Abstract
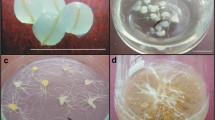
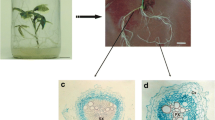
Panax ginseng Meyer is an important medicinal plant producing bioactive compounds. A droplet-vitrification method was developed for cryopreserving adventitious root cultures of mountain ginseng, and variations of this procedure were tested to determine their effects on regrowth rates and osmotic stress responses. Root regrowth rates were examined after exposing root segments to two pre-culture treatments, three loading solutions, and two vitrification solutions with different exposure periods. Pre-culturing excised segments of adventitious roots with 0.3 M sucrose produced the highest rate of regrowth after cryopreservation. Loading for 20 min with 17.5% (w/v) sucrose and 17.5% (w/v) glycerol at room temperature increased the regrowth rate of cryopreserved adventitious roots fourfold, compared with non-loaded samples. Treatments involving different vitrification solutions and exposure periods were compared, and the highest rate of regrowth (15%) after cryopreservation was achieved by incubating adventitious roots in modified plant vitrification solution 3 containing 40% (w/v) glycerol and 40% (w/v) sucrose for 10 min at room temperature, suggesting that ginseng adventitious root tips were sensitive to osmotic stress. Further study is necessary to develop optimal vitrification solutions that enhance the survival rate of cryopreserved adventitious roots of mountain ginseng.




Similar content being viewed by others
References
Ahmad P, Sarwat M, Sharma S (2008) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51:167–173
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Benson EE, Lynch PT, Jones J (1992) The detection of lipid peroxidation products in cryoprotected and frozen rice cells: consequences for post-thaw survival. Plant Sci 85:107–114
Bisht SS, Sharma A, Chaturvedi K (1989) Certain metabolic lesions of chromium toxicity in radish. Indian J Agric Biochem 2:109–115
Chen G, Ren L, Zhang J, Reed BM, Zhang D, Shen XH (2015) Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 70:38–47
Christensen LP (2008) Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99
Da Silva Cordeiro L, Simões-Gurgel C, Albarello N (2015) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. Vitro Cell Dev Biol 51:249–257
Engelman F, Takagi H (2000) Cryopreservation of tropical plant germplasm current research progress and application. Japan International Research Center for Agriculture Sciences, Tsukuba
Engelmann F, Dussert S (2013) Cryopreservation. In: Normah MN, Chin HF, Reed BM (eds) Conservation of tropical plant species. Springer, Berlin, pp 107–119
Fábián A, Jäger K, Darkó É, Barnabás B (2008) Cryopreservation of wheat (Triticum aestivum L.) egg cells by vitrification. Acta Physiol Plant 30:737–744
Fu C, Li L, Wu W, Li M, Yu X, Yu L (2012) Assessment of genetic and epigenetic variation during long-term Taxus cell culture. Plant Cell Rep 31:1321–1331
Fujikawa S, Steponkus PL (1991) Plasma membrane ultrastructural changes by vitrification procedures. Jpn J Free Dry 37:25–29
Hahn EJ, Yu KW, Paek KY (2003) Adventitious root cultures of Panax ginseng CV Meyer and ginsenoside production through large-scale bioreactor system. J Plant Biotechnol 5:1–6
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Hughes ZE, Mancera RL (2014) Molecular mechanism of the synergistic effects of vitrification solutions on the stability of phospholipid bilayers. Biophys J 106:2617–2624
Ibrahim S, Normah MN (2013) The survival of in vitro shoot tips of Garcinia mangostana L. after cryopreservation by vitrification. Plant Growth Regul 70:237–246
Jia MX, Shi Y, Di W, Jiang XR, Xu J, Liu Y (2017) ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. Vitro Cell Dev Biol 53:433–439
Jung DW, Sung CK, Touno K, Yoshimatsu K, Shimomura K (2001) Cryopreservation of Hyoscyamus niger adventitious roots by vitrification. J Plant Physiol 158:801–805
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katov II (ed) Current frontiers in cryobiology. Croatia, InTech, pp 417–438
Kim HH, Lee YG, Park SU, Lee SC, Baek HJ, Cho EG, Engelmann F (2009a) Development of alternative loading solutions in droplet-vitrification procedures. CryoLetters 30:291–299
Kim HH, Lee YG, Shin DJ, Ko HC, Gwag JG, Cho EG, Engelmann F (2009b) Development of alternative plant vitrification solutions and loading solutions in droplet-vitrification procedures. Cryobiology 59:320–334
Kim HH, Popova EV, Yi JY, Cho GT, Park SU, Lee SC, Engelmann F (2010) Cryopreservation of hairy roots of Rubia akane (Nakai) using a droplet-vitrification procedure. CryoLetters 31:473–484
Kim DS, Song M, Kim SH, Jang DS, Kim JB, Ha BK, Kim SH, Lee KJ, Kang SY, Jeong IY (2013) The improvement of ginsenoside accumulation in Panax ginseng as a result of γ-irradiation. J Ginseng Res 37:332–340
Kiselev KV, Shumakova OA, Tchernoded GK (2011) Mutation of Panax ginseng genes during long-term cultivation of ginseng cell cultures. J Plant Physiol 168:1280–1285
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species–a review. Sci Hortic (Amsterdam) 168:88–107
Kulus D, Abratowska A, Mikuła A (2018) Morphogenetic response of shoot tips to cryopreservation by encapsulation-dehydration in a solid mutant and periclinal chimeras of Chrysanthemum × grandiflorum/Ramat./Kitam. Acta Physiol Plant 40:18
Kuranuki Y, Sakai A (1995) Cryopreservation of in vitro-grown shoot tips of tea (Camellia sinensis) by vitrification. CryoLetters 16:345–352
Lambert E, Geelen D (2008) Cryopreservation of hairy root cultures from Maesa lanceolata. In: Laamanen J, Uosukainen M, Häggman H, Rantala ANS (eds) Cryopreservation of Cropspecies in Europe CRYOPLANET COST Action 871, 20th–23rd Feb 2008, Oulu, Finland
Lambert E, Goossens A, Panis B, van Labeke MC, Geelen D (2009) Cryopreservation of hairy root cultures of Maesa lanceolata and Medicago truncatula. Plant Cell, Tissue Organ Cult 96:289–296
Lee KH (1998) The pharmacology of Chinese herbs. J Nat Prod 61:1575–1576
Lü JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7:293–302
Makowska Z, Keller J, Engelmann F (1999) Cryopreservation of apices isolated from garlic (Allium sativum L.) bulbils and cloves. CryoLetters 20:175–182
Martinez-Montero ME, Harding K (2015) Cryobionomics: evaluating the concept in plant cryopreservation. In: Barh D, Khan MS, Davies E (eds) Plant omics: the omics of plant science. Springer, Berlin, pp 655–682
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Oh SY, Wu CH, Popova E, Hahn EJ, Paek KY (2009) Cryopreservation of Panax ginseng adventitious roots. J Plant Biol 52:348–354
Pandhair V, Sekhon BS (2006) Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biotechnol 15:71–78
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Park SU, Kong H, Shin DJ, Bae CH, Lee SC, Bae CH, Rha ES, Kim HH (2014) Development of vitrification protocol in Rubia akane (Nakai) hairy roots using a systematic approach. CryoLetters 35:138–144
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium Sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Quain MD, Berjak P, Acheampong E, Kioko JI (2009) Sucrose treatment and explant water content: critical factors to consider in development of successful cryopreservation protocols for shoot tip explants of the tropical species Dioscorea rotundata (yam). CryoLetters 30:212–223
Ren L, Zhang D, Jiang XN, Gai Y, Wang WM, Reed BM, Shen XH (2013) Peroxidation due to cryoprotectant treatment is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci 212:37–47
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. Brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
San José MC, Valladares S, Janeiro LV, Corredoira E (2014) Cryopreservation of in vitro-grown shoot tips of Alnus glutinosa (L.) Gaertn. Acta Physiol Plant 36:109–116
Steponkus PL, Langis R, Fujikawa S (1992) Cryopreservation of plant tissues by vitrification. In: Steponkus PL (ed) Advances in low-temperature biology. JAI Press, London, pp 1–61
Suzuki M, Ishikawa M, Okuda H, Noda K, Kishimoto T, Nakamura T, Ogiwara I, Shimura I, Akihama T (2006) Physiological changes in gentian axillary buds during two-step preculturing with sucrose that conferred high levels of tolerance to desiccation and cryopreservation. Ann Bot 97:1073–1081
Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of invitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep 16:594–599
Thinh NT (1997) Cryopreservation of germplasm of vegetatively propagated tropical monocots by vitrification. Dr Pap Fac Agric Kobe Univ Japan
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. CryoLetters 27:65–72
Turgut-Kara N, Kahraman BÜ (2015) Effects of long-term culture of Astragalus chrysochlorus callus onmorphology, genetic structure, gene expression and metabolism. Plant Biosyst 149:329–336
Uchendu EE, Muminova M, Gupta S, Reed BM (2010) Antioxidant and anti-stress compounds improve regrowth of cryopreserved Rubus shoot tips. Vitro Cell Dev Biol 46:386–393
Varghese B, Naithani SC (2008) Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss.) seeds. J Plant Physiol 165:755–765
Wang RR, Gao XX, Chen L, Huo LQ, Li MF, Wang QC (2014) Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci Hortic (Amsterdam) 176:330–339
Wen B, Wang R, Cheng H, Song S (2010) Cytological and physiological changes in orthodox maize embryos during cryopreservation. Protoplasma 239:57–67
Wesley-Smith J, Berjak P, Pammenter NW, Walters C (2013) Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: an ultrastructural study of factors affecting cell and ice structures. Ann Bot 113:695–709
Wu YL, Shen XH (2011) Cryopreservation of Dendrobium wardianum Warner. protocorms by vitrification. Chin J Cell Bio 33:279–287
Xie JT, Attele AS, Yuan CS (2006) Ginseng: beneficial and potential adverse effect. In: Yuan CS, Beiber E, Bauer BA (eds) A textbook of complementary and alternative therapies. CRC Press Company, Boca Raton, London, New York, Washington, DC, pp 71–89
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell, Tissue Organ Cult 92:251–260
Yi JY, Sylvestre I, Colin M, Salma M, Lee SY, Kim HH, Park HJ, Engelmann F (2012) Improved cryopreservation using droplet-vitrification and histological changes associated with cryopreservation of Madder (Rubia akane Nakai). Korean J Hortic Sci Technol 30:79–84
Yoon JW, Kim HH, Ko HC, Hwang HS, Hong ES, Cho EG, Engelmann F (2006) Cryopreservation of cultivated and wild potato varieties by droplet vitrification: effect of subculture of mother-plants and of preculture of shoot tips. CryoLetters 27:211–222
Yoshimatsu K, Touno K, Shimomura K (2000) Cryopreservation of medicinal plant resources: retention of biosynthetic capabilities in transformed cultures. In: Cryopreservation of tropical plant germplasm: current research progress and application. Proceedings of an international workshop, Tsukuba, Japan, October 1998. International Plant Genetic Resources Institute (IPGRI), pp 77–88
Zhang D, Ren L, Gq Chen ZJ, Reed BM (2015) ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep 34:1499–1513
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Advanced Production Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (Grant No. 315013-4).
Author information
Authors and Affiliations
Contributions
K-CL contributed to data acquisition and wrote the manuscript. H-HK and K-YP participated in interpreted data and revising for intellectual content. S-YP made substantial contributions to the conception and design of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Inhwa Yeam.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Le, KC., Kim, HH. & Park, SY. Modification of the droplet-vitrification method of cryopreservation to enhance survival rates of adventitious roots of Panax ginseng. Hortic. Environ. Biotechnol. 60, 501–510 (2019). https://doi.org/10.1007/s13580-019-00150-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00150-8