids revascularize and salvage critical ischemic limbs through anti-apoptotic and pro-angiogenic functions. Biomaterials. 2023;292: 121914.
Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol. 2018;9:6.
Amaral RL, Miranda M, Marcato PD, Swiech K. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol. 2017;8:605.
Ganguli A, Mostafa A, Saavedra C, Kim Y, Le P, Faramarzi V, et al. Three-dimensional microscale hanging drop arrays with geometric control for drug screening and live tissue imaging. Sci Adv. 2021;7:eabc1323.
Yang M, He S, Su Z, Yang Z, Liang X, Wu Y. Thermosensitive injectable chitosan/collagen/β-glycerophosphate composite hydrogels for enhancing wound healing by encapsulating mesenchymal stem cell spheroids. ACS Omega. 2020;5:21015–23.
Lewis NS, Lewis EE, Mullin M, Wheadon H, Dalby MJ, Berry CC. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J Tissue Eng. 2017;8:2041731417704428.
Carlsson J, Yuhas JM. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1–23.
Laschke M, Schank T, Scheuer C, Kleer S, Schuler S, Metzger W, et al. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater. 2013;9:6876–84.
Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng C Methods. 2010;16:735–49.
Ingram M, Techy G, Saroufeem R, Yazan O, Narayan K, Goodwin T, et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell Dev Biol Anim. 1997;33:459–66.
Manley P, Lelkes PI. A novel real-time system to monitor cell aggregation and trajectories in rotating wall vessel bioreactors. J Biotechnol. 2006;125:416–24.
Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor beta and serum factors. Proc Natl Acad Sci U S A. 1988;85:9552–6.
Bellotti C, Duchi S, Bevilacqua A, Lucarelli E, Piccinini F. Long term morphological characterization of mesenchymal stromal cells 3D spheroids built with a rapid method based on entry-level equipment. Cytotechnology. 2016;68:2479–90.
Bara JJ, McCarthy HE, Humphrey E, Johnson WE, Roberts S. Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: implications for bone and cartilage tissue engineering. Tissue Eng Part A. 2014;20:147–59.
Wilson WC Jr, Boland T. Cell and organ printing 1: protein and cell printers. The Anat Rec A Discov Mol Cell Evol Biol. 2003;272:491–6.
Ahn CB, Lee JH, Kim JH, Kim TH, Jun HS, Son KH, et al. Development of a 3D subcutaneous construct containing insulin-producing beta cells using bioprinting. Biodes Manuf. 2022;5:265–76.
Heo DN, Hospodiuk M, Ozbolat IT. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348–56.
Murphy KC, Hoch AI, Harvestine JN, Zhou D, Leach JK. Mesenchymal stem cell spheroids retain osteogenic phenotype through α 2 β 1 signaling. Stem Cells Transl Med. 2016;5:1229–37.
Kuss MA, Wu S, Wang Y, Untrauer JB, Li W, Lim JY, et al. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomed Mater Res B Appl Biomater. 2018;106:1788–98.
Lee J, Lee S, Ahmad T, Perikamana SKM, Lee J, Kim EM, et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:120192.
Karanfil AS, Louis F, Matsusaki M. Biofabrication of vascularized adipose tissues and their biomedical applications. Mater Horiz. 2023;10:1539–58.
Zhang K, Song L, Wang J, Yan S, Li G, Cui L, et al. Strategy for constructing vascularized adipose units in poly (l-glutamic acid) hydrogel porous scaffold through inducing in-situ formation of ASCs spheroids. Acta Biomater. 2017;51:246–57.
Yu SJ, Choi G, Cho Y, Lee M, Cho Y, Shin JH, et al. Three-dimensional spheroid culture on polymer-coated surface potentiate stem cell functions via enhanced cell–extracellular matrix interactions. ACS Biomater Sci Eng. 2020;6:2240–50.
Yang F, Cohen RN, Brey EM. Optimization of co-culture conditions for a human vascularized adipose tissue model. Bioengineering. 2020;7:114.
Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic differentiation in healthy and pathological conditions. Int J Mol Sci. 2016;18:41.
Marshall J, Barnes A, Genever P. Analysis of the intrinsic self-organising properties of mesenchymal stromal cells in three-dimensional co-culture models with endothelial cells. Bioeng. 2018;5:92.
Kronemberger GS, Dalmônico GM, Rossi AL, Leite PEC, Saraiva AM, Beatrici A, et al. Scaffold-and serum-free hypertrophic cartilage tissue engineering as an alternative approach for bone repair. Artif Organs. 2020;44:E288–99.
Côrtes I, Matsui RA, Azevedo MS, Beatrici A, Souza KL, Launay G, et al. A scaffold-and serum-free method to mimic human stable cartilage validated by secretome. Tissue Eng A. 2021;27:311–27.
Laschke MW, Schank TE, Scheuer C, Kleer S, Shadmanov T, Eglin D, et al. In vitro osteogenic differentiation of adipose-derived mesenchymal stem cell spheroids impairs their in vivo vascularization capacity inside implanted porous polyurethane scaffolds. Acta Biomater. 2014;10:4226–35.
Liu X, Zhao N, Liang H, Tan B, Huang F, Hu H, et al. Bone tissue engineering scaffolds with HUVECs/hBMSCs cocultured on 3D-printed composite bioactive ceramic scaffolds promoted osteogenesis/angiogenesis. J Orthop Transl. 2022;37:152–62.
Liu X, Li L, Gaihre B, Park S, Li Y, Terzic A, et al. Scaffold-free spheroids with two-dimensional heteronano-layers (2DHNL) enabling stem cell and osteogenic factor codelivery for bone repair. ACS Nano. 2022;16:2741–55.
Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–63.
Kim TG, Park SH, Chung HJ, Yang DY, Park TG. Hierarchically assembled mesenchymal stem cell spheroids using biomimicking nanofilaments and microstructured scaffolds for vascularized adipose tissue engineering. Adv Funct Mater. 2010;20:2303–9.
Yao R, Zhang R, Lin F, Luan J. Biomimetic injectable HUVEC-adipocytes/collagen/alginate microsphere co-cultures for adipose tissue engineering. Biotechnol Bioeng. 2013;110:1430–43.
Ni R, Luo C, Ci H, Sun D, An R, Wang Z, et al. Construction of vascularized tissue-engineered breast with dual angiogenic and adipogenic micro-tissues. Mater Today Bio. 2023;18:100539.
Findlay D. Vascular pathology and osteoarthritis. Rheumatology. 2007;46:1763–8.
Favreau H, Pijnenburg L, Seitlinger J, Fioretti F, Keller L, Scipioni D, et al. Osteochondral repair combining therapeutics implant with mesenchymal stem cells spheroids. Nanomedicine. 2020;29:102253.
Mackie E, Ahmed Y, Tatarczuch L, Chen K-S, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell biol. 2008;40:46–62.
Freeman FE, Allen AB, Stevens HY, Guldberg RE, McNamara LM. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res Ther. 2015;6:218.
Jang HH, Son Y, Park G, Park KS. Bone marrow-derived vasculogenic mesenchymal stem cells enhance in vitro angiogenic sprouting of human umbilical vein endothelial cells. Int J Mol Sci. 2022;24:413.
Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One. 2011;6:e28352.
Costa MH, Serra J, McDevitt TC, Cabral JM, da Silva CL, Ferreira FC. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol J. 2021;16:2000389.
Fuentes P, Torres MJ, Arancibia R, Aulestia F, Vergara M, Carrión F, et al. Dynamic culture of mesenchymal stromal/stem cell spheroids and secretion of paracrine factors. Front Bioeng Biotechnol. 2022;10:916229.
Santos RdA, Asensi KD, de Barros JHO, de Menezes RCS, Cordeiro IR, Neto JMdB, et al. Intrinsic angiogenic potential and migration capacity of human mesenchymal stromal cells derived from menstrual blood and bone marrow. Int J Mol Sci. 2020;21:9563.
Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med. 2017;113:311–22.
Sano K, Usui M, Moritani Y, Nakazawa K, Hanatani T, Kondo H, et al. Co-cultured spheroids of human periodontal ligament mesenchymal stem cells and vascular endothelial cells enhance periodontal tissue regeneration. Regen Ther. 2020;14:59–71.
Acknowledgements
This work was supported by the Ministry of Science and ICT of Korea (NRF-2021R1C1C2004576, RS-2023-00222737, NRF-2022R1I1A1A01072365 and NRF- 2022R1C1C1008610). This research was also supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Health & Welfare). (Code: KFRM 22A0105L1-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, Y., Na, J., Karima, G. et al. Mesenchymal Stem Cell Spheroids: A Promising Tool for Vascularized Tissue Regeneration. Tissue Eng Regen Med (2024). https://doi.org/10.1007/s13770-024-00636-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13770-024-00636-2