Summary
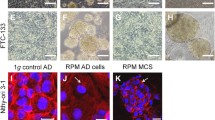
Growth patterns of a number of human tumor cell lines that form three-dimensional structures of various architectures when cultured without carrier beads in a NASA rotary cell culture system are described and illustrated. The culture system, which was designed to mimic microgravity, maintained cells in suspension under very low-shear stress throughout culture. Spheroid (particulate) production occurred within a few hours after culture was started, and spheroids increased in size by cell division and fusion of small spheroids, usually stabilizing at a spheroid diameter of about 0.5 mm. Architecture of spheroids varied with cell type. Cellular interactions that occurred in spheroids resulted in conformation and shape changes of cells, and some cell lines produced complex, epithelial-like architectures. Expression of the cell adhesion molecules, CD44 and E cadherin, was upregulated in the three-dimensional constructs. Coculture of fibroblast spheroids with PC3 prostate cancer cells induced tenascin expression by the fibroblasts underlying the adherent prostate epithelial cells. Invasion of the fibroblast spheroids by the malignant epithelium was also demonstrated.
Similar content being viewed by others
References
Acker, H. Microenvironmental conditions in multicellular spheroids grown over liquid-overlay tissue culture conditions. In: Recent results in cancer research. Vol. 95. Berlin, Heidelberg: Springer-Verlag; 1984:116–133.
Bauer, K. D.; Keng, P.; Sutherland, R. M. Isolation of quiescent cells from multicellular tumor spheroids using centrifugal elutriation. Cancer Res. 42:3956–3965; 1980.
Becker, J. L.; Prewett, T. L.; Spaulding, G. F., et al. Three-dimensional growth and differentiation of ovarian tumor cell line in high aspect rotating wall vessel: morphologic and embryologic considerations. J. Cell. Biochem. 51:283–290; 1993.
Carlsson, J. A proliferation gradient in three-dimensional colonies of cultured human glioma cells. Int. J. Cancer 20:129–136; 1977.
Dall, P.; Heider, K.-H.; Sinn, H.-P., et al. Comparison of immunohistochemistry and RT/PCR for detection of CD44v-expression, a new prognostic factor in human breast cancer. Int. J. Cancer 60:471–477; 1995.
Erickson, H. P.; Bourdon, M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Ann. Rev. Cell Biol. 5:71–92; 1989.
Fox, S. B.; Gotter, K. C.; Jackson, D. G., et al. CD44 and cancer screening. Lancet 342:548–549; 1993.
Freyer, J. P.; Sutherland, R. M. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 40:3956–3965; 1980.
Goodwin, T. J.; Jessup, J. M.; Wolf, D. A. Morphologic differentiation of colon carcinoma cell lines HT-29 and HT-29KM in rotating wall vessels. In Vitro Cell. Dev. Biol. 28A:47–60; 1992.
Goodwin, T. J.; Schroeder, W. F.; Wolf, D. A., et al. Rotating-wall vessel coculture of small intestine as a prelude to tissue modeling: aspects of simulated microgravity. Proc. Soc. Exp. Biol. Med. 202:181–192; 1993.
Ingram, M.; Buckwalter, J. G.; Jacques, D. B., et al. Immunotherapy for recurrent malignant glioma: an interim report on survival. Neurol. Res. 12:265–273; 1990.
Kaighn, M. E.; Narayan, K. S.; Ohnuki, Y., et al. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 17:16–23; 1979.
Kato, K.; Hibino, S.; Yagita, H., et al. Tenascin suppresses T-cell activation mediated by CD3 and costimulation molecules. Abs. #5085, 9th International Congress of Immunology, 23–29 July 1995, San Francisco, CA.
Laerum, O. D.; Bjerkrig, R. Monolayer and three-dimensional culture of rat and human central nervous system: normal and malignant cells and their interactions. Methods in neurosciences. Vol. 2. London: Academic Press; 1990:210–236.
Matsumura, T.; Tarin, D. Significance of CD44 gene products for cancer diagnosis and disease evaluations. Lancet 340:1053–1058; 1992.
Mueller-Lieser, W. Multicellular spheroids. J. Cancer Res. Clin. Oncol. 13:101–122; 1987.
Overduin, M.; Harvey, T. S.; Bagby, S., et al. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science 267:386–389; 1995.
Prewett, T. L.; Goodwin, T. J.; Spaulding, G. F. Three-dimensional modeling of T-24 human bladder carcinoma cell line: a new simulated microgravity culture vessel. J. Tissue Culture Methods 15:29–36; 1993.
Rutgers, D. H.; Dorien, P. P. N.; van der Linden, P. M. Cell kinetics of hypoxic cells in a murine tumor in vivo: flow cytometric determination of the radiation-induced blockage of cell cycle progression. Cell Tissue Kinet. 20:37–42; 1987.
Schwarz, R. P.; Goodwin, T. J.; Wolf, D. A. Cell culture for three-dimensional modeling in rotating wall-vessels: an application of simulated microgravity. J. Tissue Culture Methods 14:51–58; 1992.
Stamenkovic, I.; Aruffo, A.; Amiot, M., et al. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO 10:343–348; 1991.
Sutherland, R. M. Cell and microenvironment in tumor microregions. Science 240:177–184; 1988.
Sutherland, R. M.; McCredie, I. A.; Inch, W. R. Growth of multicellular spheroids in tissue culture as a model of nodular carcinoma. J. Natl. Cancer Inst. 46:113; 1971.
Sutherland, R. M.; Sordat, B.; Bamat, J., et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 46:5320–5329; 1986.
Yuhas, J. M.; Li, A. P.; Martinez, A. G., et al. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 37:3639–3643; 1977.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ingram, M., Techy, G.B., Saroufeem, R. et al. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell.Dev.Biol.-Animal 33, 459–466 (1997). https://doi.org/10.1007/s11626-997-0064-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-997-0064-8