Abstract
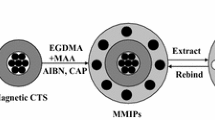
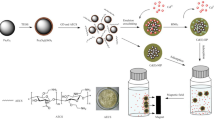
A tricyclazole selective chitosan/Fe3O4 magnetic molecularly imprinted polymer (MMIP) was synthesized using non-covalent binding polymerization involving methacrylic acid (MAA) as functional monomer, divinylbenzene (DVB-80) as crosslinker, 2,2'-azobisisobutyronitrile as initiator, acetonitrile/toluene (75:25, v/v) as porogenic solvent and tricyclazole as template. Surface morphology and magnetic characterization of the prepared imprinted and non-imprinted polymers were done using scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectrometry and vibrating sample magnetometry, respectively. The adsorption kinetic data fitted best in pseudo-second-order model. The adsorption equilibrium was achieved in 30 min and the maximum binding capacity was 4579.9 µg/g. The Freundlich isotherm model was found suitable for explaining the binding isotherm data (R2 > 0.99). Negative values of thermodynamic parameters ∆G (Gibb’s free energy), ∆H (enthalpy), and ∆S (entropy) revealed exothermic and spontaneous nature of adsorption processes. It also revealed decreased randomness at the solid–liquid interface during sorption. The scatchard plot analysis suggested heterogeneity of binding sites on MMIPs. The molecular recognition selectivity of MMIPs towards tricyclazole was much higher, as compared to its structural analogues, tebuconazole (α = 28.58) and hexaconazole (α = 37.16). The MMIPs were successfully applied to separate and enrich tricyclazole from fortified samples of rice and water, with a recovery percentage of 89.4% and 90.9%, respectively. These reusable imprinted polymers possessing high selectivity and specificity can be utilized as an adsorbent for solid-phase extraction in sample preparation for tricyclazole residue analysis in complex environmental matrices.





Similar content being viewed by others
References
Peterson LG (1990) Tricyclazole for control of Pyriculariaoryzae on rice: the relationship of the mode of action and disease occurrence and development. In: Pest management in rice, Springer, Dordrecht
Nader WF, Grote AK, Montilla EC (2014) Rice processing: the comprehensive guide to global technology and innovative products. In: Sontag J (ed) ErlingVerlag, Germany
Suneja K (2020) India adds checks to rice exports to European Union. News article published in The Economic Times
Pareja L, Colazzo M, Pérez-Parada A, Besil N, Heinzen H, Böcking B, Fernández-Alba HA (2012) Occurrence and distribution study of residues from pesticides applied under controlled conditions in the field during rice processing. J Agric Food Chem 60:4440–4448
García-Jaramillo M, Cox L, Cornejo J, Hermosín MC (2014) Effect of soil organic amendments on the behavior of bentazone and tricyclazole. Sci Total Environ 466:906–913
Kumar N, Mukherjee I, Sarkar B, Paul RK (2017) Degradation of tricyclazole: effect of moisture soil type elevated carbon dioxide and Blue Green Algae (BGA). J Hazard Mater 321:517–527
Padovani L, Capri E, Padovani C, Puglisi E, Trevisan M (2006) Monitoring tricyclazole residues in rice paddy watersheds. Chemosphere 62:303–314
Speltini A, Scalabrini A, Maraschi F, Sturini M, Profumo A (2017) Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: a review. Anal ChimActa 974:1–26
Zhao F, She Y, Zhang C, Cao X, Wang S, Zheng L, Jin M, Shao H, Jin F, Wang J (2017) Selective solid-phase extraction based on molecularly imprinted technology for the simultaneous determination of 20 triazole pesticides in cucumber samples using high-performance liquid chromatography–tandem mass spectrometry. J Chromat B 1064:143–150
Ahmadi R, Noroozian E, Jassbi AR (2020) Molecularly imprinted polymer solid-phase extraction for the analysis of 1 8-cineole in thyme and sagebrush distillates. J Iran Chem Soc 17:1153–1161
Murakami T, Iwamuro Y, Ishimaru R, Chinaka S, Hasegawa H (2018) Molecularly imprinted polymer solid-phase extraction of synthetic cathinones from urine and whole blood samples. J Sep Sci 41:4506–4514
Dong H, Zhang D, Lin H, Wang Y, Liu L, Zheng M, So J (2017) A surface molecularly imprinted polymer as chiral stationary phase for chiral separation of 1,1′-binaphthalene-2-naphthol racemates. Chirality 29:340–347
Rutkowska M, Płotka-Wasylka J, Morrison C, Wieczorek PP, Namieśnik J, Marć M (2018) Application of molecularly imprinted polymers in analytical chiral separations and analysis. Trends Anal Chem 102:91–102
Yang YJ, Liu XW, Kong XJ, Qin Z, Jiao ZH, Li SH, Li JY (2018) Preparation and evaluation of oseltamivir molecularly imprinted polymer silica gel as liquid chromatography stationary phase. Molecules 23:1881
Zhang K, Zhou T, Kettisen K, Ye L, Bülow L (2019) Chromatographic separation of hemoglobin variants using robust molecularly imprinted polymers. Talanta 199:27–31
Bougrini M, Florea A, Cristea C, Sandulescu R, Vocanson F, Errachid A, Jaffrezic-Renault N (2016) Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control 59:424–429
Liu W, Li H, Yu S, Zhang J, Zheng W, Niu L, Li G (2018) Poly (3,6-diamino-9-ethylcarbazole) based molecularly imprinted polymer sensor for ultra-sensitive and selective detection of 17-β-estradiol in biological fluids. Biosens Bioelectron 104:79–86
Kurczewska J, Cegłowski M, Pecyna P, Ratajczak M, Gajęcka M, Schroeder G (2017) Molecularly imprinted polymer as drug delivery carrier in alginate dressing. Mater Lett 201:46–49
Mokhtari P, Ghaedi M (2019) Water compatible molecularly imprinted polymer for controlled release of riboflavin as drug delivery system. Eur Polym J 118:614–618
Zheng XF, Lian Q, Yang H, Wang X (2016) Surface molecularly imprinted polymer of chitosan grafted poly (methyl methacrylate) for 5-fluorouracil and controlled release. Sci Rep 6:21409
Li GY, Jiang YR, Huang KL, Ding P, Chen J (2008) Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. J Alloys Compd 466:451–456
Zhang YL, Zhang J, Dai CM, Zhou XF, Liu SG (2013) Sorption of carbamazepine from water by magnetic molecularly imprinted polymers based on chitosan-Fe3O4. Carbohydr Polym 97:809–816
Popplewell J, Sakhnini L (1995) The dependence of the physical and magnetic properties of magnetic fluids on particle size. J Magn Magn Mater 149:72–78
Kodama RH, Berkowitz AE, McNiffJr EJ, Foner S (1996) Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett 77:394–397
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Yu Q, Deng S, Yu G (2008) Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res 42:3089–3097
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Macías-García A, García-Sanz-Calcedo J, Carrasco-Amador JP, Segura-Cruz R (2019) Adsorption of paracetamol in hospital wastewater through activated carbon filters. Sustainability 11:2672–2682
Saha P, Chowdhury S (2011) In: Mizutani M (ed) Thermodynamics. In Thech, Croatia
Lu W, Liu J, Li J, Wang X, Lv M, Cui R, Chen L (2019) Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst 144:1292–1302
Tan CJ, Wangrangsimakul S, Bai R, Tong YW (2008) Defining the interactions between proteins and surfactants for nanoparticle surface imprinting through miniemulsion polymerization. Chem Mater 20:118–127
Lu S, Cheng G, Pang X (2006) Protein-imprinted soft-wet gel composite microspheres with magnetic susceptibility II characteristics. J Appl Polym Sci 99:2401–2407
Chau HTC, Kadokami K, Ifuku T, Yoshida Y (2017) Development of a comprehensive screening method for more than 300 organic chemicals in water samples using a combination of solid-phase extraction and liquid chromatography-time-of-flight–mass spectrometry. Environ Sci Pollut Res 24:26396–26409
Kadivar M, Aliakbar A (2019) A new composite based on graphene oxide-poly 3-aminophenol for solid-phase microextraction of four triazole fungicides in water and fruit juices prior to high-performance liquid chromatography analysis. Food Chem 299:125127
Xu XY, Ye JQ, Nie J, Li ZG, Lee MR (2015) A new liquid–liquid microextraction method by ultrasound assisted salting-out for determination of triazole pesticides in water samples coupled by gas chromatography–mass spectrometry. Anal Methods 7:1194–1199
Tsochatzis ED, Menkissoglu-Spiroudi U, Karpouzas DG, Tzimou-Tsitouridou R (2010) A multi-residue method for pesticide residue analysis in rice grains using matrix solid-phase dispersion extraction and high-performance liquid chromatography-diode array detection. Anal Bioanal Chem 397:2181–2190
Jianmei W, Jie X, Xiaofeng J, Huizhen W, Hua Y, Xiaoming Z, Mingrong Q (2019) Determination of veterinary drug/pesticide residues in livestock and poultry excrement using selective accelerated solvent extraction and magnetic material purification combined with ultra-high-performance liquid chromatography–tandem mass spectrometry. J Chromato A 1617:460808
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laskar, N., Ghoshal, D. & Gupta, S. Chitosan-based magnetic molecularly imprinted polymer: synthesis and application in selective recognition of tricyclazole from rice and water samples. Iran Polym J 30, 121–134 (2021). https://doi.org/10.1007/s13726-020-00878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00878-6