Abstract
Gold nanoparticles (AuNPs) are well-known biomedical and biotechnological applications because of their interesting properties. They easily cross the cell membranes and interact with intracellular materials. This study was designed to investigate the interaction of calf spleen DNA with AuNPs at a molar ratio of 2:1 in an aqueous solution with different ionic strengths (10, 50, and 100%). AuNPs and AuNPs/DNA complex were characterized by different techniques such as UV/Vis spectrophotometry, transmission electron microscopy (TEM), dynamic light scattering (DLS), and Fourier transform IR spectrophotometry. The results revealed that the maximum absorption (λmax) of AuNPs synthesis was observed at 520 nm, and the average particle size was about 13 nm. In addition to a negative zeta potential (− 37 mV), the interaction of AuNPs with DNA was confirmed by melting point and TEM. The melting point that reflects the DNA became unstable in the presence of AuNPs, and the melting temperature decreased by about 3–5 °C with different ionic strength. Additionally, the TEM image of AuNPs/DNA complex obviously illustrated the location of AuNPs on the DNA groove. Finally, these results clearly indicate the attachment of AuNPs with DNA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is the design, production, and application of materials at atomic, molecular, and macromolecular levels to produce a new type of material at controllable size (less than 100 nm in diameter) and different shapes called nanoparticles. Nanoparticles have a unique physico-chemical property such as large surface area to volume ratio, pore structure, stability, surface plasmon resonance, and embedded effect. Consequently, in the last years nanotechnology is rapidly evolving in many research fields to discover new materials with extraordinary properties and wonderful uses [1,2,3,4].
In recent decades, gold nanoparticles (AuNPs) have received considerable attention of the researchers, when confined at the nanoscale/atomic level because they are very stable chemically, bioinert, non-toxic, and readily synthesized [5]. Moreover, their small size allows them to penetrate cells and tissues via an excellent compatibility with biomolecules; therefore, they are less likely to lead to side effects in the body. These interesting properties are having a significant impact on many scientific fields, such as chemistry, physics, biology, biomaterial sciences, and molecular biotechnology. But, particularly, medicine has some important applications that are related to diagnostic and therapeutic treatment of diseases, recognition of pathogenic agents, and drug delivery [6,7,8,9].
The science of DNA has been the center of biological science and biotechnology research since the discovery of double helical structures. Deoxyribonucleic acid (DNA) bears the hereditary information that is passed on from parents to children in organisms. DNA involves vital processes, such as gene expression, gene transcription, mutagenesis, and carcinogenesis [10, 11].
During the last decades, the interaction of DNA with AuNPs is receiving considerable interest due to their use in many important applications, such as electronics, biosensor, and catalysis, but mainly in biomedical applications like radiotherapy, drug delivery systems, and cancer treatment [12,13,14,15]. Moreover, another studies [16, 17] reported that the exploitation of biological reactivity employed in biosensor creation, nanoparticle-assisted cancer treatment, and DNA microarray optimization depends on the interactions between DNA nucleobases and noble metallic surfaces. Therefore, thermodynamic and kinetic studies of the interactions of DNA with nanoparticles acting as small ligands are key to a better understanding of those interactions to allow for their control and modulation and for the opening of new venues of research in the nanomedicine, analytic, and biologic fields. In this study, gold nanoparticles (AuNPs) were prepared, their chemical and physical characteristics were determined, and their interaction with DNA (calf spleen DNA is used in this study due to its ease of preparation and high purity and quantity) was investigated in buffer with different ionic strengths.
Materials and methods
Materials
Gold (III) chloride trihydrate (MW 393.83 g/mol), trisodium citrate (sodium citrate tribasic dehydrate MW 294.10), sodium chloride, isoamyl alcohol, chloroform, sodium dodecyl sulfate, and ethanol were purchased from the Sigma-Aldrich (Germany).
Preparation of gold nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) were prepared by chemical reduction method. Briefly, 20 ml of 1 mM HAuCl4 solution was stirred on a hot plate magnetic stirrer (MS 300 HS Mischung Scientific Co., Ltd.) just to boil. Two milliliters of 1% trisodium citrate was added drop by drop into the solution for reducing gold ions; the color gradually deepened [18].
Isolation of DNA and incubation with AuNPs
Calf spleen DNA was extracted accordingly to the method described in Walker and Rapley [19]. Stock solution of 2 mM DNA was prepared by dissolving the DNA in SSC buffer (0.015 sodium chloride and 0.015 M trisodium citrate) with different ionic strengths (100%, 50%, 10%) at 5 °C for 24 h to ensure the formation of a homogeneous solution.Then, equal volumes from AuNPs (1 mM) and DNA (2 mM) in different ionic strength were mixed and incubated at room temperature for 10 min. The pH of aqueous solutions was adjusted by 1 M HCl or 1 M NaOH.
Characterization of AuNPs and AuNPs/DNA complex
The absorption spectra of AuNPs, DNA, and AuNPs/DNA complex solutions were measured by Jenway, 6405 UV/VIS Spectrophotometer, Barloworld Scientific, Essex, UK, in the wavelength range of 200–700 nm.
The size and morphology of AuNPs were studied by transmission electron microscope (HR-TEM, Tecnai G20, FEI, Netherland). DNA/AuNPs complex was also studied by transmission electron microscope (JEOL JEM 1400) after stained with a negative stain. All the samples were diluted, and then a few drops of the sample were dried on a microscope slide for examination.
The size distribution and zeta potential for AuNPs were measured by the dynamic light scattering apparatus (Zetasizer Nano ZS Malvern Instruments Ltd., UK) at 25 °C dispersed in deionized water.
The structural characteristics of AuNPs, DNA, and AuNPs/DNA complex were determined by Fourier transform IR spectrophotometer (Basic Vector, 22FT-IR, Germany). Dried homogeneous samples and KBr were mixed and then pressed into pellets for transforming IR spectral measurement in the frequency range of 400–4,000 cm−1.
The thermal denaturation of DNA and AuNPs/DNA complex were measured by a spectrophotometer (Jenway model 6405 (Barloworld Scientific, Essex, UK)) with a jacketed cuvette. The cuvette temperature was monitored by a copper constant thermocouple with 0.1 °C resolution. The thermocouple was inserted into the cuvette through the tight-fitting Teflon stopper. The cuvette temperature was raised at a rate of 1 °C/3 min from 25 to 87 °C by circulating water as a thermo controller (Thermomix R, B. Braun Biotech International, Germany). Optical densities were recorded at wavelength 260 nm. Since examined profiles were almost linear in the melting region, melting temperature (Tm) was determined as the average of starting and final temperatures of the melting process.
Results and discussion
Gold nanoparticles (AuNPs) are considered the most successfully developed and examined metallic nanoparticles, which became the foundation for the modern nanotechnology due to their extraordinary properties. Recently, these properties expanded the applications of gold nanoparticles into various biotechnology and biomedical fields [20,21,22,23].
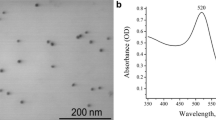
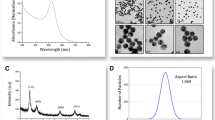
In the current study, AuNPs were prepared according to the method of Merza et al. [18] and were characterized using several techniques. AuNPs morphology and size were observed by TEM (Fig. 1). TEM image shows well-detached and not aggregated round-shaped nanoparticles that have a diameter between 12.4 and 16.4 nm. Dynamic light scattering (DLS) measurement confirmed this result as presented in Fig. 2. The figure represents a typical size distribution graph for AuNPs. As shown in the figure, the size of AuNPs is centered on 12.99 nm with relatively fine distribution. Also, DLS was used to determine the electro-kinetic surface potential for AuNPs (Fig. 3). The shape of the curve reveals a narrow dispersion of the value of zeta potential which is centered on − 37 mV. This value demonstrated that the capping and reducing molecules (trisodium citrate) present on the surface of AuNPs are negatively charged groups and achieve high stability and prevent the aggregation of the dispersed particles [24].
UV/Vis spectroscopy is one of the most obvious method for characterizing the optical properties and electronic structure of nanoparticles, biological molecules, and their interactions. Figure 4 shows the absorption spectrum of DNA solution, where DNA reveal a maximum absorption band centered around 260 nm [25]. Figure 5 shows the absorption spectra of gold nanoparticles (AuNPs) in the absence and presence of DNA at different ionic strength, where AuNPs exhibit a broad band at peak 520 nm with absorbance tail in the longer wavelength; this SPR (surface plasmon resonance) was attributed due to the dipole plasmon oscillation of the gold colloids induced by the external electric field [26, 27]. In the presence of DNA, the absorbance of AuNPs decreases markedly with the increase of ionic strength, without any change in the location of the peak (520 nm). This shows that while adding AuNPs nanoparticles to the solution of DNA, the AuNPs gets adsorbed on the surface of the DNA and is involved in the formation of a ground state complex of the type of DNA/AuNPs complex. The formed complex also has an absorption at 520 nm. These results indicate that there is an interaction between AuNPs nanoparticles and DNA in the ground state via complex formation which is electrostatic in nature. Furthermore, these results were in coincidence with another study [28] that reported that AuNPs colloid is stable, in the condition of low ionic strength due to the negative electrostatic repulsion. Increasing the ionic strength of the solution, the AuNPs colloid becomes unstable to aggregate, and the SPR decreases. But the interactions between DNA bases and AuNPs will enhance AuNPs’ stability against the salt-induced aggregation.
DNA melting is one of the most essential processes in biology and biotechnology. Figure 6 shows that the DNA melting profiles in different ionic strengths 10, 50, and 100% have a melting temperature (Tm) at 61, 69, and 82 °C, respectively. These results reveal that the effect of ionic strength on DNA melting temperature has been one of the major activities of polyelectrolyte theory, where the ionic strength contributes to the stability of the DNA and acts as shielding agents for the negatively charged strands of DNA [29,30,31]. Figure 7 shows the melting profiles for AuNPs/DNA complex in different ionic strength (10, 50, and 100%). It is obvious from the figure; DNA became unstable in the presence of AuNPs, and the melting temperature decreased by about 3–5 °C with different ionic strength. These results are in accordance with a further study [32] that reported that the decrease in Tm is due to the high affinity of mononucleotides and polynucleotides for small nanoparticles of gold and which bind externally with DNA groove. Additionally, these data were supported by the by TEM image in Fig. 8, where AuNPs with spherical shapes are obviously located on the DNA groove.
FT-IR measurements were performed in the absence and presence of AuNPs with DNA (shown only in the region of 500–4000 cm−1 (Fig. 9)), to verify the type of interaction. For only the DNA sample, two low intensities but prominent peaks appear at a wavenumber of 620 and 854 cm−1, which is due to the presence of a deoxyribose sugar unit on the DNA structure. There are two other peaks at 1046 and 1155 cm−1 which are due to the stretching vibration of C–O–C and C–C bonds. There is another peak at 1278 cm−1 which is due to the asymmetric stretching vibration of the PO2− group. Additionally, two other peaks appearing at 1428 and 1591 cm−1 are due to the bending of the C–H bond in the CH2 group. The broad peak appearing at 3451 cm−1 for only DNA is due to –N–H and –O–H stretching [33, 34]. The FT-IR analysis of AuNPs shows a broader peak observed at 3424 cm−1 which is attributed to the O–H. Smaller peaks observed at 1062, 1395, and 1590 cm−1 confirmed the existence of aromatic C-O bonds, N–H bonds, and C-H stretching as shown in Fig. 9 [35, 36]. The FT-IR analysis of DNA/AuNPs complex represented in Fig. 9 has a shape identical to that of pure DNA without any loss of or addition of peaks but a decrease in the absorbance. These results have proven that the mode of interaction between DNA and AuNPs is electrostatic in nature.
Conclusions
From the present work, it can be concluded that there is a primary interaction/attachment of DNA, with spherical AuNPs at different ionic strength. However, further studies are still needed to confirm these interactions.
Data Availability
These data are available and applicable.
References
Ramanavicˇius A, Ramanavicˇiene A, Malinauskas A (2006) Electrochemical sensors based on conducting polymer—polypyrrole. Electrochim Acta 51:6025–6037
Hagens WI, Oomen AG, deJong WH, Cassee FR (2007) What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol 49:217–229
Kathiravan A, Renganathan R (2009) Photoinduced interactions between colloidal TiO2 nanoparticles and calf thymus-DNA. Polyhedron 28:1374–1378
Pal SL, Jana U, Manna PK, Mohanta GP, Manavalan R (2011) Nanoparticle: an overview of preparation and characterization. J Appl Pharm Sci 01(06):228–234
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-sized related properties and applications towards biology, catalysts, and nanotechnology. Chem Rev 104:293–346
Henz BJ, Hawa T, Zachariah MR (2008) Mechano-chemical stability of gold nanoparticles coated with alkanethiolate SAMs. Langmuir 24(3):773–783
Jain S, Hirst DG, O’Sullivan JM (2012) Gold nanoparticles as novel agents for cancer therapy. Br J Radiol 85:101–113
Ajnai G, Chiu A, Kan T, Cheng CC, Tsai TH, Chang J (2014) Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Clin Med 6:172–178
Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ, Wang W (2017) Unique roles of gold nanoparticles in drug delivery. Target Imaging Appl Mol 22:1445
Miller EC, Miller JA (1981) Mechanisms of chemical carcinogenesis. Cancer 47:1055
Travers A, Muskhelishvili G (2015) DNA structure and function. FEBS Journal 282:2279–2295
Han YD, Park YM, Chun HJ, Yoon HC (2015) A low-cost optical transducer utilizing common electronics components for the gold nanoparticle based immune sensing application. Chem 220:233–242
Nita R, Trammell SA, Ellis GA, Moore MH, Soto CM, Leary DH, Fontana J, Talebzadeh SF, Knight DA (2016) Kinetic analysis of the hydrolysis of methyl parathion using citrate-stabilized 10 nm gold nanoparticles. Chemosphere 144:1916–1919
Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B (2019) Gold nanoparticles in cancer treatment. Mol Pharmaceutics 16(1):1–23
Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov’yov AV, Prise KM, Golding J, Mason NJ (2016) Gold nanoparticles for cancer radiotherapy. Cancer Nanotechnol 7(1):8
Iglesias E (2020) Gold nanoparticles as colorimetric sensors for the detection of DNA bases and related compounds. Mol 25:2890
He Z, Wang G, Liang X, Takarada T, Maeda M (2021) DNA base pair stacking assembly of anisotropic nanoparticles for biosensing and ordered assembly. Anal Sci 37(3):415–423
Merza KS, Al-Attabi HD, Abbas ZM, Yusr HA (2012) Comparative study on methods for preparation of gold nanoparticles. Green Sustain Chem 2:26–28
Walker JM, Rapley R (2008) Molecular biomethods handbook. Humana Press, a part of Springer Science Business Media, LLC
Tripathi RM, Shrivastav A, Shrivastav BR (2015) Biogenic gold nanoparticles: as a potential candidate for brain tumor directed drug delivery. Artif Cell Nanomedicine and Biotechnology 43:311–317
Faraday M (2007) Michael Faraday’s recognition of ruby Au: the birth of modern nanotechnology. Au Bull 40:267–269
Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R (2008) Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem 391:943–950
Luo PG, Stutzenberger FJ (2008) Nanotechnology in the detection and control of microorganisms. Adv Appl Microbiol 63:145–181
Nwokeoji AO, Kilby PM, Portwood DE, Dickman MJ (2017) Accurate quantification of nucleic acids using hyperchromicity measurements in conjunction with UV spectrophotometry. Anal Chem 89:13567–13574
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B 103(21):4212–4217
Huang X, El-Sayed MA (2010) Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res 1(1):13–28
Zuo X, Wu H, Toh J, Li SFY (2010) Mechanism of mercury detection based on interaction of single-strand DNA and hybridized DNA with gold nanoparticles. Talanta 82:1642–1646
Contreras-Trigo B, Díaz-García V, Guzmán-Gutierrez E, Sanhueza I, Coelho P, Godoy SE, Torres S, Oyarzún P (2018) Slight pH fluctuations in the gold nanoparticle synthesis process influence the performance of the citrate reduction method. Sensors 18(7):2246
Singh A, Singh N (2015) Effect of salt concentration on the stability of heterogeneous DNA. Physica A 419:328–334
Weber G, Haslam N, Essex JW, Neylon C (2009) Thermal equivalence of DNA duplexes for probe design. J Phys: Condens Matter 21(13):4106
Rouzina I, Bloomfield VA (1999) Heat capacity effects on the melting of DNA. 1. Gen Asp Biophys J 77:3242–3251
Prado-Gotor R, Grueso E (2011) A kinetic study of the interaction of DNA with gold nanoparticles: mechanistic aspects of the interaction. Phys Chem Chem Phys 13:1479–1489
Sakthikumar K, Anantharaj S, Ede SR, Karthick K, Kundu S (2016) A highly stable rhenium organosol on a DNA scaffold for catalytic and SERS applications. J Mater Chem C 4(26):6309–6320
Rolim T, Cancino J, Zucolotto V (2015) A nanostructured genosensor for the early diagnosis of systemic arterial hypertension. Biomed Microdevices 17:3
Ahmad T, Irfan M, Bhattacharjee S (2016) Study on gold nanoparticle synthesis using aqueous Elaise guineensis (oil palm) leaf extract: effect of precursor concentration. Procedia Eng 148:1396–1401
Neupane MP, Lee SJ, Park IS, Lee MH, Bae TS, Kuboki Y, Uo M, Watari F (2011) Synthesis of gelatin-capped gold nanoparticles with variable gelatin concentration. J Nanopart Res 13:491–498
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rageh, M.M., Gaber, M.H. & Mostafa, S.M. Effect of ionic strength on the interaction of AuNPs with calf spleen DNA. Gold Bull 56, 23–30 (2023). https://doi.org/10.1007/s13404-022-00322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-022-00322-y