Abstract
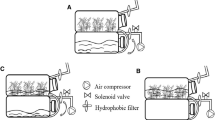
Viral pathogens reduce the quality and yield of apple (Malus domestica) fruits by 30–50%. A mass production system of virus-free apple plantlets is needed to meet the demand of the domestic fruit tree industry. In this study, we compared the production of virus-free plants in different in vitro culture systems, including a temporary immersion bioreactor (TIB), a continuous immersion bioreactor, and conventional solid and liquid culture systems (controls). Apple plantlets were immersed in the TIB once every 3 h (TIB-3) or 6 h (TIB-6). The fresh weight of apple plants was the highest in the TIB-3 system and lowest in the liquid culture. Shoots were the longest in the TIB-3 system, approximately twofold longer than those in solid culture and liquid culture. Roots of apple plants were the longest in the TIB-3 system compared with solid and liquid cultures. Root number in the TIB-3 system was also higher than that in solid and liquid cultures. Moreover, leaf area was the highest in plants grown in the TIB-3 treatment. The total stem area of TIB-3 plants was the largest at 1.46 mm2. This study suggests that the airlift bioreactor is capable of producing a large number of virus-free plants in a short time compared with conventional culture systems. Additionally, secondary xylem was well developed in the stems of plants grown in the TIB-3 system. Therefore, this system shows a high potential for producing healthy plants suitable for acclimatization.





Similar content being viewed by others

References
Akdemir H, Süzerer V, Onay A, Tilkat E, Ersali Y, Çiftçi YO (2014) Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ 117:65–76
Alvarez C, Sáez P, Sáez K, Sánchez-Olate M, Ríos D (2012) Effects of light and ventilation on physiological parameters during in vitro acclimatization of Gevuina avellana mol. Plant Cell Tissue Organ 110:93–101
Ascough GD, Fennel CW (2004) The regeneration of plant growth and development in liquid culture. S Afr J Bot 70:181–190
Benelli C, Carlo AD (2018) In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 8:317. https://doi.org/10.1007/s13205-018-1346-4
Chen J, Tang HH, Li L, Qin SJ, Wang GP, Hong N (2017) Effect of virus infection on plant growth, root development and phytohormone levels in in vitro-cultured pear plants. Plant Cell Tissue Organ 131:359–368
Chen L, Wang MR, Li JW, Feng CH, Cui ZH, Zhao L (2019) Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant Pathol 68:997–1006
Cieślińska M, Rutkowski KP (2008) Effect of Apple chlorotic leaf spot virus on yield and quality of fruits from ‘Golden Delicious’ and ‘Sampion’ apple trees. Acta Hortic 781:119–124
Cuenca B, Sanchez C, Aldrey A, Bogo B, Blanco B, Correa B, Vidal N (2017) Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ 131:307–320
Frometa OM, Morgado MME, Silva JAT, Morgado DTP, Gradaille MAD (2017) In vitro propagation of Gerbera jamesonii Bolus ex Hooker f. in a temporary immersion bioreactor. Plant Cell Tissue Organ 129:543–551
Gao M, Jiang W, Wei S, Lin Z, Cai B, Yang L, Luo C, He X, Tan J, Chen L (2015) High-efficiency propagation of Chinese water chestnut [Eleocharis dulcis (Burm. f.) Trin. ex Hensch] using a temporary immersion bioreactor system. Plant Cell Tissue Organ 121:761–772
Gatti E, Sgarbi E, Ozudogru EA, Lambardi M (2017) The effect of Plantform™ bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst 151:1129–1136
George EF, Hall MA, De Klerk GJD (2008) Micropropagation: uses and methods. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, Dordrecht, pp 29–64
Georgiev V, Schumann A, Pavlov A, Bley T (2014) Temporary immersion systems in plant biotechnology. Eng Life Sci 14:607–621
Hadidi A, Barba M (2011) Economic impact of pome and stone fruit viruses and viroids. In: Hadidi A, Barba M, Candresse TH, Jelkmann W (eds) Virus and virus-like diseases of pome and stone fruits. APS Press, College Park, pp 1–7
Hu GJ, Dong YF, Zhang ZP, Fan XD, Ren F, Li ZN (2017) Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot-tip grafting and in vitro meristem culture. J Phytopathol 165:701–706
Ko SM, Lee JH, Oh MM (2018) Development of nutrient solution for in vitro propagation of ‘M9’ apple rootstock plantlets. Hortic Sci Technol 36:202–214
Kovalchuk I, Lyudvikova Y, Volgina M, Reed BM (2009) Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell Tissue Organ 96:127–136
Kwon AR, Cui HY, Lee HS, Shin HN, Kang KS, Park SY (2015) Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol Plant 37:65. https://doi.org/10.1007/s11738-015-1812-0
Larema L, da Cruz ACF, Saldanha CW, Dias LLC, Vieira RF, de Oliveira EJ, Otoni WC (2012) Photoautotrophic propagation of Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen]. Plant Cell Tissue Organ 110:227–238
Le KC, Jeong CS, Lee H, Paek KY, Park SY (2019) Ginsenoside accumulation profiles in long- and short-term cell suspension and adventitious root cultures in Panax ginseng. Hortic Environ Biotechnol 60:125–134
Li BQ, Feng CH, Hu LY, Wang MR, Wang QC (2016) Shoot tip culture and cryopreservation for eradication of Apple stem pitting virus (ASPV) and Apple stem grooving virus (ASGV) from apple rootstocks ‘M9’ and ‘M26’. Ann Appl Biol 168:142–150
Lichtenthaler HK (1987) Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J Plant Physiol 131:101–110
Martínez-Estrada E, Islas-Luna B, Pérez-Sato JA, Bello-Bello JJ (2019) Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci Hortic 249:185–191
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Park SY, Moon HK, Murthy HN, Kim YW (2011) Improved growth and acclimatization of somatic embryo-derived Oplopanax elatus plantlets by ventilated photoautotrophic culture. Biol Plant 55:559–562
Quiala E, Canal MJ, Meijon M, Rodriguez R, Chavez M, Valledor L, Feria M, Barbon R (2012) Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ 109:223–234
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Ramirez-Madero G, Hernandez-Rincon EU (2016) Micropropagation of Stevia rebaudiana Bert. in temporary immersion systems and evaluation of genetic fidelity. S Afr J Bot 106:238–243
Ramos-Castellá A, Iglesias-Andreu LG, Bello-Bello J, Lee-Espinosa H (2014) Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell Dev Biol-Plant 50:576–581
Sáez PL, Bravo LA, Latsague MI, Sanchez-Olate ME, Ríos DG (2012) Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci Hortic 130:7–16
Schuetz M, Smith R, Ellis B (2012) Xylem tissue specification, patterning, and dirrerentiation mechanisms. J Exp Bot 64:11–31
Ševčíková H, Lhotáková Z, Hamet J, Lipavská H (2018) Mixotrophic in vitro cultivations: the way to go astray in plant physiology. Physiol Plant. https://doi.org/10.1111/ppl.12893
Sreedhar RV, Venkatachalam L, Neelwarne B (2009) Hyperhydricity-related morphologic and biochemical changes in vanilla (Vanilla planifolia). J Plant Growth Regul 28:46–57
Valdez-Tapia R, Capataz-Tafur J, López-Laredo AR, Trejo-Espino JL, Trejo-Tapia G (2014) Effect of immersion cycles on growth, phenolics content, and antioxidant properties of Castilleja tenuiflora shoots. Vitro Cell Dev Biol-Plant 50:471–477
Vives K, Andújar I, Lorenzo JC, Concepción O, Hernández M, Escalona M (2017) Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tissue Organ 131:195–199
Yeung EC (1999) The use of histology in the study of plant tissue culture systems-some practical comments. In Vitro Cell Dev Biol Plant 35:137–143
Zhang B, Song L, Bekele LD, Shi J, Jia Q, Zhang B, Jin L, Duns GJ, Chen J (2018) Optimizing factors affecting development and propagation of Bletilla striata in a temporary immersion bioreactor system. Sci Hortic 232:121–126
Ziska LH, Panicker S, Wojno HL (2008) Recent and projected increases in atmospheric carbon dioxide and the potential impacts on growth and alkaloid production in wild poppy (Papaver setigerum DC.). Clim Change 91:395. https://doi.org/10.1007/s10584-008-9418-9
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant Number 315003-5).
Author information
Authors and Affiliations
Contributions
NYK contributed to the data acquisition and wrote the manuscript. HDH, JHK, and BMK participated in the experiment and sample analysis. DK participated in data interpretation and revising of the manuscript. S-YP made substantial contributions to data interpretation, revising of the manuscript, the conception, and design of this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Heakeun Yun, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, NY., Hwang, HD., Kim, JH. et al. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 61, 779–785 (2020). https://doi.org/10.1007/s13580-020-00257-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00257-3