Abstract
With the rising occurrence of sudden heat waves, honey bees are at risk of exposure to unprecedented heat stress. We investigated the synergistic effects of imidacloprid (IMD) and high temperature on honey bees. Mini hives were treated with IMD (20 ppb for 14 days) and high temperature (41 °C for 6 h) either singly or in combination. Heat shock protein 70 and 90 genes were upregulated in bees exposed to the combined treatment compared to those exposed to each single treatment. Transcriptome analysis revealed that metabolic pathways remained intact in the high temperature treatment, whereas several metabolic pathways were altered by either the IMD treatment (downregulation of cellular respiration pathways) or the combined treatment (upregulation of protein synthesis and signaling pathways). These findings suggest that IMD and high temperature have negative synergistic effects on honey bees.
Similar content being viewed by others
1 Introduction
Honey bees, as individuals, are highly tolerant to heat stress. During flight, the temperature of the honey bee thorax rises up to 49 °C (Stabentheiner et al. 2002), and honey bees can survive for 24 h at 45 °C, far beyond the typical temperature level that other species can endure (Abou-Shaara et al. 2012; Ranga et al. 2017). Although the preferred temperature for individual honey bees is 28 °C (Schmolz et al. 2002; McAfee et al. 2020), the honey bee colony thermoregulates efficiently via active heat production, evaporation, or ventilation and maintains the in-hive temperature at 32–36 °C for proper development of the brood (Jones and Oldroyd 2006). Failure of thermoregulation during the brood and pupal stages of honey bee colonies is known to negatively affect susceptibility to pesticides (Medrzycki et al. 2010), behavioral performance (Tautz et al. 2003), synaptic organization of the brain (Groh et al. 2004), and memory of adult bees (Jones et al. 2005).
A heat wave can be defined as a period of more than five consecutive days with daily maximum temperatures being 5 °C higher than average temperatures (Frich et al. 2002). As a result of climate change, heat waves occur more frequently in general and are predicted to occur up to four times more frequently by the late twenty-first century (Lhotka et al. 2018; Marx et al. 2021). Heat waves are known to increase the mortality of humans and birds (McKechnie and Wolf 2010; Mitchell et al. 2016). It was previously reported that honey bees, through recruitment of backup foraging forces, can adapt to a simulated heat wave of 37 °C without cost (Bordier et al. 2017a, b). However, this does not necessarily mean that honey bees maintain normal physiologies under high temperatures. At the individual level, heat stress damages the fertility of queen bees and the digestive tracts of worker bees (McAfee et al. 2020; Bach et al. 2021). Additionally, frequent heat waves in conjunction with other stress factors might result in severe disorders in honey bees.
Imidacloprid (IMD) is a representative neonicotinoid insecticide that has been reported to be potentially responsible for colony collapse disorder (CCD) (Lu et al. 2014). It is known to negatively affect honey bee foraging behavior, development (Tome et al. 2020), motility (Delkash-Roudsari et al. 2020), and learning and memory (Decourtye et al. 2004). In addition, IMD exposure alters the metabolic rate of honey bees (Nicodemo et al. 2014; Cook 2019; Gooley and Gooley 2020) and affects thermoregulation (Meikle et al. 2018).
Since honey bees are usually exposed to multiple stressors concurrently in the natural agroecosystem (Barron 2015; Hristov et al. 2020), it is crucial to understand the interactions between different stressors and their combined effects. It has been reported that the combinations of two different stress factors can induce synergistic negative effects on honey bees: Nosema and virus (Zheng et al. 2015), Nosema and pesticide (Retschnig et al. 2014), two pesticides (Wang et al. 2020), and pesticide and nutritional stress (Tosi et al. 2017). IMD is known to disrupt respiratory metabolism and thermoregulation of honey bees (Tosi et al. 2016; Cook 2019; Gooley and Gooley 2020; Vergara-Amado et al. 2020). Thus, if honey bees are exposed to IMD and heat stress simultaneously, the resulting negative impacts would not be additive but rather synergistic. With a wide use for seed coating, IMD residue has been detected in nectar and pollen with average concentration of 0.9–41.9 ng/g (Stoner and Eitzer 2012; Jiang et al. 2018; Tong et al. 2018; Tosi et al. 2018; Wen et al. 2021). Considering the increased occurrence of heat waves in recent years, such a double exposure scenario is highly likely. If an IMD-exposed honey bee colony is under a sudden heat wave, the resulting failure of in-hive temperature stabilization due to deteriorated thermoregulation would affect honey bee physiology, thereby inducing a negative feedback loop between metabolic changes and in-hive temperature control.
In this study, the synergistic effects of IMD exposure and heat shock on honey bees were investigated at the colony level. To determine a colony-level response to the stressors, whole mini hives containing a queen and brood were used for the experiment. Following treatment with IMD and high temperature, singly or in combination, fluctuations in relative humidity (RH) and temperature inside hives were measured to determine their individual or combined adverse effects on thermoregulation. Transcription profiles of heat shock protein 70 and 90 genes (Hsp70 and Hsp90) and the vitellogenin gene (Vg), which are considered general stress markers for honey bees (Even et al. 2012; Kim et al. 2019), were analyzed by real-time quantitative PCR (qPCR), and any changes in metabolic pathways were investigated by whole transcriptome analysis.
2 Material and methods
2.1 IMD and high-temperature treatment of honey bees
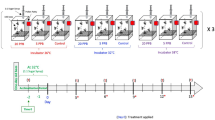
Information on reagents used in this study is listed Supplementary Table 1. Analytical standard grade IMD was purchased from Merck (Darmstadt, Germany), diluted with acetone (≥ 99.5% purity, Merck) to 20 mg/kg and stored at −20 °C until use. The storage period did not exceed 2 months. Italian hybrid honey bee colonies whose queens were all siblings were purchased from a neighboring apiary. They were kept on a rooftop apiary at the Gwanak campus of Seoul National University (Seoul, Korea, 37° 27′ 46.8″ N, 126° 57′ 06.9″ E). The experiments were performed in July to August, 2020, the summer season in Korea. Three independent experiments were performed, each of which had a single colony per treatment. For each experiment, honey bee samples were collected from four sets of treatment groups: untreated control (CC), high temperature-treated (CH), IMD-treated (IC), and both IMD and high temperature-treated (IH) groups. The procedure for the experiment is illustrated in Figure 1.
First experiment. Four mini hives (200 × 265 × 135 mm3) were prepared in the apiary. Five frames (one full of eggs and larvae, one full of pollen, and three empty cell-built frames), approximately 2000 nurse bees, and an egg-laying queen were placed into each hive. IMD-acetone stock solution (20 mg/kg) was 1000-fold diluted with 50% sucrose solution (w/v) to the final concentration of 20 μg/kg IMD with 0.1% acetone. For the control, only acetone was added to the 50% sucrose solution to obtain the final 0.1% acetone concentration. Fifty milliliters of 50% sucrose solution containing 20 μg/kg IMD, which was an average detection concentration for pollen of winter oilseed rape in the field (Wen et al. 2021), and 10 g of commercial pollen bread (composed of 20% rapeseed pollen, 20% yeast, 20% soybean flour, and 40% white sugar, Bullo Apiary, Guri-si, Korea) were provided to each of two hives, and the same amount of sucrose solution without IMD and pollen were provided to each of another two hives every day until the end of experiment. Control and IMD-treated hives were located more than 10 m away from each other with walls between them, and food was supplied just before sunset to prevent robbing from other bees. After 7 days, 1000 newly emerged bees from other regular hives were marked on thoraces with paint (Uni posca, Mitsubishi pencil, Japan) and introduced to each hive; thus, the total number of worker bees/hive became approximately 3000. After 14 days, the entrances of all four hives were blocked by plastic screen slides with holes to prevent bees from escaping from the hives during the following heat stress period. Every thermal treatment started at 9 a.m. A set of one control hive and one IMD-treated hive was placed into a growth chamber, while the other set of hives was placed into another growth chamber. One growth chamber was set for normal temperature conditions at 25 °C for 30 min and then at 30 °C for 7 h under 60% RH and light conditions (1_CC and 1_IC). The temperature 30 °C was considered a typical sunny day temperature in the summer of Seoul, Korea. For the high temperature treatment, the other growth chamber was set at 25 °C for 30 min, 30 °C for 30 min, 35 °C for 30 min, and then 41 °C for 6 h under 60% RH and light conditions (1_CH and 1_IH). During incubation, temperature and humidity were constantly recorded using in-hive sensors (BroodMinder, Stoughton, Wisconsin, USA) attached to the brood frames in each hive. After incubation, bees were immediately flash-frozen with liquid nitrogen, and marked bees were sorted.
Second experiment. A set of one control mini hive and one IMD-treated mini hive was prepared outside the apiary following the 1st experimental procedure. After 13 days, 100 marked bees were collected from each hive and flash-frozen (2_CC and 2_IC). After 14 days, the two mini hives were heat-treated following the protocol from the 1st experiment, and honey bees from the respective hives were catalogued as 2_CH and 2_IH.
Third experiment. This was a replicate of the 2nd experiment.
2.2 Quantitative real-time PCR
Total RNA was extracted from pooling of eight honey bee abdomens (three replicates) from every experimental condition of each colony (total n = 36, pooling of eight bees × 3 replicates from a colony × 4 experimental conditions × 3 independent experiments) with TRI reagent (MRC, Cincinnati, OH, USA) following the instructions of the manufacturer. After DNase I treatment (TAKARA Korea Biomedical Inc., Seoul, Korea), cDNA was synthesized using Superscript IV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and oligo(dT)20 primer (Macrogen, Seoul, Korea) at 55 °C following the instruction manual. The synthesized cDNAs were used as templates for qPCR.
qPCR was conducted using SYBR Premix Ex Taq (TAKARA Korea Biomedical Inc) in 40 cycles of the following thermal program: 30 s at 95 °C, 15 s at 56 °C and 30 s at 72 °C. cDNA (12.5 ng) was added to a 10 μl total reaction volume. The ADP-ribosylation factor 1 (arf1) and Ras-related protein Rab-1A (rab1a) genes were used as reference genes for normalization of target gene expression in pesticide-treated honey bees (Kim et al. 2022a). Transcription levels of Hsp70, Hsp90, and the Vg were measured to evaluate stress levels of each experimental group following treatment (Kim et al. 2019). Information on the primer sets is listed in Supplementary Table 2.
2.3 Transcriptome analysis
For transcriptome analysis, the total RNA from honey bee abdomen samples from the 1st experiment was used. Three biological replicates (pooling of eight bees per replicate) from each of the 1_CC, 1_CH, 1_IC, and 1_IH experimental groups (total 12 samples) were sequenced. The RNA quality and quantity of each sample were analyzed using a Bioanalyzer (Agilent Technologies, USA). TruSeq mRNA library kits (Illumina, Inc, San Diego, CA, USA) were used to construct RNA-Seq libraries with insert sizes of 300 bp from 2 μg of total RNA for each experimental group. Pooled libraries were sequenced using the Illumina HiSeq X platform with paired-end (PE) reads of 151 bp. Low-quality and duplicated reads and adapter sequences were removed using Trimmomatic ver. 0.39 (Bolger et al. 2014) with default parameters. Sequences contaminated by bacteria, viruses and humans were removed using BBDuk (ver. 38.87) with default parameters. For the expression profiling of genes, trimmed RNA-Seq reads were mapped to the A. mellifera (DH4) genome sequences (v3.1, enBank acc. GCF_003254395.2) using HISAT2 ver. 2.1.0 (Kim et al. 2015), and then RNA reads mapped to protein coding sequences were counted using HTSeq-count ver. 0.11.2 (Anders et al. 2015). The bioconductor package DESeq2 (Love et al. 2014) was used to identify differentially expressed genes (DEGs). Genes with p values of < 0.05 and |Log2(fold change)|≥ 1 were considered to be DEGs.
Up- or downregulated sets of DEGs were uploaded as a query into g:GOSt in g:Profiler (https://biit.cs.ut.ee/gprofiler/gost) for functional annotation (Raudvere et al. 2019). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with a false discovery rate (FDR) < 0.05 were considered to be significant.
2.4 Statistical analyses
To compare multiple sample data, one-way ANOVA was performed with Tukey’s multiple comparison test as a post hoc analysis. All statistical analyses and graph designs were performed using Prism 6.0 (GraphPad, San Diego, USA).
3 Results
3.1 Transcription profiles of Hsps and Vg
As representative markers for the stress response of honey bees, the transcription levels of Hsp70, Hsp90, and Vg were determined. The overall transcription profiles of Hsp70 and Hsp90 were almost identical in all experiments (Figure 2). Transcription levels significantly increased in the CH group (p = 0.012, 0.0001, and 0.008 for Hsp70 in the 1st, 2nd, and 3rd experiments, respectively, and p = 0.0001 and 0.006 for Hsp90 in the 2nd and 3rd experiments), whereas no difference was observed in the IC group compared to the CC group. The IH group showed significantly higher transcription levels of Hsp70 and Hsp90 than the CC group in all experiments. The transcription levels of Hsp70 and Hsp90 in the IH group were significantly higher than those in the CH group (p = 0.010, 0.036, and 0.0001 for Hsp70 and p = 0.019, 0.044, and 0.004 for Hsp90 in the 1st, 2nd, and 3rd experiments, respectively) and the IC group (p = 0.0003, < 0.0001, and < 0.0001 for Hsp70 and p = 0.0002, < 0.0001, and < 0.0001 for Hsp90 in the 1st, 2nd, and 3rd experiments, respectively) in all three experiments, suggesting synergistic effects of IMD and high temperature. The transcription levels of Vg significantly increased following heat treatment (p = 0.0001, 0.030, and 0.0004 in the 1st, 2nd, and 3rd experiments, respectively) but were not significantly altered by IMD treatment. The simultaneous treatments of IMD and high temperature upregulated Vg transcription levels compared to those of the CC group in the 1st and 3rd experiments (p = 0.001 and 0.003) and those of the IC group in the 1st and 2nd experiments (p < 0.0001 and = 0.049), but the levels were statistically indistinguishable from those of the CH group except in the 1st experiment (p = 0.003).
3.2 DEGs following IMD and high-temperature treatment
Venn diagrams were drawn with DEGs between the CC group and the CH/IC/IH groups (Figure 3A and B). High-temperature treatment resulted in the least upregulation of genes (141 genes in CH), followed by IMD treatment (872 genes in IC) and the combined treatment of high temperature and IMD (1609 genes in IH, Figure 3A). The IH group shared large fractions [84% (119 in 141 genes) and 87% (761 in 872 genes)] of upregulated DEGs with the CH and IC groups, respectively (Figure 3A). Following the IMD/high temperature combined treatment, 783 genes were uniquely upregulated, accounting for 45% of the total upregulated DEGs. The pattern of downregulated genes was almost identical to that of the upregulated genes (Figure 3B). A total of 163, 825, and 1502 genes were downregulated in CH, IC, and IH, respectively. The IH group shared a total of 75% (123 in 163 genes) and 73% (606 in 825 genes) of downregulated DEGs in the CH and IC groups, respectively. Double treatment with IMD and high temperature led to the downregulation of 814 genes that were not identified in either the IMD- or high temperature-treated group.
Venn diagrams of differentially expressed genes (DEGs) from honey bee abdomen transcriptome analysis and subsequent significant pathways from GO and KEGG enrichment analysis. Upregulated (A) or downregulated (B) genes of each group were obtained by comparison with the control group. Up- (C) or downregulated pathways (D) with FDR < 0.05 were considered to be significant. The values in parentheses indicate the proportion of the DEGs or pathways to the total number of DEGs or pathways from the three groups
The ten most up- or downregulated genes following the treatments in each group are listed in Table I. Upregulation of several Hsps was observed in the high temperature-treated groups: 10 kDa heat shock protein (Hsp10), mitochondrial, protein lethal (2) essential for life (Hsp20), heat shock protein 60A (Hsp60), and endoplasmin (Hsp90B1) in the CH group and Hsp60 and heat shock 70 kDa protein 4 in the IH group. Vg was upregulated in the CH group (3.0-fold), whereas it was downregulated in the IC group (7.1-fold). Both the IC and IH groups shared four commonly upregulated genes: cytoplasmic probable aspartate aminotransferase, mitochondrial 2-oxoglutarate dehydrogenase, membrane-associated progesterone receptor component 1, and mitochondrial pyruvate carboxylase. Four genes related to cellular respiration, NADH dehydrogenase 1, 4, and 5 and cytochrome c oxidase subunit III, were commonly downregulated in both the IC and IH groups.
3.3 Functional annotation of DEGs
Enrichment analysis of GO and KEGG pathways was performed with DEGs obtained from the CC groups vs. the CH, IC, and IH groups (Table II). Only two upregulated pathways were identified following high-temperature treatment, whereas 10 and 99 metabolic pathways were upregulated following IMD treatment and the combined treatment of IMD and high temperature, respectively (Figure 3C). Among the two upregulated pathways in the CH group, the “protein processing in endoplasmic reticulum” pathway was commonly upregulated in IH (Table II), suggesting that its upregulation is likely attributed to high temperature. Eight upregulated pathways (e.g., “ribosome” and “thermogenesis”) were commonly annotated in the IC and IH groups (Table II), which suggests that those pathways may be induced by IMD treatment. Contrary to the increased number of upregulated pathways in the IH group, the number of downregulated pathways in the IH group was much lower than that observed in the IC group (4 vs. 33, Figure 3D). There was no commonly downregulated pathway between any of the groups.
All the downregulated GO terms or pathways in the IC group were related to cellular respiration. The upregulated GO terms or pathways in the IH group were mainly related to protein synthesis and modification, olfactory sensing, and overall cell signaling along with the thermogenesis pathway.
To investigate any synergistic effect of IMD under high-temperature conditions, KEGG pathway enrichment analysis was performed with DEGs from the comparisons of CH vs. IH and IC vs. IH. A total of 52 KEGG pathways were upregulated, and one pathway was downregulated in the CH vs. IH comparison. In contrast, only three KEGG pathways were upregulated in the IC vs. IH comparison (Supplementary Table 3). The top 20 upregulated pathways are presented in Figure 4. Several pathways upregulated in the CC vs. IH comparison (e.g., insulin signaling pathway, axon guidance, Hippo signaling pathway, Rap1 signaling pathway, and regulation of actin cytoskeleton) were also observed to be significantly upregulated in the CH vs. IH group comparison.
4 Discussion
4.1 Effects of high temperature on honey bee colonies as a single stressor
Following the simulated heat wave of 41 °C for 6 h, the inner temperatures of bee hives were effectively maintained at temperatures at least 2 °C lower than outside temperatures (Figure S1), indicating that the thermoregulation of workers efficiently lowered inner hive temperature. The inner temperatures of bee hives were maintained at 33.8 to 37.0 °C in the summer when ambient temperatures rose up to 39 °C (Fahrenholz et al. 1989). Under high-temperature conditions, honey bee workers actively evaporate water and ventilate by fanning to adjust hive temperature to the range required for proper brood development (Jones and Oldroyd 2006). When inner hive temperature reaches 35 °C, honey bees start to show fanning behavior, and with higher temperatures, some of them move out to the hive entrance and begin fanning (Southwick and Moritz 1987). Honey bees also recruit spare bees as water foragers following heat shock (Bordier et al. 2017a, b). Although bees were confined inside their hives in this experiment, aggressive fanning behavior was observed at the entrances of the hives following heat treatment, which would reduce inner hive temperature and increase water evaporation.
The total number of DEGs in the CH group relative to the CC group was 5.7-fold and 10.2-fold lower than those of the IC and IH groups, respectively, which suggests that worker bees tolerated the heat wave without a large alteration in gene expression profile (Figure 3). High temperature tolerance appears to be due to the highly developed heat shock response (HSR) system of honey bees (Elekonich 2009; Bach et al. 2021). Heat shock proteins (Hsps) are representative proteins in the HSR pathway that function as chaperones to prevent protein misfolding under stress conditions (Whitley et al. 1999). It has been reported that Hsp20s, Hsp40, Hsp60, Hsp70, Grp78, Hsp82, and Hsp90 are highly upregulated in honey bees in response to heat shock (Koo et al. 2015; Kim et al. 2019; Bach et al. 2021; Shih et al. 2021). With their roles as regulators of general stress responses (Santoro 2000; King and MacRae 2015), the transcription levels of Hsps have been commonly used to estimate the stress level of honey bees (Even et al. 2012; Koo et al. 2015; Kim et al. 2019). Although heat shock-related pathways were not enriched, extensive upregulation of Hsps in transcriptome analysis and Hsp70 and Hsp90 in qPCR analysis following heat shock indicated that the HSR system of honey bees actively defends against heat stress without extensively altering the expression profiles of other genes (Table I, Table II, and Figure 2). The “protein processing in endoplasmic reticulum” pathway, upregulated in both the CH and IH groups under heat-treated conditions, is related to the HSR that enables proteins to fold properly under the oxidative conditions induced by heat shock (Kalmar and Greensmith 2009). Its upregulation was previously reported in the digestive tracts of heat-shocked honey bees (Bach et al. 2021). A high transcription level of Vg, which is known to protect honey bees from oxidative stress resulting from heat shock (Seehuus et al. 2006; Bordier et al. 2017a, b; Kim et al. 2019), was also observed under high temperature conditions in both transcriptome and qPCR data (Table I and Figure 2). Another upregulated pathway, “the longevity regulating pathway—multiple species,” expresses Hsps and stress resistance genes at the end of the HSR cascade to protect organisms from stressful conditions (Uno and Nishida 2016). It has been reported that antiviral and regenerative signaling pathways were altered in honey bees individually exposed to heat shock (McMenamin et al. 2020; Bach et al. 2021). Considering this, the observation of only two enriched pathways related to the stress response indicates that honey bees are capable of regulating heat stress in a selective and efficient way as a superorganism.
4.2 Effects of IMD on honey bee colonies as a single stressor
Despite no apparent changes in phenotypes, in-hive temperatures, or RH following chronic exposure of honey bees to 20 ppb IMD for 14 days (Figure S1), honey bee physiology appeared to be severely affected by IMD treatment from the perspective of the gene transcription profile in that a large number of pathways were affected in the IC group (Figure 3). The notable feature in the enriched pathways identified following IMD exposure was the general downregulation of cellular respiration-related pathways (Table II). It has been reported that IMD negatively affects mitochondrial respiration in honey bees (Nicodemo et al. 2014). IMD is known to show biphasic effects on the physiology of honey bees depending on concentration (Cook 2019; Kim et al. 2022b). Chronic exposure to low concentrations of IMD leads to an increase in the metabolic rate of honey bees. In contrast, chronic exposure to high concentrations of IMD results in a reduction in metabolic rate (Meikle et al. 2018; Gooley and Gooley 2020; Kim et al. 2022b), which resembles the case of the IC group in this study.
Downregulation of pathways in the electron transfer system of mitochondrial respiration (e.g., “electron transfer activity,” “NADH dehydrogenase activity,” “ATP synthesis coupled electron transport,” and “oxidative phosphorylation”) was the most apparent feature in the DEG profiles and enriched pathway analysis for the IC group. In contrast, several genes related to gluconeogenesis (e.g., aspartate aminotransferase, 2-oxoglutarate dehydrogenase, and pyruvate carboxylase) (DeRosa and Swick 1975; Smith et al. 1992; Kumashiro et al. 2013) were significantly overexpressed in the IC group (Tables I and II). It was previously reported that chronic exposure of honey bees to low IMD concentrations (5 and 20 ppb) induced diabetic symptoms (e.g., downregulation of insulin signaling pathway and reduction of body weight) (Kim et al. 2022b). The energy metabolism disorder in diabetes is known to increase the rate of gluconeogenesis (Magnusson et al. 1992). Considering this, the upregulation of gluconeogenesis in the IC group might be a compensating response to the shortage of chemical energy due to the induced diabetic symptoms and reduced cellular respiration.
4.3 Synergistic effects of IMD and high temperature on honey bee colonies as combined stressors
Hsps were highly overtranscribed following high-temperature treatment, as discussed in Sect. 4.2, but the transcriptional responses of Hsps to IMD exposure in honey bees are known to vary depending on the concentration, duration, and condition of the treatments (Koo et al. 2015; Kim et al. 2019; Manzi et al. 2020). In this study, the transcription levels of Hsp70 and Hsp90 were not affected by IMD treatment alone (i.e., IC), but they were significantly higher following IMD and high temperature combined treatment (i.e., IH) compared to high temperature treatment alone (i.e., CH) (Figure 2), indicating that both stressors interact with each other, producing synergistic adverse effects on honey bees.
Additional evidence for synergism of IMD and high temperature was observed from the transcriptome analysis. The expression profiles of a much larger number of genes (783 upregulated and 814 downregulated genes) were altered in the IH group following the combined treatment, and most of their enriched pathways were not shared by either the CH or IC groups (Figure 3). The GO term “ribosome” upregulated in the IC group was more significantly upregulated in the IH group (FDR 0.025 vs. 0.00083). The upregulation of other pathways was related to protein synthesis and modification, which appear to be induced by the reactive oxygen species generated by both IMD and high temperature treatments (He et al. 2021; Zhao et al. 2021). Although cellular respiration-related pathways, which were downregulated following IMD treatment alone in the IC group, were not enriched in the IH group, the inclusion of NADH dehydrogenase subunits 1, 4, and 5 and cytochrome c oxidase subunit III in the top 10 list of downregulated genes (Table I) indicates that cellular respiration is still substantially affected following the combined treatment of IMD and high temperature.
Upregulation of various signaling pathways was more obvious in the functional annotation of DEGs when comparing the IH group to the CH group, which demonstrates the large synergistic adverse effects of IMD on honey bees exposed to high temperature (Figure 4). Energy metabolism pathways such as the “insulin signaling pathway” and “insulin resistance,” which were not identified in the functional annotations of IMD-treated honey bees either in this (IC group) or a previous study (Kim et al. 2022b), were significantly upregulated when honey bees were exposed to both stressors simultaneously. This finding indicates that exposure to high temperature conditions can aggravate disorders in the insulin signaling pathway potentially induced by IMD, which eventually disrupt the energy metabolism of worker bees (Cook 2019). Considering that disrupted energy metabolism most likely impairs the in-hive temperature regulation system of fanning and ventilation and that in the long term, insulin resistance can disturb foraging activity and labor division of worker bees (Ament et al. 2008; Kim et al. 2022b), the combined effects of IMD and high temperature can deteriorate the overall health of honey bee colonies in a synergistic way. This notion is supported by the fact that both IMD and high temperature are known to induce precocious onset of foraging (Medina et al. 2018; Colin et al. 2019). One of the possible mechanisms for the precocious foraging under high temperature and IMD exposure may be the low nutritional status caused by the disruption of the energy metabolism (Cho et al. 2022; Kim et al. 2022b), since the nutritional status of bees is known to influence influence foraging onset independently from the social regulations (Toth et al. 2005). The precocious foraging of bees induced by stresses could accelerate failure of honey bee colonies through the recruitment of young foragers and therefore the reduction of the adult population (Perry et al. 2015). The disruption of insulin pathway is also contributable considering the importance of the pathway in labor division of honey bees (Ament et al. 2008). The upregulation of “regulation of actin cytoskeleton,” “axon guidance,” “focal adhesion,” and “axon regeneration” appears to be related to the regeneration of damaged tissues, as reported for the Hippo signaling pathway involved in the regeneration of honey bee digestive tracts under heat shock (Bach et al. 2021).
In contrast to the comparison of CH vs. IH, only two KEGG pathways were upregulated in the comparison of IC vs. IH (Figure 4), which is similar to the comparison of CC vs. CH, as discussed in Sect. 4.1. Taken together, this finding suggests that the relative synergistic effects of IMD on heat-stressed honey bees (i.e., CH vs. IH) are larger than those of high temperature on IMD-exposed honey bees (i.e., IC vs. IH).
Along with the changes in gene expression profiles, a failure of thermoregulation following the combined treatment of IMD and high temperature was observed in the 3rd experiment (Figure S1), with the inner temperatures of the hives reaching up to 41 °C. In addition to the potential synergistic negative effects in diverse physiologies, such high temperatures can damage the overall fitness of honey bee colonies. Heat stress up to 40 °C during the pupal stage leads to precocious foraging behavior and forewing length reduction in honey bee workers (Medina et al. 2018). Pupae developing at 37 °C exhibit brain plasticity disorder (Groh et al. 2004), and the body weights of worker bees maturing at 35 °C were lower than those of bees maturing at 25 and 30 °C and field bees (Cho et al. 2022). All of these results suggest that thermoregulation failure of IMD-treated honey bees under heat stress conditions, along with the synergistic negative effects on honey bee metabolic pathways, likely results in developmental disorders of honey bees.
5 Conclusions
Honey bees, as a colony, coped well with the heat stress through the active thermoregulation and upregulation of heat stress response pathways. However, transcriptional analysis suggested that a pre-exposure to IMD could influence the heat stress response of honey bees, aggravating the adverse effects of IMD on honey bee physiology such as energy metabolic pathway. Large-scale tracking of in-hive temperatures and colony development following IMD exposure and heat stress should be performed to investigate the possible synergistic effects of the two stress factors on honey bee colony thermoregulation and performances. Such studies will be important for further exploring the effects of environmental pollution and climate change on honey bees.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Code availability
Not applicable.
References
Abou-Shaara HF, Al-Ghamdi AA, Mohamed AA (2012) Tolerance of two honey bee races to various temperature and relative humidity gradients. Environ Exp Biol 10(4):133–138
Ament SA, Corona M, Pollock HS, Robinson GE (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci 105(11):4226–4231
Anders S, Pyl PT, Huber W (2015) HTSeq-a Python framework to work with high-throughput sequencing data. Bioinform 31(2):166–169
Bach DM, Holzman MA, Wague F, Miranda JL, Lopatkin AJ et al (2021) Thermal stress induces tissue damage and a broad shift in regenerative signaling pathways in the honey bee digestive tract. J Exp Biol 224(18): jeb242262
Barron AB (2015) Death of the bee hive: understanding the failure of an insect society. Curr Opin Insect Sci 10:45–50
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinform 30(15):2114–2120
Bordier C, Dechatre H, Suchail S, Peruzzi M, Soubeyrand S et al (2017a) Colony adaptive response to simulated heat waves and consequences at the individual level in honeybees (Apis mellifera). Sci Rep-Uk 7(1):1–11
Bordier C, Suchail S, Pioz M, Devaud JM, Collet C et al (2017b) Stress response in honeybees is associated with changes in task-related physiology and energetic metabolism. J Insect Physiol 98:47–54
Cho S, Lee SH, Kim S (2022) Determination of the optimal maturation temperature for adult honey bee toxicity testing. Comp Biochem Physiol C Toxicol Pharmacol 257:109359
Colin T.o., W.G. Meikle, X. Wu, A.B. Barron. (2019) Traces of a neonicotinoid induce precocious foraging and reduce foraging performance in honey bees. Environ Sci Technol 53(14):8252–8261
Cook SC (2019) Compound and dose-dependent effects of two neonicotinoid pesticides on honey bee (Apis mellifera) metabolic physiology. Insects 10(1):18
Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S et al (2004) Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic Biochem Phys 78(2):83–92
Delkash-Roudsari S, Chicas-Mosier AM, Goldansaz SH, Talebi-Jahromi K, Ashouri A et al (2020) Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifespan in honey bees (Apis mellifera L.). Ecotox Environ Safe 204:111108
DeRosa G, Swick RW (1975) Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J Biol Chem 250(20):7961–7967
Elekonich MM (2009) Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones 14(2):219–226
Even N, Devaud JM, Barron AB (2012) General stress responses in the honey bee. Insects 3(4):1271–1298
Fahrenholz L, Lamprecht I, Schricker B (1989) Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J Comp Physiol B 159(5):551–560
Frich P, Alexander LV, Della-Marta P, Gleason B, Haylock M et al (2002) Observed coherent changes in climatic extremes during the second half of the twentieth century. Clim Res 19(3):193–212
Gooley ZC, Gooley AC (2020) Exposure to field-realistic concentrations of imidacloprid at different ambient temperatures disrupts non-flight metabolic rate in honey bee (Apis mellifera) foragers. B Insectol 73(2):161–170
Groh C, Tautz J, Rössler W (2004) Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc Natl Acad Sci 101(12):4268–4273
He B, Liu Z, Wang Y, Cheng L, Qing Q et al (2021) Imidacloprid activates ROS and causes mortality in honey bees (Apis mellifera) by inducing iron overload. Ecotox Environ Safe 228:112709
Hristov P, Shumkova R, Palova N, Neov B (2020) Factors associated with honey bee colony losses: A mini-review. Vet Sci 7(4):166
Jiang J, Ma D, Zou N, Yu X, Zhang Z et al (2018) Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere 201:159–167
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee Apis mellifera. J Comp Physiol A 191(12):1121–1129
Jones JC, Oldroyd BP (2006) Nest thermoregulation in social insects. Advances in Insect Physiology 33:153–191
Kalmar B, Greensmith L (2009) Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61(4):310–318
Kim D, Landmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357-U121
Kim S, Cho S, Lee SH (2022a) Selection of stable reference genes for real-time quantitative PCR in honey bee pesticide toxicity studies. J Apic Res 61(1):26–36
Kim S, Kim JH, Cho S, Clark JM, Lee SH (2022b) Chronic exposure to field-realistic doses of imidacloprid resulted in biphasic negative effects on honey bee physiology. Insect Biochem Mol Biol 144:103759
Kim S, Kim K, Lee JH, Han SH, Lee SH (2019) Differential expression of acetylcholinesterase 1 in response to various stress factors in honey bee workers. Sci Rep-Uk 9(1):1–10
King AM, MacRae TH (2015) Insect heat shock proteins during stress and diapause. Annu Rev Entomol 60:59–75
Koo J, Son TG, Kim SY, Lee KY (2015) Differential responses of Apis mellifera heat shock protein genes to heat shock, flower-thinning formulations, and imidacloprid. J Asia-Pac Entomol 18(3):583–589
Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL et al (2013) Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62(7):2183–2194
Lhotka O, Kyselý J, Farda A (2018) Climate change scenarios of heat waves in Central Europe and their uncertainties. Theor Appl Climatol 131(3):1043–1054
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):1–21
Lu CS, Warchol KM, Callahan RA (2014) Sub-lethal exposure to neonicotinoids impaired honey bees winterization before proceeding to colony collapse disorder. B Insectol 67(1):125–130
Magnusson I, Rothman DL, Katz L, Shulman R, Shulman G (1992) Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Investig 90(4): 1323–1327
Manzi C, Vergara-Amado J, Franco LM, Silva AX (2020) The effect of temperature on candidate gene expression in the brain of honey bee Apis mellifera (Hymenoptera: Apidae) workers exposed to neonicotinoid imidacloprid. J Therm Biol 93:102696
Marx W, Haunschild R, Bornmann L (2021) Heat waves: a hot topic in climate change research. Theor Appl Climatol 146(1):781–800
McAfee A, Chapman A, Higo H, Underwood R, Milone J et al (2020) Vulnerability of honey bee queens to heat-induced loss of fertility. Nat Sustain 3(5):367–376
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6(2):253–256
McMenamin AJ, Daughenbaugh KF, Flenniken ML (2020) The heat shock response in the western honey bee (Apis mellifera) is antiviral. Viruses 12(2):245
Medina RG, Paxton RJ, De Luna E, Fleites-Ayil FA, Medina LAM et al (2018) Developmental stability, age at onset of foraging and longevity of Africanized honey bees (Apis mellifera L.) under heat stress (Hymenoptera: Apidae). J Therm Biol 74:214–225
Medrzycki P, Sgolastra F, Bortolotti L, Bogo G, Tosi S et al (2010) Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. J Apic Res 49(1):52–59
Meikle WG, Adamczyk JJ, Weiss M, Gregorc A (2018) Effects of bee density and sublethal imidacloprid exposure on cluster temperatures of caged honey bees. Apidologie 49(5):581–593
Mitchell D, Heaviside C, Vardoulakis S, Huntingford C, Masato G et al (2016) Attributing human mortality during extreme heat waves to anthropogenic climate change. Environ Res Lett 11(7):074006
Nicodemo D, Maioli MA, Medeiros HCD, Guelfi M, Balieira KVB et al (2014) Fipronil and imidacloprid reduce honeybee mitochondrial activity. Environ Toxicol Chem 33(9):2070–2075
Perry CJ, Søvik E, Myerscough MR, Barron AB (2015) Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc Natl Acad Sci 112(11):3427–3432
Ranga P, Prakash R, Mrinal N (2017) Sibling Drosophila species (Drosophila leontia and Drosophila kikkawai) show divergence for thermotolerance along a latitudinal gradient. Evol Ecol 31(1):93–117
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P et al (2019) g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47(W1):W191–W198
Retschnig G, Neumann P, Williams GR (2014) Thiacloprid-Nosema ceranae interactions in honey bees: Host survivorship but not parasite reproduction is dependent on pesticide dose. J Invertebr Pathol 118:18–19
Santoro MG (2000) Heat shock factors and the control of the stress response. Biochem Pharmacol 59(1):55–63
Schmolz E, Hoffmeister D, Lamprecht I (2002) Calorimetric investigations on metabolic rates and thermoregulation of sleeping honeybees (Apis mellifera carnica). Thermochim Acta 382(1–2):221–227
Seehuus S-C, Norberg K, Gimsa U, Krekling T, Amdam GV (2006) Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci 103(4):962–967
Shih SR, Bach DM, Rondeau NC, Sam J, Lovinger NL et al (2021) Honey bee sHSP are responsive to diverse proteostatic stresses and potentially promising biomarkers of honey bee stress. Sci Rep-Uk 11(1):1–12
Smith BC, Clotfelter LA, Cheung JY, LaNoue KF (1992) Differences in 2-oxoglutarate dehydrogenase regulation in liver and kidney. Biochem J 284(3):819–826
Southwick EE, Moritz RF (1987) Social control of air ventilation in colonies of honey bees Apis mellifera. J Insect Physiol 33(9):623–626
Stabentheiner A, Kovac H, Schmaranzer S (2002) Honeybee nestmate recognition: the thermal behaviour of guards and their examinees. J Exp Biol 205(17):2637–2642
Stoner KA, Eitzer BD (2012) Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 7(6):e39114
Tautz J, Maier S, Groh C, Rössler W, Brockmann A (2003) Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci 100(12):7343–7347
Tome HVV, Schmehl DR, Wedde AE, Godoy RSM, Ravaiano SV et al (2020) Frequently encountered pesticides can cause multiple disorders in developing worker honey bees. Environ Pollut 256:113420
Tong Z, Duan J, Wu Y, Liu Q, He Q et al (2018) A survey of multiple pesticide residues in pollen and beebread collected in China. Sci Total Environ 640:1578–1586
Tosi S, Costa C, Vesco U, Quaglia G, Guido G (2018) A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci Total Environ 615:208–218
Tosi S, Démares FJ, Nicolson SW, Medrzycki P, Pirk CW et al (2016) Effects of a neonicotinoid pesticide on thermoregulation of African honey bees (Apis mellifera scutellata). J Insect Physiol 93:56–63
Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P (2017) Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc R Soc b: Biol Sci 284:20171711
Toth AL, Kantarovich S, Meisel AF, Robinson GE (2005) Nutritional status influences socially regulated foraging ontogeny in honey bees. J Exp Biol 208(24):4641–4649
Uno M, Nishida E (2016) Lifespan-regulating genes in C. elegans. NPJ aging and mechanisms of disease 2(1):1–8
Vergara-Amado J, Manzi C, Franco LM, Contecha SC, Marquez SJ et al (2020) Effects of residual doses of neonicotinoid (imidacloprid) on metabolic rate of queen honey bees Apis mellifera (Hymenoptera: Apidae). Apidologie 51(6):1091–1099
Wang Y, Zhu YC, Li W (2020) Interaction patterns and combined toxic effects of acetamiprid in combination with seven pesticides on honey bee (Apis mellifera L.). Ecotox Environ Safe 190:110100
Wen X, Ma C, Sun M, Wang Y, Xue X et al (2021) Pesticide residues in the pollen and nectar of oilseed rape (Brassica napus L.) and their potential risks to honey bees. Sci Total Environ 786:147443
Whitley D, Goldberg SP, Jordan WD (1999) Heat shock proteins: a review of the molecular chaperones. J Vasc Surg 29(4):748–751
Zhao H, Li G, Guo D, Li H, Liu Q et al (2021) Response mechanisms to heat stress in bees. Apidologie 52(2):388–399
Zheng H-Q, Gong H-R, Huang S-K, Sohr A, Hu F-L et al (2015) Evidence of the synergistic interaction of honey bee pathogens Nosema ceranae and deformed wing virus. Vet Microbiol 177(1–2):1–6
Funding
This work was equally supported by grant PJ015778012022 from the Rural Development Administration (RDA) of Korea and grant NRF-2021R1A6A3A01088066 from the National Research Foundation of Korea. Susie Cho were supported in part by the Brain Korea 21 program.
Author information
Authors and Affiliations
Contributions
Sanghyeon Kim: conceptualization, formal analysis, funding acquisition, investigation, visualization, writing—original draft. Susie Cho: investigation. Si Hyeock Lee: project administration, supervision, funding acquisition, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Cedric Alaux
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Cho, S. & Lee, S.H. Synergistic effects of imidacloprid and high temperature on honey bee colonies. Apidologie 53, 67 (2022). https://doi.org/10.1007/s13592-022-00980-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-022-00980-z