Abstract
In this work, the performance of carotenoids extraction from Rhodotorula spp red yeast (strain ELP2022) using supercritical CO2 (CO2-SFE) was compared to the traditional technique with organic solvent. For this purpose, the yeast was cultured in liquid medium, pre-treated with glass beads in 0.1 M NaHCO3, and lyophilized. The extraction by CO2-SFE was carried out using a bench scale equipment at 300, 400, and 500 bar whilst maintaining a constant CO2 flow rate (6 mL/min) and temperature (40 °C) resulting in an average extraction yields of 60.8 ± 1.1, 68.0 ± 1.4, and 67.6 ± 1.4 µg of total carotenoids per g of yeast (dry weight), respectively. Based on these results, three other experiments at 400 bar and a CO2 flow rate of 6 L/min were also performed. In specific, the temperature was increased up to 60 °C, and ethanol as a co-solvent was added at 40 and 60 °C. The results showed that the temperature does not have a significant effect on the extraction of carotenoids. On the contrary, the yields improved significantly in the presence of the co-solvent, and the percentage of recovery reached the mean values of 71.70% ± 1.4 and 73.86% ± 1.9 at 40 and 60 °C, respectively. Furthermore, from chromatographic analysis, four major peaks were observed and identified as torularhodin, torulene, γ-carotene, and β-carotene which represented about 53.4%, 6.4%, 8.3%, and 26.9% of total carotenoids, respectively. Therefore, these promising results show the potential of this green technique for obtaining high-value products, such as carotenoids, from yeasts and therefore avoiding the use of dangerous solvents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carotenoids are a group of pigments naturally present in plants, algae, and microorganisms that have beneficial health activities due to their antimicrobial, antioxidant, anticancer, and immune response-stimulating properties [1,2,3,4,5].
Therefore, carotenoids have a significant commercial interest, and the related worldwide market in 2019 was about $ 1.5 billion [6].
For their production, mainly chemically synthesised processes are currently used, as the extraction of carotenoids from natural matrices is still limited [7].
There is, however, a growing interest in the recovery of carotenoids from green biotechnological processes and eco-sustainable matrices such as agro-food waste [8,9,10,11].
In this context of valorization of eco-sustainable matrices, red yeasts, due to their ability to grow even in large quantities on the latter low-cost substrates, represent an excellent alternative source for producing carotenoids [12]. Many studies evaluate the production of carotenoids from yeasts by investigating different microbial growth factors [13,14,15]. The type and quantity of carotenoids synthesised by yeasts vary according to the species. In the literature, it is widely reported that yeasts belonging to the genera Rhodotorula and Sporobolomyces, in addition to β-carotene, synthesise specific carotenoids such as torulene and torularhodin [11, 16, 17]. Since plants do not synthesise them, the latter two are scarcely present in human and animal diets and could therefore be successfully used as food additives [18, 19].
In addition, they are characterised by robust antioxidant, antitumor, and antimicrobial activity, on par with other important molecules such as polyphenols [20, 21] which points them toward potential use in the pharmaceutical and cosmetic industries [16, 22,23,24]. However, to fully exploit the biotechnological potential of these bioactive compounds, effective and efficient extraction processes need to be developed [9].
Traditionally, carotenoid extraction techniques involve using organic solvents such as petroleum ether, hexane, chloroform, diethyl ether, acetone, benzene, methanol, ethanol, and acetone [25]. Despite their different degrees of toxicity, many of these solvents have been tested and used for their ability to extract carotenoids from yeasts in several studies [10, 15, 26, 27].
An alternative technique for extracting carotenoids is using fluids in supercritical conditions [28]. A fluid is considered supercritical when its temperature and pressure are above the critical point, having a liquid-like density, high diffusivity, and low viscosity like a gas. Thanks to these properties, a fluid under supercritical conditions can diffuse into a matrix more remarkably than organic solvents, enhancing the efficiency of carotenoids extraction [29, 30].
Carbon dioxide (CO2-SFE) is the most used in biotechnological applications amongst supercritical fluids. This toxic waste-free process does not require solvent removal in the extracts [31]. Furthermore, the parameters can be adjusted so that the process temperature does not cause the degradation of thermolabile compounds such as carotenoids [32].
Data regarding the extraction of carotenoids from various vegetable matrices and microalgae using CO2-SFE are available in the literature [33,34,35,36,37].
Conversely, based on our knowledge, there are only a few data regarding the extraction of carotenoids from yeasts. However, an early study by Lim et al. dated 2002, investigated using CO2-SFE to separate astaxanthin from the yeast Phaffia rhodozyma [38].
Subsequently, Wang et al. (2012) extracted β-carotene from red yeast cells [39]. Martinez et al. (2020) [9] evaluated how pulsed electric field technology can improve carotenoids extraction from Rhodotorula glutinis. In other studies, the CO2-SFE extraction efficacy of astaxanthin from enzymatically pretreated cells of two different yeasts (Phaffia rhodozyma and Xanthophyllomyces dendrorhous) was evaluated [40, 41].
This preliminary study aimed to evaluate the extraction of carotenoids from a new isolate of red yeast Rhodotorula spp. with CO2-SFE. Different operating parameters were adopted, and the extraction yields were compared with those obtained by conventional organic solvent extraction. Fresh yeast biomass was subjected to chemical and mechanical pretreatment optimised by us, which facilitated and improved carotenoid extraction in both methods. The total carotenoids in the extracts were determined by the spectrophotometric method, and the different carotenoids present were separated and characterised by HPLC analysis.
2 Materials and methods
2.1 Culture of Rhodotorula and biomass pretreatment
The red yeast, strain ELP2022, used in this study was isolated from the surface of fresh cheese in the year 2022. It was characterised for its metabolic pattern in the YT MicroPlate™ by a semi-automated system for rapid identification (Biolog, Inc) and identified as Rhodotorula spp (Supplementary information, fig. S1). The pure culture was cryopreserved at − 80 °C in 30% glycerol at ENEA Trisaia Research Center.
To produce the microbial biomass, a loop of yeast strain was picked up from glycerol stocks and streaked onto Potato Dextrose Agar (PDA, Sigma-Aldrich, Italy) plates. After incubation of 120 h, a single red colony was used to inoculate 1 L Erlenmeyer baffled flasks containing 200 mL of the YPD medium (10 g/L yeast extract, 20 g/L peptone, and 20 g/L dextrose). Next, the flasks were incubated for 96 h at 26 °C and 130 rpm in a thermostatic orbital shaker (Thermo Scientific Forma, model 420). After this culture time, the liquid was recovered, and the biomass was separated by centrifugation (Beckman Avanti® J-25 centrifuge) at 9000 g for 10 min.
Subsequently, to ensure the extraction of carotenoids, the cell wall of the yeasts was pretreated by the following method. Briefly, the fresh biomass derived from 200 mL of culture was suspended in 16 mL of 0.1 M NaHCO3. Then, the suspension was added in a 50 mL Falcon tube filled with 8 g of glass beads (SiLibeads® type S) with a 1–1.3 mm diameter.
All materials used for pretreatment were sterilised as a precaution to avoid bacterial contamination. The Falcon tube was shaken horizontally at 130 rpm at 40 °C under dark conditions to prevent loss of pigments by degradation.
After 24 h, to monitor cell lysis, 10 µL of cell suspension was mixed with an equal volume of Trypan Blue stain and was observed with differential phase-contrast microscopy (Olympus, model BX60). The broken cell number was determined using a Burker chamber, and their percentage was calculated to the total cell number.
Finally, the cell suspension was separated from the glass beads using a mesh sieve and centrifuged at 9000 g for 10 min. The pellet was washed three times with deionised water and dried using a freeze-dryer (Martin Christ, model Alpha 1–4). The lyophilised biomass was weighed with an analytical balance (Kern, model 870) and stored at 4 °C.
2.2 Carotenoids extraction procedure with the organic solvent
To extract the carotenoids, lyophilised pretreated cells (0.2 g) were suspended in 10 mL of a mixture of [acetone:methanol] (7:3 v/v) and vortexed for 5 min. The resulting suspension was centrifuged at 8900 g, 4 °C for 10 min (Beckman Coulter-centrifuge Allegra TM 2IR). The supernatant was recovered, and the extraction process was repeated 4 times on the solid suspension to reach a complete extraction. The entire solvent (40 mL) was concentrated at 35 °C with a rotary evaporator (Steroglass Rotary Evaporator Instruments Kentron-Strike 202). After complete solvent evaporation, the weight of the red pigments was determined and kept at − 20 °C until use for the quantification of total carotenoids and HPLC analysis. All operations were conducted under dark conditions to avoid loss of pigments through degradation.
2.3 CO2 supercritical extraction on a bench scale system
The extraction unit used for the trials was the bench scale equipment (Applied Separations Spe-ed SFE-2, Allentown, PA) that could reach temperatures and pressures up to 250 °C and 680 bar.
The system is fed by a cylinder containing CO2 and, as schematically represented in Fig. 1, consists of the following parts: two pumps (P-01 and P-02) that respectively compress the gas and the co-solvent at working pressures; an oven inside which there is a flow preheater (PH) and the extraction vessel (EX) which is loaded with the glass beads and the pretreated yeast biomass; a separator (vial) in which the extracts are recovered. Along the line, there are manual valves (V) and probes for pressure (PC) and temperature (TC) monitoring.
In and out pressures were modulated using manual valves (Wika Transmitter, Milano, Italia), whilst CO2 feed was controlled up to 10 L/min using a flowmeter (LPN/S80 ALG 2.5, Sacofgas, Italia).
In addition, the system could use a co-solvent from a specific line fed with a syringe pump (Speed SFE Modifier Pump Module-PN 7170-Applied Separations).
Stainless steel tubes with different capabilities (32, 50, and 300 mL) can be used as extraction vessels. To avoid material transport, metal filters with 5-micron pores were used.
All parameters were controlled by EasyCom2011 software version 2.0.5.16 (WIKA Alexander Wiegand SE & Co. KG).
In specific, pretreated biomass (about 0.3 g) was loaded into a 50 mL extraction vessel (14 mm inner diameter) for all experiments. In addition, to prevent biomass agglomeration and increase the contact surface area between biomass and CO2, 44 g of glass microspheres with a diameter of 3 mm were inserted into the vessel. The extractions were all carried out for 90 min with a constant flow of CO2 (6 L/min).
The influence of three different pressures (300, 400, and 500 bar) at 40 °C on carotenoid extraction yield was studied.
Experimental evidence described below identifies 400 bar pressure as the best performing. Therefore, all further investigations were conducted on this pressure parameter. Both the effect of temperature (40 °C and 60 °C) and the use of ethanol as an extraction co-solvent (flow rate of 0.5 mL/min) on carotenoid recovery were evaluated. One experimental condition was changed at a time, and the investigation was conducted in triplicates. Extracts were recovered in amber vials, weighed, and stored at 4 °C for subsequent analysis.
2.4 Determination of total carotenoids and chromatographic profile
After each extraction cycle, the sample was collected in an amber vial, and the total carotenoid content was measured using the following spectrophotometric method.
The samples of red pigments were resuspended in ethanol stabilised with 0.2% BHT (butylhydroxytoluene) (w/v) and directly used to measure the absorbance at 453 nm using a spectrophotometer (Thermo Scientific—Multiscan GO). The total carotenoids amount was calculated as follows:
where A is the absorbance; V is the total volume of sample solution (mL); \({E}_{1cm}^{1\%}\) is the specific extinction coefficient of β-carotene for ethanol (2620 mL g−1 cm−1).
The carotenoid yield (CY) was expressed in terms of µg/g and was given following the formula:
where TC is the total carotenoids (µg) in the extract and Wlb is the weight (g) of lyophilised biomass.
Furthermore, the effect of operating parameters with SFE-CO2 was evaluated by comparison of the carotenoids yields with those obtained using organic solvent. It was expressed as a recovery percentage and was given by the following formula:
where CY(SFE) and CYwhere CY(SFE) and CY(CS) are the total carotenoids yields obtained using the CO2-SFE process and conventional solvent, respectively.
The different carotenoids present in the extract were separated by an Agilent 1200 series high-performance liquid chromatography (HPLC) system consisting of an in-line degasser (G1379B), binary pump (G1312B), auto-sampler (G1367B), column temperature controller (G1316A), UV–Vis detector (G1314B), and a diode array detector (DAD) (G1315A). Before analysis, the extracts were resuspended in 1 mL ethanol with 0.2% (w/v) BHT, filtered through a 0.22 μm Millipore filter, and added into 2 mL vials. The separation was achieved on a C-18 reverse-phase analytical column (Zorbax RX-C18 4.6 × 250 mm, 5 μm) following the method reported by Ghiraldi et al. (2020) [42]. Specifically, the column was kept at 25 °C using an injection volume of 20 μL and a 1 mL/min flow rate. Acetone (solvent A) and water (solvent B) were used as mobile phase at the following gradient elution: 75% A (initial composition), min 0–10 linear gradient from 75% A to 95% A; min 10–17 at 95% A; min 17–20 linear gradient from 95% A to 100% A; min 20–30 at 100% A; min 30–35 linear gradient back to the initial conditions at 75% A.
Direct UV absorption detection was performed at the characteristic wavelength for β-carotene (453 nm). Online spectra were recorded between 350 and 650 nm, and an identification tentative was performed comparing, for each peak, the retention times (RT) and the wavelengths for the maximum absorbance (λ max) with literature data. Synthetic β-carotene, purchased from Sigma-Aldrich (PHR1239-1G), was dissolved in hexane and diluted in ethanol with BHT to prepare standard stock solutions. All data were collected and analysed using the software OpenLAB CDS Chemstation Edition Rev. C.01.10(201).
Each sample was analysed three times, so carotenoids yields and recovery percentage averages with standard deviations were calculated. The means were separated by Tukey’s HSD test when the analysis of variance showed statistical significance (α = 0.05).
3 Results and discussion
3.1 Yeast culture and pretreatment
In the present study, a combined method for cell lysis, using glass beads and sodium bicarbonate, was coupled with both conventional [acetone:methanol] (7:3 v/v) and SFE-CO2 extraction.
It has been recognised that cell disruption represents an essential step for the extraction of intracellular compounds from yeast cells, exerting significant effects on their recovery and purification [43]. Monks et al. (2013) [44], in their work focused on the cell disruption of Sporidiobolus salmonicolor (CBS 2636) for releasing carotenoids by different treatment methods, observed that the maximum amount of carotenoids was obtained after enzymatic hydrolysis. From our preliminary results (data not shown), the extraction yields of carotenoids were significantly increased by using pretreated biomass over untreated one.
In accordance with the findings reported by da Fonseca et al. (2011) [45], the efficacy of our pretreatment method could be mainly due to the use of glass beads rather than to the sodium bicarbonate effect. Indeed, these authors, evaluating different cell disruption methods of the yeast Phaffia rhodozyma, concluded that the method with Na2CO3 was not effective; whilst the agitation with glass pearls increased the extraction yields.
The percentage of damaged cells, which appeared internally coloured by Trypan Blue assay (Fig. 2), was higher than 98% after pretreatment.
3.2 Chromatographic profile of carotenoids in extracts of red yeast ELP2022 strain
The separation of the carotenoids present in the extracts, performed by HPLC, allowed observing 4 prominent peaks (Fig. 3a) representing about 95% of the total areas of the peaks.
These peaks can be potentially identified by their characteristic UV/Vis spectrum and their retention time as torularhodin (retention time = 12.061 min), torulene (retention time = 15.553 min), γ-carotene (retention time = 15.759 min), and β-carotene (retention time = 17.031 min). In addition, the β-carotene peak in the extract was confirmed by the chromatographic profile of the β-carotene standard, which exhibited the peak at the same retention time (Fig. 3b).
Torularhodin was the major component and represented about 53.4% of total carotenoids (Table 1). It had maximum adsorption at 498 nm (Supplementary information, fig. S2) and a shorter retention time, compared to the other carotenoids, probably due to its carboxylic group (Fig. 4) [46]. β-carotene was the second most abundant component with an average percentage of about 26.9% and maximum adsorption at 453 nm. Torulene and γ-carotene represented about 6.4% and 8.3% with a maximum adsorption at 483 and 462 nm, respectively.
3D chemical structures of main carotenoids present in red yeast extracts (strain ELP2022) with maximum absorption referred to the spectrum in acetone. Images obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/search/search.cgi)
In several independent studies, similar profiles for carotenoid extracts have been reported. However, their concentrations and their percentage ratios may vary depending on the metabolic synthesis of the particular species of Rhodotorula used and are strongly influenced by the culture conditions [11, 13].
Elfeky et al. (2019) showed that the production of specific carotenoids compounds by Rhodotorula glutinis could be enhanced under a high C/N ratio when ammonium sulphate was used as a nitrogen source in combination with a low C/S ratio [47].
3.3 Total carotenoids extraction by CO2 supercritical fluid bench-scale instrument (CO2-SFE)
CO2-SFE can be an efficient, green, and suitable technique for the recovery of carotenoids compounds from red yeasts. In this study, the first approach evaluated the effect of different extraction pressures maintaining a constant CO2 flow rate (6 L/min) and temperature (40 °C) regarding total carotenoids.
The results in Table 2 show that the extraction carried out at 400 bar increased yields in comparison to yields at 300 bar. In specific, the average extraction yields were 60.8 ± 1.1, 68.0 ± 1.4, and 67.6 ± 1.4 µg/g DW (dry weight) at 300, 400, and 500 bar, respectively.
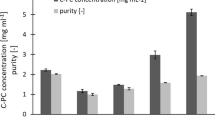
Conversely, by further increasing the pressure up to 500 bar, differences in recovery percentage were not observed, as shown in Fig. 5.
Although carotenoids extraction could increase with pressure as it improves solubility and cell destruction [37, 48], our study did not verify this prediction, probably because the pretreatment already completely destroyed the yeast cells.
Based on the results of this preliminary approach, three other experiments at 400 bar and a CO2 flow rate of 6 mL/min were also performed. Specifically, the temperature was increased to 60 °C, and ethanol as co-solvent was added at 40 and 60 °C.
From the results shown in Fig. 6, the temperature did not significantly affect the extraction of carotenoids.
Indeed, the effect of temperature on solubility is complex and difficult to predict.
At constant pressure, increasing the temperature, the solubility of most solutes increases. However, the density of the solvent decreases, reducing its solvating capacity [49].
Furthermore, the thermal instability of carotenoids that increases with temperature increasing [50] is not irrelevant.
These results are in line with the work of Wei et al. (2005) [51], in which it is stated that the effect of temperature on the solubility of carotenoids was insignificant as compared to the effect of pressure.
On the contrary, the extraction yields improved significantly with the addition of co-solvent, and the recovery percentage reached the mean values of 71.70% ± 1.4 and 73.86% ± 1.9 at 40 and 60 °C, respectively (Fig. 6).
This observation is widely confirmed in the literature. Although carotenoids are very low polarity compounds, their high molecular weight can cause reductions of solubility in CO2. Therefore, adding ethanol may increase the dissolution of heavier substances and thereby improving their extraction [52, 53].
Finally, considering that the total content of carotenoids, extracted from the yeast using the organic solvent, was 123.2 ± 3.1 (μg/g DW) and the minimum amount of carotenoids extracted using CO2-SFE was 60.8 ± 1.1 (μg/g DW), the minimum recovery rate is 49.37%.
These yields were higher than those reported by Martinez et al. (2020) [9]. These authors investigated the pulsed electric fields (PEF) as pretreatment technology coupled with CO2-SFE achieving very low yields. However, like us, they also observed significant yield increases by adding ethanol as a co-solvent.
The difference between our results and those of Martinez et al. (2020) is most likely due to the different pretreatment performed.
However, the results align with the previous work of Lim et al. (2002) [38]. Here, the authors destroyed the Phaffia rhodozyma red yeast cells using a bead mill, obtaining high yields for all extractions performed at operating parameters similar to ours.
Despite the wide availability of data regarding carotenoid synthesis in red yeast, information is lacking for this specific interest area where supercritical CO2 was investigated as an alternative solvent.
Based on our knowledge, in addition to the two already discussed works, two other independent studies regarding the extraction of astaxanthin from enzymatically lysed cells of Phaffia rhodozyma and Xanthophyllomyces dendrorhous by SFE-CO2 were reported [40, 41]. In particular, Harit et al. (2020), under the best conditions investigated, reached a maximum value of ~ 45% astaxanthin extractability.
Therefore, combining supercritical CO2 extraction with different pretreatment methods (i.e. chemical, mechanical, and enzymatic) represents an essential focus for further research to maximise the recovery of carotenoids from red yeasts.
4 Conclusions
In this work, the extraction of carotenoids from a red yeast belonging to the Rhodotorula genus (strain ELP2022) was carried out using CO2 under supercritical conditions. After pretreatment, the yeast biomass was dried and subsequently subjected to extractions. From the comparison of carotenoids yields with those obtained by the traditional extraction method, a positive effect of the pressure up to 400 bar is highlighted. On the other hand, the increase in temperature from 40 to 60 °C was irrelevant.
Furthermore, adding ethanol as a co-solvent allowed for increased carotenoid extraction.
Four major compounds were highlighted from the chromatographic profile of the extracts, and they were identified as torularhodin, torulene, γ-carotene, and β-carotene.
Considering the lack of data in the literature on the extraction of carotenoids from yeast using CO2-SFE, the present study shows the potential of this green technique in combination with an economical and environmentally friendly biomass pretreatment.
Moreover, this approach avoids the presence of residual solvents of class 1 and 2 in the extracts, which should be respectively not employed or limited in pharmaceuticals according to ICH guidelines Q3C(R8).
Therefore, the present study provides valuable information for subsequent investigations involving the recovery and application of these pigments for cosmetic and pharmaceutical purposes.
Data availability
Data generated during the current study will be made available from the corresponding authors on reasonable request.
References
Ambrico A, Trupo M, Magarelli R et al (2020) Effectiveness of dunaliella salina extracts against bacillus subtilis and bacterial plant pathogens. Pathogens 9:1–14. https://doi.org/10.3390/pathogens9080613
Amengual J (2019) Bioactive properties of carotenoids in human health. Nutrients 11:1–6. https://doi.org/10.3390/nu11102388
Tinoi J, Rakariyatham N, Deming RL (2005) Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem 40:2551–2557. https://doi.org/10.1016/j.procbio.2004.11.005
Moratilla-Rivera I, Marta S, Antonio J (2023) Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int J Mol Sci 24(4):3748. https://doi.org/10.3390/ijms24043748
Montesano V, Negro D, De Lisi A et al (2021) Agronomic performance and phenolic profile of Tritordeum (x Tritordeum martinii A. Pujadas) lines. Cereal Chem 98:382–391. https://doi.org/10.1002/cche.10378
Igreja WS, Maia FDA, Lopes AS (2021) Biotechnological production of carotenoids using low cost-substrates is influenced by cultivation parameters : a review. Int J Mol Sci 22:8819. https://doi.org/10.3390/ijms22168819
Martínez-Cámara S, Ibañez A, Rubio S et al (2021) Main carotenoids produced by microorganisms. Encyclopedia 1:1223–1245. https://doi.org/10.3390/encyclopedia1040093
Kot AM, Błażejak S, Kurcz A et al (2016) Rhodotorula glutinis—potential source of lipids, carotenoids, and enzymes for use in industries. Appl Microbiol Biotechnol 100:6103–6117. https://doi.org/10.1007/s00253-016-7611-8
Martínez JM, Schottroff F, Haas K et al (2020) Evaluation of pulsed electric fields technology for the improvement of subsequent carotenoid extraction from dried Rhodotorula glutinis yeast. Food Chem 323:126824. https://doi.org/10.1016/j.foodchem.2020.126824
Saini RK, Keum YS (2018) Carotenoid extraction methods: a review of recent developments. Food Chem 240:90–103. https://doi.org/10.1016/j.foodchem.2017.07.099
Tang W, Wang Y, Zhang J, Cai Y, He Z (2019) Biosynthetic pathway of carotenoids in Rhodotorula and strategies for enhanced their production. J Microbiol Biotechnol 29(4):507–517. https://doi.org/10.4014/jmb.1801.01022
Mezzomo N, Ferreira SRS (2016) Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem 2016:1–16. https://doi.org/10.1155/2016/3164312
Allahkarami S, Sepahi AA, Hosseini H, Razavi MR (2021) Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol Reports 32:e00687. https://doi.org/10.1016/j.btre.2021.e00687
Cardoso LAC, Jäckel S, Karp SG et al (2016) Improvement of Sporobolomyces ruberrimus carotenoids production by the use of raw glycerol. Bioresour Technol 200:374–379. https://doi.org/10.1016/j.biortech.2015.09.108
Hladnik L, Vicente FA, Grilc M, Likozar B (2022) β-Carotene production and extraction: a case study of olive mill wastewater bioremediation by Rhodotorula glutinis with simultaneous carotenoid production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03081-0
Kot AM, Błazejak S, Gientka I et al (2018) Torulene and torularhodin: “new” fungal carotenoids for industry? Microb Cell Fact 17:49. https://doi.org/10.1186/s12934-018-0893-z
Mussagy CU, Gonzalez-Miquel M, Santos-Ebinuma VC, Pereira JFB (2022) Microbial torularhodin – a comprehensive review. Crit Rev Biotechnol 43(4):540–558. https://doi.org/10.1080/07388551.2022.2041540
Ungureanu C, Ferdes M (2012) Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv Sci Lett 18:50–53. https://doi.org/10.1166/asl.2012.4403
Zoz L, Carvalho JC, Soccol VT et al (2015) Torularhodin and torulene: bioproduction, properties and prospective applications in food and cosmetics - a review. Brazilian Arch Biol Technol 58:278–288. https://doi.org/10.1590/S1516-8913201400152
Magrone T, Spagnoletta A, Bizzoca A et al (2019) Polyphenol effects on splenic cytokine response in post-weaning contactin 1-overexpressing transgenic mice. Molecules 24:1–14. https://doi.org/10.3390/molecules24122205
Nesci S, Oppedisano SA, F, (2023) Inflammation, mitochondria and natural compounds together in the circle of trust. Int J Mol Sci 24:6106. https://doi.org/10.3390/ijms24076106
Sandmann G (2022) Carotenoids and their biosynthesis in fungi. Molecules 27(4):1431. https://doi.org/10.3390/molecules27041431
Ungureanu C, Barbulescu L, Dumitriu C et al (2021) Titanium industrial residues surface modification towards its reuse as antimicrobial surfaces. Environ Sci Pollut Res 28:38224–38237. https://doi.org/10.1007/s11356-021-13359-x
Li J, Qian H, Pi F, Wang B-X (2022) Bioavailability evaluation of the intestinal absorption and liver accumulation of torularhodin using a rat postprandial model. Food Funct 13:5946–5952. https://doi.org/10.1039/D1FO03707B
Liu D, Ding L, Sun J et al (2016) Yeast cell disruption strategies for recovery of intracellular bio-active compounds — a review. Innov Food Sci Emerg Technol 36:181–192. https://doi.org/10.1016/j.ifset.2016.06.017
Park PK, Kim EY, Chu KH (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Purif Technol 53:148–152. https://doi.org/10.1016/j.seppur.2006.06.026
Aksu Z, Eren AT (2007) Production of carotenoids by the isolated yeast of Rhodotorula glutinis. Biochem Eng J 35:107–113. https://doi.org/10.1016/j.bej.2007.01.004
Crampon C, Boutin O, Badens E (2011) Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind Eng Chem Res 50:8941–8953. https://doi.org/10.1021/ie102297d
Manjare SD, Dhingra K (2019) Supercritical fluids in separation and purification: a review. Mater Sci Energy Technol 2:463–484. https://doi.org/10.1016/j.mset.2019.04.005
Akanda MJH, Sarker MZI, Ferdosh S et al (2012) Applications of supercritical fluid extraction (SFE) of palm oil and oil from natural sources. Molecules 17:1764–1794. https://doi.org/10.3390/molecules17021764
Molino A, Mehariya S, Iovine A et al (2018) Extraction of astaxanthin and lutein from microalga haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar Drugs 16(11):432. https://doi.org/10.3390/md16110432
Georgiopoulou I, Tzima S, Louli V, Magoulas K (2022) Supercritical CO2 extraction of high-added value compounds from Chlorella vulgaris: experimental design, modelling and optimization. Molecules 27(18):5884. https://doi.org/10.3390/molecules27185884
Döker O, Salgin U, Şanal I et al (2004) Modeling of extraction of β-carotene from apricot bagasse using supercritical CO2 in packed bed extractor. J Supercrit Fluids 28:11–19. https://doi.org/10.1016/S0896-8446(03)00006-8
Shi J, Yi C, Ye X et al (2010) Effects of supercritical CO2 fluid parameters on chemical composition and yield of carotenoids extracted from pumpkin. LWT - Food Sci Technol 43:39–44. https://doi.org/10.1016/j.lwt.2009.07.003
Di Sanzo G, Mehariya S, Martino M et al (2018) Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from haematococcus pluvialis microalgae. Mar Drugs 6(9):334. https://doi.org/10.3390/md16090334
Molino A, Larocca V, Di Sanzo G et al (2019) Extraction of bioactive compounds using supercritical carbon dioxide. Molecules 24(4):782. https://doi.org/10.3390/molecules24040782
Ludwig K, Rihko-Struckmann L, Brinitzer G et al (2021) β-Carotene extraction from Dunaliella salina by supercritical CO2. J Appl Phycol 33:1435–1445. https://doi.org/10.1007/s10811-021-02399-y
Lim G-B, Lee S-Y, Lee E-K et al (2002) Separation of astaxanthin from red yeast Phaffia rhodozyma by supercritical carbon dioxide extraction. Biochem Eng J 11:181–187. https://doi.org/10.1016/S1369-703X(02)00023-2
Lou WS, Liu W, Wang HX, Lv CH (2012) The extraction of β-carotene from red yeast cells by supercritical carbon dioxide technique. Adv Mater Res 554–556:949–952. https://doi.org/10.4028/www.scientific.net/AMR.554-556.949
Hasan M, Azhar M, Nangia H et al (2016) Influence of high-pressure homogenization, ultrasonication, and supercritical fluid on free astaxanthin extraction from β-glucanase-treated Phaffia rhodozyma cells. Prep Biochem Biotechnol 46:116–122. https://doi.org/10.1080/10826068.2014.995807
Harith ZT, Lima M de A, Charalampopoulos D, Chatzifragkou A (2020) Optimised production and extraction of astaxanthin from the yeast Xanthophyllomyces dendrorhous. Microorganisms 8(3):430. https://doi.org/10.3390/microorganisms8030430
Ghilardi C, Sanmartin Negrete P, Carelli AA, Borroni V (2020) Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour Bioprocess 7:52. https://doi.org/10.1186/s40643-020-00341-7
Šovljanski O, Saveljić A, Tomić A et al (2022) Carotenoid-producing yeasts: selection of the best-performing strain and the total carotenoid extraction procedure. Processes 10(9):1699. https://doi.org/10.3390/pr10091699
Monks LM, Rigo A, Mazutti MA et al (2013) Use of chemical, enzymatic and ultrasound-assisted methods for cell disruption to obtain carotenoids. Biocatal Agric Biotechnol 2:165–169. https://doi.org/10.1016/j.bcab.2013.03.004
da Fonseca dos Santos RA , Rafael da Silva R, Kalil SJ et al (2011) Different cell disruption methods for astaxanthin recovery by Phaffia rhodozyma. African J Biotechnol 10:1165–1171. https://doi.org/10.5897/AJB10.1034
Moliné M, Libkind D, Van Broock M (2012) Production of torularhodin, torulene, and β-carotene by rhodotorula yeasts. Methods Mol Biol 898:275–283. https://doi.org/10.1007/978-1-61779-918-1_19
Elfeky N, Elmahmoudy M, Zhang Y et al (2019) Lipid and carotenoid production by Rhodotorula glutinis with a combined cultivation mode of nitrogen, sulfur, and aluminium stress. Appl Sci 9(12):2444. https://doi.org/10.3390/app9122444
Yu T, Niu L, Iwahashi H (2020) High-pressure carbon dioxide used for pasteurization in food industry. Food Eng Rev 12:364–380. https://doi.org/10.1007/s12393-020-09240-1
Abou Elmaaty T, Sayed-Ahmed K, Elsisi H, Magdi M (2022) Optimization of extraction of natural antimicrobial pigments using supercritical fluids: a review. Processes 10(10):2111. https://doi.org/10.3390/pr10102111
Derrien M, Aghabararnejad M, Gosselin A et al (2018) Corresponding author : Yacine Boumghar using response surface methodology. LWT - Food Sci Technol. https://doi.org/10.1016/j.lwt.2018.03.016
Wei PC, May CY, Ngan MA, Hock CC (2005) Supercritical fluid extraction of palm carotenoids. Am J Environ Sci 1:264–269. https://doi.org/10.3844/ajessp.2005.264.269
Pereira CG, Meireles MAA (2010) Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food Bioprocess Technol 3:340–372. https://doi.org/10.1007/s11947-009-0263-2
de Andrade LM, Charalampopoulos D, Chatzifragkou A (2018) Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J Supercrit Fluids 133:94–102. https://doi.org/10.1016/j.supflu.2017.09.028
Funding
Open access funding provided by Ente per le Nuove Tecnologie, l'Energia e l'Ambiente within the CRUI-CARE Agreement. Open Access Funding provided by ENEA, Italian National Agency for New Technologies, Energy and Sustainable Economic Development, within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this work and agreed to publish this version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Larocca, V., Martino, M., Trupo, M. et al. Evaluation of carbon dioxide supercritical fluid extraction (CO2-SFE) on carotenoids recovery from red yeast cells. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04434-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04434-z