Abstract
Phycocyanin is an interesting alternative to synthetic food colorants. Various methods to obtain phycocyanin from Arthrospira (Spirulina) biomass have been described in the literature, including ultrasonication, glass bead extraction and freeze-thawing. In this study, three optimized procedures were implemented to assess their efficacy in obtaining phycocyanin from Arthrospira maxima biomass, facilitating a comparative analysis of their effectiveness. After harvesting the biomass, extraction processes were conducted utilizing ultrasonication followed by flocculation with chitosan in various organic acid solutions, as well as glass bead extraction and freeze-thawing techniques, each followed by centrifugation. The obtained extracts were analyzed spectrophotometrically across the wavelength range of 280 to 800 nm. The freeze-thawing method yielded the highest C-PC contents at 17.03 ± 0.53%, followed closely by the ultrasonication method at 15.21 ± 0.41%. The highest purity of 2.02 ± 0.01 was attained through ultrasonication and subsequent flocculation with chitosan in acetic acid. Conversely, employing chitosan dissolved in citric or lactic acid for flocculation resulted in greenish extracts containing high amounts of chlorophyll.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years consumer awareness of the potential risks posed by certain food additives has increased. This is particularly evident in the case of synthetic food colorants, as several of these dyes have been shown to elevate the risk of developing cancer or immunological diseases (Martelli et al. 2014). Consequently, laws prohibit the use of known harmful substances in food production. However, other synthetic food dyes remain available for industrial purposes due to their cost-effectiveness, high efficacy, reliability, and chemical stability (Chen et al. 1998). Specifically concerning blue food colorants, the industry faces a shortage of alternatives to synthetic dyes (Newsome et al. 2014).
One alternative could be phycocyanin (PC), a protein found in cyanobacteria (C-PC) and Rhodophyta (R-PC), where it plays a major role in photosynthesis as an accessory pigment of bright cobalt-blue color (Horváth et al. 2013; Singh et al. 2015). The PC molecule consists of two subunits, α (ca. 19 kDa) and β (ca. 21 kDa). Within the cell, the PC mostly occurs in its trimeric (αβ)3 or hexameric (αβ)6 form, displaying a ring-like structure of one ring (trimer) or two stacked rings (hexamer) (Abalde et al. 1998). PC is hydrophilic and belongs to the group of phycobiliproteins (PBP). The bright blue color of PC is due to the covalently bound chromophore phycocyanobilin (PCB), a tetrapyrrole derivative attached to the apoprotein by thioether bounds at the 84th amino acid in both the α and the β subunit. Additionally, a third PCB group is attached to the 155th amino acid of the β subunit. The amino acid sequence of PC mostly forms helical areas, displaying a topological structure similar to the heme group in the myoglobin molecule (Stec et al. 1999). In cyanobacteria, C-PC hexamers are part of the so-called phycobilisomes (PBS). These PBS are protein structures with antenna-like protein stacks consisting of C-PC and phycoerythrin (PE), another PBP of reddish color. The antennas are attached to a third light-blue PBP, allophycocyanin (APC), which itself is attached to photosystem II within the thylakoid membrane of cyanobacteria and eukaryotic chloroplasts. These PBS enable the utilization of light energy by electron transfer for the photosystem II, making it possible for the cyanobacterium to perform photosynthesis (Samsonoff and MacColl 2001; Singh et al. 2015). Moreover, it also has been demonstrated that photosystem I is also provided with energy from phycobilins (Mullineaux 2008; Singh et al. 2015).
In addition to its use as a food colorant, phycocyanin (PC) is recognized for its antioxidative capacity and is therefore a subject of ongoing research. The incorporation of phycocyanin into a mayonnaise product enhanced its antioxidative capacity, qualifying the resulting product as a functional food (Khorsand et al. 2021). Studies investigating the impact of C-PC in the diet of European seabass under heat stress conditions have demonstrated its ability to enhance the fish's resistance to heat stress (Islam et al. 2021). Similarly, research on Nile tilapia subjected to heat stress has yielded comparable findings, albeit with a much higher dosage of C-PC per kilogram of feed used in the experiment (El-Araby et al. 2022). Furthermore, the beneficial effects of supplementing feed with C-PC have been observed in mammals, particularly rabbits (Abdelnour et al. 2020).
The majority of phycocyanin (PC) for industrial demand is typically extracted from cyanobacteria, specifically Arthrospira platensis or Arthrospira maxima, commonly referred to as Spirulina (Eriksen 2008; Sekar and Chandramohan 2008; Moraes et al. 2011). Like all cyanobacteria, Spirulina performs oxygenic photosynthesis to obtain energy for the synthesis of sugar molecules. Spirulina consists of unicellular species that collectively form long helically-shaped filaments (Tomaselli 1997). For optimal growth, Spirulina requires a temperature between 30 and 35°C, and a pH between 9 and 11 (Usharani et al. 2012). The natural habitats of Spirulina are tropical or subtropical water bodies with high concentrations of carbonates and bicarbonates (Rajasekaran et al. 2016). The beneficial value of Spirulina for human nutrition is contributed by the high protein content of 55-70 % by reference to the dry matter content (Oliveira et al. 1999; Babadzhanov et al. 2004; Aouir et al. 2017). Besides, Spirulina is rich in polysaccharides, unsaturated fatty acids, vitamins, minerals and antioxidative substances like C-PC (Rajasekaran et al. 2016; Jung et al. 2019). It is also assumed, that the intake of large amounts of intact bacteria cells has a positive impact on the competence of the immune system and displays anti-inflammatory, antioxidative, and anti-carcinogenic properties (Hayashi et al. 2006; Eriksen 2008). That makes the cyanobacterium itself a valuable food for human nutrition. The United States Food and Drug Administration allowed the use of Spirulina products in 2013 for various food categories like bakery products, ice cream, beverages, and chewing gums (FDA 2013). The market volume for Spirulina products in 2016 was estimated to be 700 million US dollars and predicted to reach 2 billion US dollars by 2026 (Soni et al. 2021).
For the extraction of phycocyanin, various methods have been described, including freeze-thawing (Doke 2005; Prabhath et al. 2019; Tan et al. 2020), glass bead extraction (Moraes et al. 2011), and ultrasonication (Furuki et al. 2003). A common problem in C-PC extraction is the presence of chlorophyll in the extract (Doke 2005; Günerken et al. 2015; Li et al. 2020). In this study, three different extraction methods were compared to evaluate the C-PC yield, purity, and selectivity obtained by these methods. Fresh biomass harvested from one single culture was used to perform all three extractions simultaneously. Freeze-thawing and glass bead disruption were performed followed by centrifugation. As an interesting alternative, ultrasonication and subsequent flocculation with chitosan-acid solution was established. This method offers the advantage of replacing the expensive centrifugation step with flocculation, which can be more easily scaled up.
Materials and methods
Spirulina cultivation
Arthrospia maxima UTEX 2342 (purchased from Culture Collection of Algae, University of Texas, Austin, USA). It was cultivated in a 10 L algabag (algatec GbR, Germany) in half-concentrated Spirulina medium (by Culture Collection of Algae Göttingen, Germany, version of March 2007) at 25°C for 33 days. The culture was aerated and the light intensity was set at 63 µmol photons m-2 s-1 emitted by VALOYA C75 DIM spectrum AP67 (Valoya Ltd, Finland). Biomass increase was measured using photometric absorption measurement at 800 nm (OD800).
Biomass harvesting
The biomass was harvested during the exponential growth phase at OD800 = 1.32 and concentrated by filtering through a 40 µm mesh tissue. The concentrated biomass was then washed twice by adding deionized water in 50 mL tubes (1:2 w/v), thoroughly shaken, centrifuged (3,500 rpm, 10 min), and the supernatant was discarded. The washed biomass was stored at 4°C for 18 h. Subsequently, the dry matter content was measured thermo-gravimetrically and the biomass was used for C-PC extraction. In total, 28.83 g of wet biomass with a dry matter content of 12.58 % were obtained and used for the three different extraction procedures described below.
Ultrasonication-assisted extraction
For cell lysis and C-PC extraction using ultrasonication, 15 g of the harvested biomass were mixed with 135 g of deionized water (1:10, w/w) to adjust the dry matter content to 1.26 %. The cell suspension was then processed using a UP100H ultrasonic processor with MS7D sonotrode (0,8 s interval, 100 % intensity) and D7K flow-through cell (Hielscher Ultrasonics GmbH, Germany). The processing was carried out using a peristaltic pump (100 mL s-1) and ultrasonicated for 27 min (equivalent to 18 flow-through cycles). After confirming successful cell disruption by microscopy, the cell suspension was divided into 3 subsamples, each with a volume of 50 mL, and stored at 4°C for 1 h. Each subsample was then mixed with 5 g of a 1 % chitosan Heppix AS solution (Separ Chemie GmbH, Germany). The chitosan was dissolved beforehand in either acetic acid, citric acid, or lactic acid (10 % acid concentration each). After adding the different chitosan-acid solutions, all three subsamples were stirred for 10 min at 80 rpm and then filtered with a 60 µm plankton sieve. The resulting filtrates were considered the C-PC extracts, and the pH and absorption spectrum were measured.
Freeze-thawing
The remaining biomass (10 g) was mixed with 40 mL CaCl2 solution (10 g L-1). An aliquot of 2 mL was taken and used for glass bead extraction (as described in the next chapter). The remaining cell suspension was divided into several micro reaction tubes and frozen at -80°C. After 18 h, the cell suspension was thawed at room temperature for 4 h and then refrozen at -80°C. After another 20 h, the cell suspension was thawed for 4 h and then centrifuged (10,000 rpm, 30 min, 4°C). The supernatants were measured photometrically.
Glass bead extraction
A 2 mL aliquot of the cell suspension in CaCl2 solution (10 g L-1), as mentioned before, was used for glass bead extraction. For every replicate, 500 µL of the cell suspension was pipetted into a micro reaction tube already filled with 500 mg of glass beads (Ø 0.25 – 0.5 mm; Verder Scientific GmbH & Co. KG, Germany). The micro reaction tubes were applied to a bead mill (Retsch bead mill MM301; Verder Scientific) and underwent cell lysis using 4 disruption cycles with 30 Hz for 25 s each with 30 s of cooling phase in between. After disruption, the samples where kept on ice and then centrifuged (10,000 rpm, 30 min, 4°C). The bluish supernatants were measured photometrically.
Photometric analysis
The C-PC concentration and purity were determined by measuring the absorption spectrum from 280 to 800 nm using a Genesys 50 UV/VIS spectrophotometer (Thermo-Fisher Scientific Inc., USA). Prior to measurement, the extracts were appropriately diluted with deionized water to ensure they fell within the linear measuring range. Concentrations and purities were calculated using equations originally proposed by Bennett and Bogorad (1973):
where CC-PC is C-PC concentration in the extract; Ax is absorption of the final extract at the wavelength x; purityC-PC is the purity of the C-PC in the extract calculated as the ratio of absorptions at 620 nm and 280 nm. C-PC content is expressed as percentage (w/w) (representing gram C-PC per 100 gram dry matter). The selectivity as the ratio of the absorption at 620 nm and 680 nm was elected as an indicator to evaluate the presence of undesired chlorophyll a in the extract.
where AX is absorption of the final extract at the wavelength X.
The mean absorption spectra of all five extracts were generated by calculating the arithmetic mean of the measured wavelengths for each extract. Subsequently, the five mean absorption spectra were normalized by setting the absorption at 620 nm as 1 (equivalent to 100 %) and assigning relative values to all other absorptions based on this reference point. To achieve this, the lowest measured absorption was subtracted from every absorption in the spectrum. Then, each of these values was multiplied by the reciprocal of the absorption at 620 nm (after subtracting the lowest measured absorption).
Statistical analysis
The glass bead extraction as well as the freeze-thawing extraction were conducted as separate triplicates (n=3). The three results were utilized to compute the arithmetic mean (x̄) and standard deviation (SD). Conversely, the ultrasonication-assisted extraction was not performed in separate triplicates. Instead, the cell suspension was prepared in a single batch. Subsequent to cell disruption, this suspension was divided into three subsamples, and each was treated with a different chitosan-acid solution for flocculation. The extract resulting from each of the three different treatments was measured three times (n=3). Following measurement, the three results for each extract were used to calculate the arithmetic mean (x̄) and standard deviation (SD).
Results
The five resulting C-PC extracts (ultrasonication with chitosan-acetic acid, chitosan-citric acid, and chitosan-lactic acid flocculation solution, glass bead extraction, and freeze thawing) were analyzed spectrophotometrically. The dry matter content of the initial biomass (washed and concentrated) was 12.58 ± 0.84 %. The total yield of dry matter from the culture was 0.76 g L-1. For the ultrasonication-assisted extraction, the dry matter content of the cell suspension was adjusted to 1.26 % with deionized water. For both glass bead extraction and freeze-thawing extraction, the dry matter content was adjusted to approximately 2.5 % with CaCl2 solution.
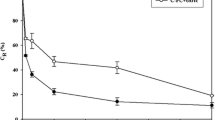
The C-PC concentrations of the five different extracts were: 5.11 ± 0.16 mg mL-1 (freeze-thawing), 2.98 ± 0.20 mg mL-1 (glass bead extraction), 2.23 ± 0.06 mg mL-1 (ultrasonication and subsequent chitosan-acetic acid flocculation), 1.17 ± 0.08 mg mL-1 (ultrasonication and subsequent chitosan-citric acid flocculation), and 1.48 ± 0.02 mg mL-1 (ultrasonication with subsequent chitosan-lactic acid flocculation). The highest purity of C-PC was observed in the ultrasonicated sample with subsequent chitosan-acetic acid flocculation (2.02 ± 0.01) and the freeze-thawed sample (1.94 ± 0.01). The lowest purity was found in the ultrasonicated samples followed by chitosan-citric and lactic acid flocculation (1.00 ± 0.06 and 1.28 ± 0.06, respectively). The extract obtained by glass bead extraction and centrifugation had a purity of 1.58 ± 0.01. C-PC concentration and purity of the five final extracts are displayed in Fig. 1. With regard to the dry matter content used for the different extraction methods, the highest C-PC content was 17.03 ± 0.53 % for the freeze-thawed samples, followed by ultrasonication with subsequent chitosan-acetic acid flocculation (15.21 ± 0.41 %). The C-PC content was lower for glass bead extraction (10.92 ± 0.74 %) and for the ultrasonication followed by chitosan-lactic acid flocculation (10.11 ± 0.13 %) and chitosan-citric acid flocculation (8.02 ± 0.58 %). The results are presented in Fig. 2.
C-PC concentration and purity of extracts obtained from A. maxima by different extraction methods: ultrasonication followed by flocculation with chitosan in acetic acid (A), citric acid (B), lactic acid (C); glass bead extraction followed by centrifugation (D); freeze-thawing followed by centrifugation (E); values display the arithmetic mean of the triplicates (n=3); error bars show the standard deviations
C-PC contents of A. maxima biomass obtained by different extraction methods: ultrasonication followed by flocculation with chitosan in acetic acid (A), citric acid (B), lactic acid (C); glass bead extraction followed by centrifugation (D); freeze-thawing followed by centrifugation (E); values display the arithmetic mean of the triplicates (n=3); error bars show the standard deviations
The normalized absorption spectra of the five different extracts (Fig. 3) all showed a distinct peak with maximum absorption at 620 nm (ultrasonication with flocculation) or 616 nm (glass bead extraction and freeze-thawing). The ultrasonicated samples with flocculated with chitosan-citric and lactic acid solution additionally showed higher absorption in the area from 280 nm to 480 nm and around 680 nm. The glass bead extracted and the freeze-thawed samples displayed a marginal higher absorbance at around 650 nm.
Normalized absorption spectra of C-PC extracts obtained from A. maxima by different extraction methods: ultrasonication followed by flocculation with chitosan in acetic acid (A), citric acid (B), lactic acid (C); glass bead extraction followed by centrifugation (D); freeze-thawing followed by centrifugation (E)
The ratio of the absorption of 620 nm to 680 nm used as an indicator for the selectivity of the extraction method was highest for freeze-thawing (27) and glass bead extraction (22) (Fig. 4). The bluish extract of the ultrasonicated sample treated with chitosan-acetic acid solution had a ratio of around 15. The ultrasonicated samples treated with chitosan-citric and lactic acid flocculation had values of ca. 4 and 2, respectively.
Ratio of absorption at 620 nm (A620) to absorption at 680 nm (A680) as an indicator for the selectivity of C-PC extracts obtained from A. maxima by different extraction methods: ultrasonication followed by flocculation with chitosan in acetic acid (A), citric acid (B), lactic acid (C); glass bead extraction followed by centrifugation (D); freeze-thawing followed by centrifugation (E); values display the A620/ A680-values of the normalized spectra
The final pH of the ultrasonicated extracts was 4.03 (for chitosan-acetic acid flocculation), 3.14 (for chitosan-citric acid flocculation), 3.02 (for chitosan-lactic acid flocculation). The pH of the extract obtained by bead mill extraction was 7.14, while the freeze-thawing extract had a pH of 6.49. The freeze-thawed extract, the extract from glass bead extraction and the one from ultrasonication using chitosan-acetic acid solution for flocculation exhibited an intense bluish colour (‘cobalt blue’), while the ultrasonicated samples with citric and lactic acid-containing flocculation solution displayed a more greenish proportion (‘aqua green’) in their color composition (Fig. 5).
Discussion
While A. platensis is well known for its C-PC content and has been the subject of various studies regarding extraction methods, publications on the extraction of C-PC from A. maxima are less numerous. However, at the cellular level, both, A. platensis and A. maxima exhibit remarkable similarity, despite minor differences in cell morphology and the trichomes have been described (Tomaselli 1997). The chemical composition of both species is also very similar with respect to protein, carbohydrate, and lipid content, as well as the fatty acid composition. Contrarily, A. maxima demonstrated a better growth when culturing temperature was chosen to be between 20 and 40°C. At optimal culturing temperature of 30°C, the protein content in A. maxima was slightly higher than in A. platensis, while not varying significantly at other temperatures (Oliveira et al. 1999).
CPC concentration and content
The freeze-thawing method, which has been described as one of the most effective and simplest methods for C-PC extraction (Tan et al. 2020), provided the highest C-PC concentration (5.11 mg mL-1) in this study. This value exceeds most reported values for C-PC concentration in literature for C-PC extracts from A. maxima (Nisticò et al. 2022) and A. platensis (Silveira et al. 2007; Moraes et al. 2011; Aoki et al. 2021). For glass bead extraction, conducted with the same biomass concentration as the freeze-thawing extraction, the C-PC concentration was found to be 2.98 mg mL-1. The lower C-PC concentration in the glass bead extracts (approximately 60% compared to freeze-thawing approach) can be attributed to less effective cell disruption. The ultrasonication method, which utilized lower biomass concentrations, led to extracts with C-PC concentrations ranging from 2.23 to 1.17 mg mL-1. The C-PC concentration in the resulting extract is strongly influenced by the dry matter concentration used, while the C-PC yield per dry matter is not. However, Tan et al (2020) stated that in freeze-thawing extraction, even a dry matter contents of 4 % can lead to reduced C-PC contents compared to 0.5 and 2 %, which gave higher C-PC contents not significantly differing among each other. Initial dry matter contents of more than 8 % were shown to result in high concentrated cell suspensions in which extractant efficiency is reduced (Silveira et al. 2007).
In this study the freeze-thawing method obtained the highest C-PC content (17.03 %) indicating the most effective extraction procedure. This was followed by the ultrasonication method combined with flocculation using chitosan in acetic acid (15.21 %). These values exceed those reported in most other publications for C-PC extraction from A. platensis using various methods and for extraction from A. maxima via stirring for 24 h (Nisticò et al. 2022). A comparison of the results from this study with those from other publications can be found in Tab. 1. In all of these publications, deionized water or sodium phosphate buffers were used for C-PC extraction. However, in this study, a calcium chloride solution was used because previous experiments (results not shown) demonstrated its superiority over other extractants (deionized water, phosphate buffers). Therefore, the high C-PC contents obtained via freeze-thawing can be partially attributed to the choice of extractant. This contrasts with the results on the efficiency of different extractants, which found no significant differences between sodium chloride solution, calcium chloride solution, deionized water, and phosphate buffer (pH 7) in terms of the C-PC concentration obtained (Silveira et al. 2007). Interestingly, Tan et al. (2020) who also employed the freeze-thawing extraction method, reported a similar C-PC content of 17.28 % (and also a similar purity). However, Ruiz-Domínguez et al. (2019) using spray-dried A. maxima powder demonstrated that less common cell disruption methods such as high-pressure homogenization and microwaving, but also freeze-thawing, could result in even higher C-PC contents of 29.2 %, 21.1%, and 22.6 %, respectively. This is more than the assumed average C-PC content of 14-20 % in Spirulina dry matter (Ali and Saleh 2012; Vernès et al. 2015), indicating that both, the high efficiency of the extraction methods and a high protein or C-PC content of the Spirulina cells due to the strain characteristics or the optimization of culturing conditions contributed to the high C-PC contents. For A. platensis, even simple cell lysis in deionized water could result in a higher C-PC content of 21.1 % (Aoki et al. 2021) using A. platensis NIES-39 strain and SOT medium for cultivation.
The wide range of results reported in literature and this study cannot be solely attributed to the different extraction methods employed, but rather to variations in protein content within the cells. The protein content of A. platensis typically ranges from 55 to 70 % of dry matter (Oliveira et al. 1999; Babadzhanov et al. 2004; Aouir et al. 2017). The C-PC content in A. platensis has been reported to lie between 14 % (Ali and Saleh 2012) and 20 % (Vernès et al. 2015) of dry matter, indicating that more than 20 % of the entire proteome in this cyanobacterium is contributed by C-PC. On the other hand, the chemical composition of Spirulina is strongly dependent on the culturing conditions used to grow the biomass (Oliveira et al. 1999; Olguín et al. 2001; Markou et al. 2012; Marrez et al. 2014). For instance, the protein content in A. maxima has been shown to increase at lower temperatures (20-30°C) during cultivation, while higher temperatures lead to an increase in carbohydrate content of the cells (Oliveira et al. 1999). The choice of culture medium also influences the protein content, with the BG-11 medium resulting in higher protein amounts in the biomass compared to modified BG-11 and Zarrouk Medium (Marrez et al. 2014). Standardized media often yield higher protein contents in the final biomass than media utilizing secondary raw materials, likely due to the lack of accessible nitrogen, leading to nitrogen deficiency in the cells when cultivated with non-standardized media (Olguín et al. 2001; Marrez et al. 2014). Additionally, the various available strains display a wide variety in chemical composition which can be attributed to their natural habitat (Aouir et al. 2017). The protein content of Spirulina cells also depends on the light intensity applied during cultivation. Studies have shown that reduced illumination contributed to higher protein contents in A. platensis (Olguín et al. 2001; Markou et al. 2012). Varying culture conditions make the comparability of different extraction methods published in literature difficult.
Purity
The extract obtained by ultrasonication with subsequent chitosan-acetic acid flocculation displayed the highest purity with 2.02. Second highest purity was found in the freeze-thawed extract (1.94). These values are higher than in most other publications for C-PC from A. maxima (Nisticò et al. 2022) and A. platensis (Doke 2005; Silveira et al. 2007; Martínez et al. 2017; Prabhath et al. 2019; Aoki et al. 2021). Higher values for the purity can be achieved by further purification with ammonium sulphate precipitation, dialysis or additional filtration steps (Athiyappan et al. 2024). A purification method combining ammonium sulphate precipitation and reversed phase high-performance liquid chromatography was shown to increase the purity of a C-PC extract from 0.89 to 4.35 (Zhou et al. 2024). Amarante et al. (2020) used ion exchange chromatography with pH gradient elution to obtain C-PC extracts with purities of 4.2 and 3.5. An increase in purity can affect the bioactive properties of the phycocyanin. Zhou et al. (2024) have demonstrated that analytical grade C-PC shows higher antioxidative capacity, anti-inflammatory activity and emulsifying activity compared to the food-grade crude extract. In general, purity values of higher than 0.7 are considered food grade and a purity values of more than 4.0 are considered analytical grade (Rito-Palomares et al. 2001). Therefore, all the extracts obtained in this study are food grade.
Since the purity is defined as the ratio between phycocyanin (A620) and aromatic amino acids (A280), a lower purity indicates a relatively increased contaminant protein concentration in the extract. This can be the consequence of a higher degree of cell disruption corresponding to a lower selectivity of the whole extraction method due to the division of particles that otherwise would be easily separated from the extract (Furuki et al. 2003). Also, nucleotides and nucleic acids contribute to the absorption at 280 nm (Voet et al. 1963). Compared to incubation in deionized water, the extraction of C-PC by ultrasonication was demonstrated to rapidly decrease the purity value of the resulting extract. The purity also decreased when ultrasonication lasted for longer than 20 min which is assumed to be the consequence of the release of proteins from cell organelles (Tavakoli et al. 2021). Furthermore, the pH influencing the water-solubility of disintegrated cell proteins can contribute to a varying C-PC purity. In fact, protein isolates from A. platensis were shown to have their lowest solubility at a pH of 3 corresponding to the isoelectric point of the protein isolate (Devi et al. 1981). The solubility of the proteins is increasing when pH is decreased (pH 2) and also when the pH is increased (pH 4-10) (Benelhadj et al. 2016). This is, more or less, in accordance with the purities found in this study, where the extract obtained by ultrasonication with chitosan-acetic acid flocculation (pH 4.03) had the highest purity, followed by the freeze-thawed extract (pH 6.49), and the bead milled extract (pH 7.14) showing the lowest purity of the three bluish extracts. The two ultrasonicated extracts with citric acid and lactic acid containing flocculation solution had pH values close to the isoelectric point of spirulina protein isolate (3.14 and 3.02, respectively), but lower purities. Since the C-PC concentration of these two extracts was also lower compared to the extract obtained by ultrasonication and flocculated with chitosan-acetic acid solution, the pH of the final extract apparently affected the C-PC stability causing it to denaturize. The maximum stability for C-PC lies between pH 5 and 7.5 (Sarada et al. 1999; Chaiklahan et al. 2012). At pH 4, a slight and at pH 3, a massive decrease of C-PC concentration could be observed. This is attributed to unfolding of the protein structure leading to precipitation (Wu et al. 2016). A change in the protein conformation by proteolytic digestion of C-PC was demonstrated to decrease the absorption at 620 nm while simultaneously increasing the absorption in the UV range (with a peak at 350 nm) which is associated with the folding of the PCB chromophores (Debreczeny et al. 1989). This can explain the higher absorption in the area of 280 to 380 nm for the ultrasonicated samples as a result of degradation due to the low pH.
The method used to separate the extract from the cell debris can also contribute to different purity values of the extract. The ultrasonicated samples were processed by chitosan flocculation and filtration instead of centrifugation. Based on the methodology, the final extract may contain residues of chitosan. Since chitosan is a well-known coagulating agent due to its high number of charged amino acid groups, it can bind proteins and flocculate them out of the extract (Li et al. 1992). This has maybe furtherly contributed to the high purity of the ultrasonicated extract with chitosan-acetic acid flocculation. In this case, the low purity for the ultrasonicated samples containing citric acid and lactic acid is rather implicated by the decrease in C-PC concentration due to the low pH than the increase of protein concentration. However, the presence of chitosan in the extract can also cause practical problems for the further processing in cosmetic or food industry (unwanted flocculation or chemical reactions). Concerning the food legislations, at least the use of chitosan originating from shrimp shells could be problematic. On the other hand, the chitosan from the white button mushroom (Agaricus bisporus) has already been classified GRAS (generally recognized as safe) for certain applications in food industry by the FDA (FDA 2022). For Europe, Chitosan extracts from fungi (A. bisporus or Aspergillus niger) have been authorized as food supplements by the European Commission (EC 2017).
Selectivity
In previous extraction experiments (results not shown), the quality was often reduced by the presence of chlorophyll a resulting in a greenish or greyish color of the extract. Therefore, an indicator to display the ratio of C-PC and chlorophyll a using the ratio of absorptions at 620 nm (absorption maximum of C-PC) and at 680 nm (an absorption maximum of chlorophyll a) was established in this study. This A620/A680-ratio, that was considered the selectivity, was highest for the extracts obtained by freeze-thawing and glass bead extraction (27 and 22, respectively). The selectivity for the ultrasonicated extracts was lower than 20 in all three samples (flocculation with chitosan dissolved in acetic acid, citric acid, and lactic acid).
Excessive cell disruption can cause higher proportions of unwanted substances in the final extract, since the increased degree of cell constituent destruction leads to the solution of substances that are aimed to be separated from the extract with the solid parts (Furuki et al. 2003). There are extraction methods that have already been shown to reduce the abundance of chlorophyll a in C-PC extracts from A. platensis, e.g. pulsed electric fields treatment (PEF) (Jaeschke et al. 2019; Li et al. 2020) or high-pressure processing (HPP) (Li et al. 2020) when compared to ultrasonication. In comparison to homogenisation of the biomass with mortar and pestle, the freeze-thawing method yielded C-PC extracts with lower chlorophyll contents (Sarada et al. 1999), indicating, that rough physical methods show lower selectivity. On the other hand, bead milling is associated with a reduced selectivity, due to the formation of small cell debris particles (Günerken et al. 2015). Contrary to this, the glass bead extracts in this study, showed a high selectivity compared to the ultrasonicated samples, which can best be observed looking at the normalized absorption spectres. But regarding the low C-PC content of 10,92 % obtained in this experiment, a non-completed cell disruption can contribute to a selectivity higher than expectable. Acid extraction also was described to result in C-PC extracts with significant amounts of chlorophyll (Doke 2005). This is supported by the fact, that the ultrasonicated extracts with low pH values (ranging from 3 - 4) showed the highest chlorophyll a contamination in this study. Furthermore, the used extraction solution has an influence on the chlorophyll concentration in the final extract. Sodium chloride solutions with concentrations of more than 5 g L-1 were shown to significantly reduce the chlorophyll concentration in C-PC extracts compared to less concentrated NaCl-solutions and deionized water (Li et al. 2020). In this study, freeze-thawing and glass bead extraction were carried out using calcium chloride (10 g L-1), which is in accordance to the findings mentioned before. Moreover, the addition of 1.5 % calcium chloride to a phosphate buffer was demonstrated to increase the C-PC yield and purity while reducing the greenish coloration of the extract (Lijassi et al. 2024). The ultrasonicated samples had lower selectivity values than the extracts yielded with freeze-thawing and glass beads. Because of the subsequent flocculation with chitosan as part of the extraction procedure, calcium chloride solution was not suitable as the extractant. Instead, deionized water was used for the ultrasonication, and can therefore explain the lower selectivity. The separation technique can also contribute to lower selectivity, since the ultrasonication was carried out accompanied by subsequent flocculation and filtration, while for freeze-thawing and glass bead extraction centrifugation was applied. However, previous experiments, that are not shown in this study, showed, that centrifugation of the ultrasonicated samples resulted in green-brownish extracts indicating a failed separation of extract and cell debris.
Actually, the contamination of C-PC extracts with high amounts of chlorophyll can bias the calculation of C-PC concentration, because the absorption of chlorophyll can interfere with the absorption of phycocyanin and allophycocyanin (Yacobi et al. 2015). Therefore, some publications have suggested the adaption of the common formula for the calculation of the C-PC concentration taking the potential presence of chlorophyll a in the final extract into account (Lauceri et al. 2017; Fabre et al. 2022). However, this bias only applies for less concentrated C-PC extracts (Yacobi et al. 2015; Lauceri et al. 2017). In literature, the adaption of the formula apparently has as yet not prevailed.
Conclusion
With the freeze-thawing method, C-PC extracts with very high C-PC concentrations and purities can be achieved. However, the repeated freeze-thawing method is very time- and energy-consuming for high biomass throughputs and therefore not suitable for industrial applications. Moreover, the final extracts contain large amounts of calcium chloride, which, depending on the further use, would have to be removed in an additional process step. This also applies for the bead mill extraction method. Additionally, the purity of the extracts obtained by glass bead extraction was lower than for the other methods used in this study. Ultrasonication-assisted extraction followed by flocculation with chitosan-acetic acid solution can be an interesting alternative for the extraction of C-PC from A. maxima. The advantages of this method are the high C-PC yield and purity. Furthermore, the ultrasonication and subsequent flocculation processes can be scaled up easily to industrial scale. Disadvantages are the relatively low selectivity and the contamination of the extract with chitosan and acetic acid from the flocculation solution.
Data availability
All external data used for this work is publicly accessible.
References
Abalde J, Betancourt L, Torres E, Cid A, Barwell C (1998) Purification and characterization of phycocyanin from marine cyanobacterium Synechococcus sp. IO9201. Plant Sci 136:109–120
Abdelnour SA, Swelum AA, Salama A, Al-Ghadi MQ, Qattan SYA, El-Hack MEA, Khafaga AF, Alhimaida AR, Almutairi BO, Ammari AA, El-Saadony MT (2020) The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital J Anim Sci 19:1046–1056
Ali SK, Saleh AM (2012) Spirulina - an overview. Int J Pharm Pharmaceut Sci 4:9–15
Aoki J, Sasaki D, Asayama M (2021) Development of a method for phycocyanin recovery from filamentous cyanobacteria and evaluation of its stability and antioxidant capacity. BMC Biotechnol 21:40
Aouir A, Amiali M, Bitam A, Benchabane A, Raghavan VG (2017) Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. Food Meas 11:913–923
Athiyappan KD, Routray W, Paramasivan B (2024) Phycocyanin from Spirulina: a comprehensive review on cultivation, extraction, purification, and its application in food and allied industries. Food and Humanity 2:100235
Babadzhanov AS, Abdusamatova N, Yusupova FM, Faizullaeva N, Mezhlumyan IG, Malikova MK (2004) Chemical composition of Spirulina platensis cultivated in Uzbekistan. Chem Nat Compd 40:276–279
Benelhadj S, Gharsallaoui A, Degraeve P, Attia H, Ghorbel D (2016) Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem 194:1056–1063
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Chaiklahan R, Chirasuwan N, Bunnag B (2012) Stability of phycocyanin extracted from Spirulina sp.: influence of temperature, pH and preservatives. Process Biochem 47:659–664
Chen Q, Mou S, Hou X, Riviello JM, Ni Z (1998) Determination of eight synthetic food colorants in drinks by high-performance ion chromatography. J Chromatogr A 827:73–81
de Amarante MCA, Corrêa Júnior LCS, Sala L, Kalil SJ (2020) Analytical grade C-phycocyanin obtained by a single-step purification process. Process Biochem 90:215–222
Debreczeny M, Gombos Z, Csizmadia V, Várkonyi Z, Szalontai B (1989) Chromophore conformational analysis in phycocyanin and in related chromopeptides by surface enhanced Raman spectroscopy. Biochem Biophys Res Commun 159:1227–1232
Devi MA, Subbulakshmi G, Devi KM, Venkataraman LV (1981) Studies on the proteins of mass-cultivated, blue-green alga (Spirulina platensis). J Agric Food Chem 29:522–525
Doke JM (2005) An Improved and Efficient method for the extraction of phycocyanin from Spirulina sp. Int J Food Eng 1:5
El-Araby DA, Amer SA, Attia GA, Osman A, Fahmy EM, Altohamy DE, Alkafafy M, Elakkad HA, Tolba SA (2022) Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 546:737413
Eriksen NT (2008) Production of phycocyanin - a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
European Commission (2017) Commission implementing regulation (EU) 2017/2470. Off J European Union 60:72–201
Fabre JF, Niangoran NUF, Gaignard C, Buso D, Mouloungui Z, Valentin R (2022) Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. Eur Food Res Technol 248:1583–1599
Furuki T, Maeda S, Imajo S, Hiroi T, Amaya T, Hirokawa T, Ito K, Nozowa H (2003) Rapid and selective extraction of phycocyanin from Spirulina platensis with ultrasonic cell disruption. J Appl Phycol 15:319–324
Günerken E, D’Hondt E, Eppink MHM, Garcia-Gonzalez L, Elst K, Wijffels RH (2015) Cell disruption for microalgae biorefineries. Biotechnol Adv 33:243–260
Hayashi O, Ono S, Ishii K, Shi YH, Hirahashi T, Katoh T (2006) Enhancement of proliferation and differentiation in bone marrow hematopoietic cells by Spirulina (Arthrospira) platensis in mice. J Appl Phycol 18:47–56
Horváth H, Kovács AW, Riddick C, Présing M (2013) Extraction methods for phycocyanin determination in freshwater filamentous cyanobacteria and their application in a shallow lake. Eur J Phycol 48:278–286
Islam MJ, Kunzmann A, Henjes J, Slater MJ (2021) Can dietary manipulation mitigate extreme warm stress in fish? The case of European seabass, Dicentrarchus labrax. Aquaculture 545:737153
Jaeschke DP, Mercali GD, Marczak LDF, Müller G, Frey W, Gusbeth C (2019) Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour Technol 283:207–212
Jung F, Krüger-Genge A, Waldeck P, Küpper J-H (2019) Spirulina platensis, a super food? J Cell Biotech 5:43–54
Khorsand H, Shadafza S, Shariati FP (2021) Enriching antioxidant properties of mayonnaise sauce by the extracted phycocyanin pigment from Spirulina platensis microalgae, 17th National Chemical Congress and Exhibition, Mashhad, Iran
Lauceri R, Bresciani M, Lami A, Morabito G (2017) Chlorophyll a interference in phycocyanin and allophycocyanin spectrophotometric quantification. J Limnol 77:169–177
Li Q, Dunn ET, Grandmaison EW, Goosen MFA (1992) Applications and properties of chitosan. J Bioact Compat Pol 7:370–397
Li Y, Zhang Z, Paciulli M, Abbaspourrad A (2020) Extraction of phycocyanin—a natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques. J Food Sci 85:727–735
Lijassi I, Arahou F, Koudi STH, Wahby A, Benaich S, Rhazi L, Wahby I (2024) Optimized extraction of phycobiliproteins from Arthrospira platensis: quantitative and qualitative assessment of C-phycocyanin, allophycocyanin, and phycoerythrin. Braz J Chem Eng
Markou G, Chatzipavlidis I, Georgakakis D (2012) Effects of phosphorus concentration and light intensity on the biomass composition of Arthrospira (Spirulina) platensis. World J Microb Biot 28:2661–2670
Marrez DA, Naguib MM, Sultan YY, Daw ZY, Higazy AM (2014) Evaluation of chemical composition for Spirulina platensis in different culture media. Res J Pharmaceut Biol Chem Sci 5:1161–1171
Martelli G, Folli C, Visai L, Daglia M, Ferrari D (2014) Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications. Process Biochem 49:154–159
Martínez JM, Luengo E, Saldaña G, Álvarez I, Raso J (2017) C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res Int 99:1042–1047
Minchev I, Petkova N, Milkova-Tomova I (2020) Ultrasound-assisted extraction of chlorophylls and phycocyanin from Spirulina platensis. Biointerface Res Appl Chem 11:9296–9304
Moraes CC, Sala L, Cerveira GP, Kalil SJ (2011) C-phycocyanin extraction from Spirulina platensis wet biomass. Braz J Chem Eng 28:45–49
Mullineaux CW (2008) Phycobilisome-reaction centre interaction in cyanobacteria. Photosynth Res 95:175–182
Newsome AG, Culver CA, Van Breemen RB (2014) Nature’s palette: the search for natural blue colorants. J Agric Food Chem 62:6498–6511
Nisticò DM, Piro A, Oliva D, Osso V, Mazzuca S, Fagà FA, Morelli R, Conidi C, Figoli A, Cassano A (2022) A combination of aqueous extraction and ultrafiltration for the purification of phycocyanin from Arthrospira maxima. Microorganisms 10:308
Olguín EJ, Galicia S, Angulo-Guerrero O, Hernández E (2001) The effect of low light flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour Technol 77:19–24
Oliveira MD, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquacult Int 7:261–275
Prabhath GPWA, Shukla SP, Kumar K, Nuwansi KKT (2019) Salinity mediated enhancement in protein and pigment content in Spirulina (Arthrospira) platensis. Indian J Biotech 18:323–329
Rajasekaran C, Ajeesh CPM, Balaji S, Shalini M, Siva R, Das R, Fulzele DP, Kalaivani T (2016) Effect of modified Zarrouk’s medium on growth of different Spirulina strains. Walailak J Ag Technol Biol Sci 13:67–75
Rito-Palomares M, Nuñez L, Amador D (2001) Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. J Chem Technol Biot 76:1273–1280
Ruiz-Domínguez MC, Jáuregui M, Medina E, Jaime C, Cerezal P (2019) rapid green extractions of C-phycocyanin from Arthrospira maxima for functional applications. Appl Sci 9:1987
Samsonoff W, MacColl R (2001) Biliproteins and phycobilisomes from cyanobacteria and red algae at the extremes of habitat. Arch Microbiol 176:400–405
Sarada R, Pillai MG, Ravishankar GA (1999) Phycocyanin from Spirulina sp: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem 34:795–801
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Silveira ST, Burkert JFM, Costa JAV, Burkert CAV, Kalil SJ (2007) Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour Technol 98:1629–1634
Singh NK, Sonani RR, Rastogi RP, Madamwar D (2015) The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J 14:268–289
Soni RA, Sudhakar K, Rana RS, Baredar P (2021) Food supplements formulated with Spirulina. In: Mandotra SK, Upadhyay AK, Ahluwalia AS (eds) Algae. Springer, Singapore, pp 201–226
Stec B, Troxler RF, Teeter MM (1999) Crystal structure of C-phycocyanin from Cyanidium caldarium provides a new perspective on phycobilisome assembly. Biophys J 76:2912–2921
Tan HT, Khong NMH, Khaw YS, Ahmad SA, Yusoff FM (2020) Optimization of the freezing-thawing method for extracting phycobiliproteins from Arthrospira sp. Molecules 25:3894
Tavakoli S, Hong H, Wang K, Yang Q, Gahruie HH, Zhuang S, Li Y, Liang Y, Tan Y, Luo Y (2021) Ultrasonic-assisted food-grade solvent extraction of high-value added compounds from microalgae Spirulina platensis and evaluation of their antioxidant and antibacterial properties. Algal Res 60:102493
Tomaselli L (1997) Morphology, ultrastructure and taxonomy of Arthrospira (Spirulina) maxima and Arthrospira (Spirulina) platensis. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. Taylor & Francis, London, pp 1–16
United States Food and Drug Administration (2013) PART 73 – Listing of color additives exempt from certificatioN. Code of Federal Regulations, Title 21, Volume 1, Food and Drugs Chapter I Sec. 73.530, content current as of 02/16/2024
United States Food and Drug Administration (2022) GRAS Notice No. GRN 00099. GRAS Notice Inventory, content current as of 01/30/2024
Usharani G, Saranraj P, Kanchana D (2012) Spirulina cultivation: A Review. Int J Pharm Biol Arch 3:1327–1341
Vernès L, Granvillain P, Chemat F, Vian M (2015) Phycocyanin from Arthrospira platensis. Production, extraction and analysis. Curr Biotech 4:481–491
Voet D, Gratzer WB, Cox RA, Doty P (1963) Absorption spectra of nucleotides, polynucleotides, and nucleic acids in the far ultraviolet. Biopolymers 1:193–208
Wu HL, Wang GH, Xiang WZ, Li T, He H (2016) Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int J Food Prop 19:2349–2362
Yacobi YZ, Köhler J, Leunert F, Gitelson A (2015) Phycocyanin-specific absorption coefficient: Eliminating the effect of chlorophylls absorption. Limnol Oceanogr - Meth 13:157–168
Zhou Y, Huang Z, Liu Y, Li B, Wen Z, Cao L (2024) Stability and bioactivities evaluation of analytical grade C‐phycocyanin during the storage of Spirulina platensis powder. J Food Sci
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was developed within the project Phycokult funded by the Agency for Renewable Resources (FNR e.V.) and the German Federal Ministry of Food and Agriculture (BMEL), respectively.
Author information
Authors and Affiliations
Contributions
Conceptualisation by Jan Kuhnholz; Investigation by Jan Kuhnholz and Anja Noke; Methodology by Jan Kuhnholz, Till Glockow, Verena Siebecke, Anh Thu Le, Long-Dinh Tran; Writing by Jan Kuhnholz; Supervision by Anja Noke; Revision: Jan Kuhnholz and Anja Noke
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuhnholz, J., Glockow, T., Siebecke, V. et al. Comparison of different methods for extraction of phycocyanin from the cyanobacterium Arthrospira maxima (Spirulina). J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03224-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03224-y