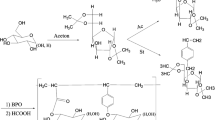
Abstract
Ten new carbohydrate-based aromatic polyurethanes have been synthesized using isomannide and/or a new 1,2,3-triazole isomannide derivative, IV, as the diol source in the polymerization reactions. As the isocyanate source, 1,4-phenylene diisocyanate or 2,4-toluene diisocyanate was used. To synthesize compound IV, isomannide was tosylated, the tosyl groups were substituted with azide, the triazole ring was formed by reaction with 2,4-pentane dione and finally carbonyl groups were converted into oximes. The synthesis of copolymers and terpolymers was performed using different proportions of diisocyanate and diol sources in DMF. The structural elucidation of the synthesized compounds and polymers was performed using spectroscopic techniques such as FTIR, 1D- and 2D-NMR and HRMS. The thermal behaviour and molecular weight distribution of polymers were analyzed by thermal gravimetric analysis and gel permeation chromatography, respectively. The surface properties of the polymers were analyzed using scanning electron microscopy. A comparative study on thermal behaviour of the synthesized polymers showed that polymers containing a higher amount of compound IV displayed better thermal stability. The average molecular weights of copolymers were observed to vary from 7,900 (PUR-3) to 21,000 (PUR-4) whereas for terpolymers, values of 19,000 (PUR-5), 5,100 (PUR-6), 22,000 (PUR-8) and 28,000 (PUR-9) were found. The diisocyanate source was found to have more effect on the surface properties of polymers than the diol source: when 2,4-toluene diisocyanate was used in polymer synthesis, the resultant polymers had a spongy morphology with cavities while a polymer matrix containing spheres and platelets was obtained when 1,4-toluene diisocyanate was used.
Graphical abstract













Similar content being viewed by others
References
Paraskevopoulou P, Smirnova I, Athamneh T, Papastergiou M, Chriti D, Mali G, Čendak T, Chatzichristidi M, Raptopoulos G, Gurikov P (2020) Mechanically strong polyurea/polyurethane-cross-linked alginate aerogels. ACS Appl Polym Mater 2:1974–1988
Momtaz M, Barikani M, Razavi-Nouri M (2015) Effect of ionic group content on thermal and structural properties of polycaprolactone-based shape memory polyurethane ionomers. Iran Polym J 24:505–513
Mustafov SD, Sen F, Seydibeyoglu MO (2020) Preparation and characterization of diatomite and hydroxyapatite reinforced porous polyurethane foam biocomposites. Sci Rep 10:1–9
Gunatillake PA, Dandeniyage LS, Adhikari R, Bown M, Shanks R, Adhikari B (2019) Advancements in the development of biostable polyurethanes. Polym Rev 59:391–417
Shokrolahi F, Yeganeh H (2014) Soft segment composition and its influence on phase-separated morphology of PCL/PEG-based poly (urethane urea)s. Iran Polym J 23:505–512
Skleničková K, Abbrent S, Halecký M, Kočí V, Beneš H (2020) Biodegradability and ecotoxicity of polyurethane foams: a review. Crit Rev Env Sci Tec 15:1–46
Namviriyachote N, Muangman P, Chinaroonchai K, Chuntrasakul C, Ritthidej GC (2020) Polyurethane-biomacromolecule combined foam dressing containing asiaticoside: fabrication, characterization and clinical efficacy for traumatic dermal wound treatment. Int J Biol Macromol 143:510–520
Segan S, Jakobi M, Khokhani P, Klimosch S, Billing F, Schneider M, Martin D, Metzger U, Biesemeier A, Xiong X, Mukherjee A, Steuer H, Keller BM, Joos T, Schmolz M, Rothbauer U, Hartmann H, Burkhardt C, Lorenz G, Schneiderhan-Marra N, Shipp C (2020) Systematic investigation of polyurethane biomaterial surface roughness on human immune responses in vitro. Biomed Res Int 11:2020
Jiang L, Ren Z, Zhao W, Liu W, Liu H, Zhu C (2018) Synthesis and structure/properties characterizations of four polyurethane model hard segments. R Soc Open Sci 5:180536
Cooper SL, Guan J (2016) Advances in polyurethane biomaterials. Elsevier Science
Akindoyo JO, Beg M, Ghazali S, Islam M, Jeyaratnam N, Yuvaraj A (2016) Polyurethane types, synthesis and applications: a review. Rsc Adv 6:114453–114482
Borcan F, Len A, Dehelean CA, Dudás Z, Ghiulai R, Iftode A, Racoviceanu R, Soica CM (2021) Design and assessment of a polyurethane carrier used for the transmembrane transfer of acyclovir. Nanomaterials 11:51
Behl A, Parmar VS, Malhotra S, Chhillar AK (2020) Biodegradable diblock copolymeric PEG-PCL nanoparticles: synthesis, characterization and applications as anticancer drug delivery agents. Polymer 207:122901
Nsengiyumva O, Miller SA (2019) Synthesis, characterization, and water-degradation of biorenewable polyesters derived from natural camphoric acid. Green Chem 21:973–978
Amjed N, Bhatti IA, Zia KM, Iqbal J, Jamil Y (2020) Synthesis and characterization of stable and biological active chitin-based polyurethane elastomers. Int J Biol Macromol 154:1149–1157
Sharif A, Hoque ME (2019) Renewable resource-based polymers. Bio Polym Nanocompos 2019(1):28
Lavilla C, Alla A, Martínez De Ilarduya A, Benito E, García-MartíN MdG, Galbis J, MuñOz-Guerra S (2011) Carbohydrate-based polyesters made from bicyclic acetalized galactaric acid. Biomacromol 12:2642–2652
Lavilla C, Alla A, De Ilarduya AM, Benito E, García-Martín MdG, Galbis J, Muñoz-Guerra S (2012) Carbohydrate-based copolyesters made from bicyclic acetalized galactaric acid. J Polym Sci A 50:1591–1604
Lavilla C, Alla A, De Ilarduya AM, Benito E, García-Martín M, Galbis J, Muñoz-Guerra S (2012) Bio-based poly (butylene terephthalate) copolyesters containing bicyclic diacetalized galactitol and galactaric acid: Influence of composition on properties. Polymer 53:3432–3445
Ay K, Cetin F, Yuceer L (2007) The Wittig-cyclization procedure: acid promoted intramolecular formation of 3-C-branched-chain 3,6-anhydro furano sugars via 2’-oxopropylene derivatives. Carbohydr Res 342:1091–1095
Kök G, Karayıldırım T, Ay K, Ay E (2010) The Knoevenagel-Doebner reaction on 1,2-O-(2, 2, 2-trichloroethylidene) derivatives of d-gluco-and d-manno-furanose. Molecules 15:7724–7731
Yetgin CE, Oskay M, Ay K (2014) Synthesis and characterization of chloralose-derived thiosemicarbazones and semicarbazones and investigation of their antimicrobial properties. J Carbohydr Chem 33:238–251
Singh H, Sindhu J, Khurana JM (2013) Synthesis of biologically as well as industrially important 1,4,5-trisubstituted-1,2,3-triazoles using a highly efficient, green and recyclable DBU-H2O catalytic system. Rsc Adv 3:22360–22366
Huo J, Hu H, Zhang M, Hu X, Chen M, Chen D, Liu J, Xiao G, Wang Y, Wen Z (2017) A mini review of the synthesis of poly-1, 2, 3-triazole-based functional materials. Rsc Adv 7:2281–2287
Agalave SG, Maujan SR, Pore VS (2011) Click chemistry: 1,2,3-triazoles as pharmacophores. Chem Asian J 6:2696–2718
Halay E, Ay E, Şalva E, Ay K, Karayıldırım T (2018) Synthesis of triazolylmethyl-linked nucleoside analogs via combination of azidofuranoses with propargylated nucleobases and study on their cytotoxicity. Chem Heterocycl Compd 54:158–166
Suyama K, Tachi H (2017) Near ultraviolet-sensitive polyurethanes networked with photolabile carbamoyloxime linker units. J Photopolym Sci Technol 30:247–252
Liu WX, Zhang C, Zhang H, Zhao N, Yu ZX, Xu J (2017) Oxime-based and catalyst-free dynamic covalent polyurethanes. J Am Chem Soc 139:8678–8684
Wu ZY (2020) Synthesis and properties of moisture-cured reactive polyurethane containing castor oil and oxime compounds. Polymers 12:1838
Buruiana EC, Olaru M, Simionescu BC (2004) Photobase-generating new polyurethanes with oxime-urethane groups in the main chain. J Appl Polym Sci 94:2324–2332
Chen LY, Guillarme S, Saluzzo C (2013) Dianhydrohexitols: new tools for organocatalysis. Application in enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes. ARKIVOC 3:227–244
Barros TG, Pinheiro S, Williamson J, Tanuri A, Gomes M Jr, Pereira HS, Brindeiro R, Neto JB, Antunes O, Muri EM (2010) Pseudo-peptides derived from isomannide: inhibitors of serine proteases. Amino Acids 38:701–709
Kim HJ, Kang MS, Knowles JC, Gong MS (2014) Synthesis of highly elastic biocompatible polyurethanes based on bio-based isosorbide and poly (tetramethylene glycol) and their properties. J Biomater Appl 29:454–464
Medimagh R, Smaidia MM, Bennour H, Chatti S (2015) Synthesis and evaluation of the thermal properties of biosourced poly (ether) ureas and copoly (ether) ureas from 1,4:3,6-dianhydrohexitols. Polym Int 64:513–520
Kumar AS, Ghule VD, Subrahmanyam S, Sahoo AK (2013) Synthesis of thermally stable energetic 1,2,3-triazole derivatives. Chem Eur J 19:509–518
Shou Q, Yuan R, Ma G, Liang X, Wan J, Xian M, Wang Q (2019) Chiral nanostructures of isosorbide- and isomannide-based polyurethanes. Polymer 164:118–125
Acknowledgements
The authors are also grateful to Prof. Dr. Stephen T. ASTLEY for English language editing of manuscript. This project was financially supported by the Manisa Celal Bayar University Research Projects Coordination Office Unit. Project Number: 2016-060.
Funding
The funding has been received from Celal Bayar Üniversitesi with Grant no. Research Projects Coordination Office Unit. Project Number: 2016-060.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayrak, F., Ay, E., Oral, A. et al. Synthesis of 1,2,3-triazole group-containing isomannide-based aromatic new polyurethanes. Iran Polym J 31, 413–423 (2022). https://doi.org/10.1007/s13726-021-01001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-021-01001-z