Abstract
Neuroimaging abnormalities are common in chronically infected HIV-positive individuals. The majority of studies have focused on structural or functional brain outcomes in samples infected with clade B HIV. While preliminary work reveals a similar structural imaging phenotype in patients infected with clade C HIV, no study has examined functional connectivity (FC) using resting-state functional magnetic resonance imaging (rs-fMRI) in clade C HIV. In particular, we were interested to explore HIV-only effects on neurocognitive function using associations with rs-fMRI. In the present study, 56 treatment-naïve, clade C HIV-infected participants (age 32.27 ± 5.53 years, education 10.02 ± 1.72 years, 46 female) underwent rs-fMRI and cognitive testing. Individual resting-state networks were correlated with global deficit scores (GDS) in order to explore associations between them within an HIV-positive sample. Results revealed ten regions in six resting-state networks where FC inversely correlated with GDS scores (worse performance). The networks affected included three independent attention networks: the default mode network (DMN), sensorimotor network, and basal ganglia. Connectivity in these regions did not correlate with plasma viral load or CD4 cell count. The design of this study is unique and has not been previously reported in clade B. The abnormalities related to neurocognitive performance reported in this study of clade C may reflect late disease stage and/or unique host/viral dynamics. Longitudinal studies will help to clarify the clinical significance of resting-state alterations in clade C HIV.
Similar content being viewed by others
Introduction
HIV-associated neurocognitive disorders (HAND) are a common sequel of HIV infection and persist despite the use of combination antiretroviral therapy (cART) (Heaton et al. 2010). In HIV-infected individuals, the relative contribution of HIV disease mechanisms, cART, and host variables to the expression of cognitive impairment remains unclear. The diagnosis of HIV-associated cognitive impairment requires testing of multiple neurocognitive domains, including attention, information processing, language, complex perceptual motor skills (or psychomotor processing), learning, memory, and simple motor skills (McArthur 2004; Gartner 2000; De Francesco et al. 2016). The incidence of severe forms of HAND has declined through the effects of cART, but milder forms remain persistent, and may be increasing in prevalence. It is unclear whether these milder forms are a precursor of more severe disorder; or whether the frequency is inflated by limitations in the diagnostic process (Gisslen et al. 2011). A valid imaging biomarker in neuropsychologically impaired individuals would help to clarify some of these concerns through a more direct link to HIV neuropathology.
Prior studies reveal variability in the reported prevalence of HAND. These may be the result of differences in the adaptation and use of neuropsychological testing across sites, cART-experience and regimen, HIV disease stage and degree of viral suppression, and demographic characteristics of the study samples (such as gender, age, and pre-morbid cognitive reserve) (Bouwman et al. 1998; Antinori et al. 2007). The net effect is limited insight into the association between neuropathological HIV effects and clinical impairment. HIV genotype has also been proposed as a source of variability in HAND since the subtypes of HIV are not equally distributed on a global basis. While HIV-B is prevalent in North America and Europe, HIV-C is dominant in Sub-Saharan Africa and Southeast Asia (Rotta and Almeida 2011). Most of the neuroimaging work conducted to date in HIV has focused on structural and functional markers of HAND in HIV-B. Structural neuroimaging of HIV-C in South Africa confirm neurovirulence, with smaller volumes in the total gray matter (GM), white matter (WM), and thalamus in HIV-infected participants compared to healthy controls (Heaps et al. 2012). A further study comparing volumes between HIV-B and HIV-C samples with respect to local controls revealed no effect of HIV subtype (Ortega et al. 2013). Again, there are limited data linking neuroimaging signature of HIV-C and cognitive abilities. Specifically, there are no studies exploring the resting-state functional properties of the brain in HIV-C individuals. Resting-state functional magnetic resonance imaging (rs-fMRI) is a non-invasive imaging technique that permits investigation of resting-state functional connectivity (RSFC), which adds additional information to existing structural imaging modalities. Previous studies indicate high sensitivity of RSFC to impaired brain function across multiple clinical disorders (Van den Heuvel and Pol 2010; Wang et al. 2013; Zhou et al. 2013).
HIV-B infected individuals exhibit altered connectivity in the attention network (Ortega et al. 2015), thalamus (Qiu et al. 2011), and the default mode network (DMN) (Thomas et al. 2013; Wang et al. 2011), when compared to HIV- controls. Only Wang et al. (2011) identified a link between connectivity and cognitive performance in HIV-B where connectivity in the lateral occipital cortex network was correlated with a task involving visual-motor coordination. While these previous studies focused on between group effects, Ann et al. (2016) focused on RSFC between HAND and non-HAND participants using a seed-based analysis centered in the bilateral precuneus. They found reduced connectivity between the bilateral precuneus and prefrontal cortex in the HAND participants compared to cognitively intact HIV+ individuals. Additionally, increased connectivity in the DMN correlated with better performance on tests of learning and memory. While informative, these studies included mixed samples of cART-naïve individuals, and the methodological approach did not examine whole-brain correlations between RSFC and cognitive function.
We designed our study to identify HIV-specific effects on RSFC in a cohort of treatment-naïve HIV-infected individuals by inserting Global Deficit Scores into the general linear model (GLM). Our research further adds to the literature by specifically investigating these effects in HIV-C, for which there are limited data. We hypothesized that worse cognitive function would correlate with altered connectivity through a range of resting-state networks, and that these patterns would correspond to the typical cognitive phenotype of treatment-naïve HIV.
Methods
Participants
We included 61 HIV+ and 50 HIV- demographically similar control participants from a larger study (Paul et al. 2014), for the purposes of neurocognitive ascertainment. Data from only the HIV+ participants were then used to explore the associations between RSFC and cognition. All participants provided consent to participate in the study as prescribed by the Human Research Ethics Committee at the University of Cape Town. Exclusion criteria included schizophrenia and bipolar mood disorder, neurological disorders such as multiple sclerosis and other central nervous system (CNS) conditions, head injury with more than 30 min loss of consciousness, clinical evidence of opportunistic CNS infections, and substance use disorder as defined by the Mini-International Neuropsychiatric Interview (MINI-Plus) (Lecrubier et al. 1997). Additionally, participants with possible pregnancy, claustrophobia, or metal implants were disqualified from participation in the study as these factors would affect scanning.
Participants were recruited from two primary care HIV clinics in Cape Town, South Africa, prior to cART initiation. As such, all were cART-naïve at the time of participation. Inclusion criteria included an age range of 18 to 45 years, and Xhosa as the primary language. HIV status was determined by an enzyme-linked immune assay (ELISA) and confirmed by either a Western blot, plasma HIV RNA, or a second antibody test. HIV subtype was determined by polymerase chain reaction (PCR) of the tat exon 1 region (HXB2 position 5831–6045), using the Promega GoTaq Flexi Kit (Promega, Madison, WI). This approach has been described in Paul et al. (2014, 2017).
Neuroimaging scans were acquired and blood samples were taken during a separate visit 1 week after neuropsychological testing and prior to commencing cART.
Neuropsychological assessment
All cognitive tests were performed by a psychometrist fluent in both Xhosa and English. Tests included grooved pegboard test, the Hopkins Verbal Learning Test-Revised (HVLT-R) (Brandt and Benedict 1991), Rey Complex Figure Test (RFC) (Knight and Kaplan 2003), Brief Visuospatial Memory Test-Revised (BVMT-R) (Benedict et al. 1996), Mental Alternation Test (MAT) (Salib and McCarthy 2002), Wechsler Memory Scale III (WMS III) (Wechsler 1997), Trail Making Test A (TMTA) (Corrigan and Hinkeldey 1987), Color Trails Test (CTT) (D'Elia and Satz 1996), Stroop Color Word Test (Golden 1978), Wisconsin Card Sorting Test (WCST) (Grant and Berg 1948), and the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999).
Cognitive performances recorded for the HIV-infected group were converted to T scores based on comparisons to the HIV-negative sample. T scores were then converted to global deficit scores (GDS) as described previously (Carey et al. 2004). The GDS, within the HIV+ group only, were then used to explore the resting-state signature of HIV infection.
MRI scanning protocol
A 3T Allegra MRI scanner (Siemens, Erlangen, Germany) located at the Cape Universities Brain Imaging Centre (CUBIC), in Cape Town, South Africa, was utilized for all scans. For each subject, a gradient echo planar imaging (EPI) sequence was used to acquire rs-fMRI data: voxel resolution = 4 × 4 × 4 mm3; FOV = 256 × 256 × 144 mm3; 36 slices; 164 volumes; TR/TE = 2200/27 ms; flip angle = 90o. Additionally, T1-weighted (T1w) structural images were acquired using an MPRAGE sequence for each subject: voxel resolution = 1 × 1 × 1 mm3; FOV = 256 × 256 × 176 mm3; 176 slices; TR/TE = 2400/2.38 ms; TI = 1000 ms; flip angle = 8o.
Pre-processing
Processing and analyses of all imaging data were performed using tools in AFNI (Cox 1996) and FSL (v5.0) (Smith et al. 2004), as well as in-house scripts. Prior to processing of the functional data, each subject’s T1w volume was skull-stripped by means of in-house scripts and AFNI’s 3dSkullStrip, for later use in alignment and registration. Each subject’s skull-stripped anatomical was visually checked to ensure the quality of the processing, with adjustments performed as necessary.
The main processing of each subject’s rs-fMRI data was implemented using a set of procedures specified with AFNI’s afni_proc.py tool as follows. First, the first four time points were removed from each EPI time series, spikes were truncated, and slice timing correction was applied to adjust for the temporal offset between slice acquisitions. The EPI volumes were coregistered using 6 degrees of freedom (3 translation and 3 rotation) to adjust for motion during scanning and aligned to the T1w anatomical image. Volumes exceeding 0.3 mm of bulk motion were excluded from further analyses. The average subject motion for the remainder of the volumes was found to be 0.10 ± 0.05 mm. Linear affine alignment of each subject’s motion-adjusted EPI volumes to their T1w volumes was performed, which was nonlinearly warped to the standard Talairach-Tournoux template; these transforms were concatenated with the motion adjustment, and a single transform was applied to map the EPI data into standard space, maintaining their initial spatial resolution. Visual inspection of the alignment between the rs-fMRI data and the Talairach template was performed for each subject as an additional measure of quality control.
The T1w volumes were segmented into tissue masks of gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Signals from eroded maps of both WM and CSF were regressed out from the EPI time series along with motion, motion derivatives, and linear and quadratic trends. The EPI data were then spatially smoothed using a 6-mm full-width at half-maximum (FWHM) Gaussian kernel. Low-frequency fluctuations (LFFs) were calculated by bandpass filtering the time series in the standard interval 0.01–0.1 Hz, in order to reduce contributions of physiological processes in the BOLD signal.
Group independent component analysis (ICA) was preformed using FSL’s MELODIC tool (Smith et al. 2004), in order to decompose the 4D time series data and to identify resting-state networks (RSNs) of high temporal correlation. A standard size of dimension reduction was used, producing 20 independent components (ICs). The ICs were compared with a standard set of RSN template maps from the Functional Connectome Project (Biswal et al. 2010) using 3dMatch in FATCAT (Taylor and Saad 2013), with results also inspected visually. In this case, 14 ICs were identified as known functional networks, and these were thresholded at Z > 4 to provide network spatial masks of high RSFC. The remaining components (representing mainly non-GM tissue, edge alignment artifacts, small motion effects, etc.) were not included in further analyses.
Statistical analyses
General linear modeling (GLM) was implemented with nonparametric permutation testing in FSL to examine the association between the RSFC and GDS. The design matrix was set up to evaluate correlations between the connectivity measures of each RSN and participant GDS, while controlling for possible confounders. In this study, subject age, gender, and education (number of years) were considered as potential confounders. Voxelwise connectivity Z scores were estimated for each subject and each network using FSL’s dual_regression function (Beckmann et al. 2009). FSL’s randomize function (Winkler et al. 2014) was then used to perform permutation tests for voxelwise GLMs using the specified design matrix, resulting in test statistic maps. AFNI’s AlphaSim tool was used to determine the minimum cluster size of significant regions within the respective RSN with a corrected p < 0.05/N, where N is the number of networks identified, and alpha = 0.01. Significant clusters were then identified and extracted using FATCAT’s 3dROIMaker.
For each participant, an average RSFC value (i.e., Z score) was calculated for each significant cluster. SPSS (version 20; IBM, Armonk, NY) was used to determine Pearson correlation coefficients between the RSFC and GDS. Outliers, defined as the mean ± 3 times the standard deviation (SD), was removed from these calculations.
Results
Sample characteristics
Fifty-six participants were included in the subsequent analyses as excessive motion eliminated five participants (Table 1).
Resting-state networks
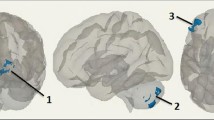
A total of 14 ICs were identified as standard RSNs. The IC number, network, and corresponding functional connectome project map are provided in Table 2. The RSNs were thresholded at a Z > 4 and binarized to form network masks (Fig. 1).
Z score maps (thresholded Z > 4) representing the 14 RSNs identified by the ICA for 56 HIV+ subjects: visual occipital lobe (Vis1), dorsal attention (dAtt), right executive (R-Exec), default mode network (DMN), dorsal DMN (dDMN), salience (Sal), visual lingual gyrus (Vis2), sensorimotor (SenMot), ventral attention (vAtt), left executive (L-Exec), attention (Att), auditory (Aud), cerebellum (Cer), and basal ganglia (Bas)
Resting-state connectivity correlations with GDS
Analyses revealed regions in 6 of the 14 identified networks where RSFC was significantly associated with cognitive impairment in HIV-infected participants. In all cases, the functional connectivity (FC) of the significant clusters was inversely related to GDS. Table 3 shows the peak coordinates of these regions of interest (ROIs) in each RSN, as well as the anatomical structure at the peak. Several networks contained more than one significant cluster.
Figures 2 and 3 show the cluster locations (blue) within the networks (hot colors). Additionally, the scatterplots of the RSFC-GDS correlations are shown for each cluster.
(Left) Brain maps of clusters that exhibited significant associations between resting state connectivity (blue) in the dorsal attention network (hotter colors) for 56 HIV+ subjects. Peak is situated in a the right precentral gyrus of the dAtt network, b the right superior frontal gyrus of the DMN, c–e the left postcentral gyrus, right precentral gyrus, and right medial frontal gyrus of the SenMot network. (Right) Correlation graphs show relationships between average Z scores and GDS with r and corrected p values
(Left) Brain maps of clusters that exhibited significant associations between resting state connectivity (blue) in the dorsal attention network (hotter colors) for 56 HIV+ subjects. Peak is situated in the right inferior temporal gyrus and right superior frontal gyrus in the vAtt network (a—b), the right inferior frontal gyrus and right inferior parietal lobule in the Att network(c–d), and the right thalamus in the Bas (e). (Right) Correlation graphs show relationships between average Z scores and GDS with r and corrected p values
Discussion
We describe a unique association between neurocognitive function and RSFC in treatment-naïve HIV-C-infected adults. We found that reduced RSFC was correlated with cognitive deficits in the attention and sensorimotor networks, DMN, and basal ganglia. Our study is unique in the fact that no previous rs-fMRI studies identified regions of altered connectivity by inserting measures of cognition into the GLM analysis—even in the frequent clade B studies. As such, the present study is the first to report global cognitive function as a predictor of differences in resting-state network activation. In this way, rs-fMRI may hold promise as a sensitive biomarker of neurocognitive status.
The fact that RSFC was significantly affected in the attention networks is to be expected as impaired attention in HIV-infected individuals is common (Berger and Arendt 2000; Selnes 2005; Watkins and Treisman 2015; Cysique and Brew 2009; Nath et al. 2008; Heaton et al. 2004). Of the previous rs-fMRI studies, only one (Ortega et al. 2015) reported reduced RSFC between HIV-infected individuals and healthy controls in attention networks. However, they did not find any correlations between RSFC and neurocognitive impairment. In contrast, our results show reduced RSFC with increased cognitive impairment in five regions of the attention networks. These differences might be explained by methods, the absence of cART use in our samples, or the clinical and demographic composition of the samples.
We also found that cognitive deficits correlated with reduced RSFC in the thalamus, a structure known to be affected in several motor disorders, such Parkinson’s disease and Huntington’s chorea, (Mink 1996; Groenewegen 2003). The basal ganglia also play a role in emotional processing (Cancelliere and Kertesz 1990; Paulmann et al. 2008), learning and memory (Graybiel 2005; Packard and Knowlton 2002), and executive function (Graybiel 2000). Structural MRI has frequently shown that reduced subcortical volumes in HIV-infected individuals are related to neurocognitive impairment (Dal Pan et al. 1992; Aylward et al. 1993; Paul et al. 2002; Ances et al. 2006). The findings reported by Qiu et al. (2011) support our results. Specifically, Qiu et al. (2011) reported reduced regional homogeneity in the thalamus. Again, thalamic involvement may reflect a subcortical pattern commonly seen in untreated HIV.
Reduced RSFC in the sensorimotor network also correlated with worse global cognition. Juengst et al. (2007) performed task-based fMRI requiring simple sensorimotor activity in a between-group study of HIV-infected individuals with a range of HAND severity and healthy controls. While group differences were not identified, the hemodynamic response was delayed in the HIV-infected participants with minor neurocognitive disorder (MNCD) and HIV-associated dementia (HAD), which is consistent with alterations in neuronal functioning. By contrast, other rs-fMRI studies have not revealed altered RSFC in the sensorimotor network in HIV-infected patients. The absence of cognitive performance in the analytic models may account for these outcomes.
Our results of reduced RSFC in the DMN are supported by other studies focused on RSFC (Thomas et al. 2013) and regional homogeneity (Wang et al. 2011). Additionally, Ann et al. (2016) found positive correlations between the right inferior operculum and superior frontal gyrus, the specific region of the DMN identified in our study, with the memory and learning neuropsychiatric domains.
While the aforementioned studies did not specifically mention clade, they were performed in higher-income countries where clade B is prevalent. However, the different designs of these reports make it difficult to definitively ascribe altered RSFC findings to clade effects. In spite of this, our findings of altered connectivity with GDS in the attention network, thalamus, and DMN are reflected in these studies, irrespective of design and clade. In contrast, only our study found that RSFC in the sensorimotor network was affected. Table 4 summarizes the methodologies, demographics, and major findings of the cited articles. Firstly, there are several demographic differences between these studies and our own, namely that most of the participants in our sample were female with fewer years of education than the prior studies. Secondly, our sample only included treatment-naïve individuals, as compared to the other studies mostly including cART-experienced samples. These differences may account for the level of RSFC disruption observed in the present study. Thirdly, viral clade differences may be present. Previous work from our team confirms that HIV-C is neurovirulent, despite earlier preclinical evidence suggesting lower neurotoxicity of clade C due to a polymorphism in the Tat C31 dicysteine motif. We have previously reported outcomes from the sample included in this study demonstrating no differences in cognition or structural neuroimaging when individuals with the Tat C31S substitution are compared to individuals without the Tat defect (Paul et al. 2014). Results from the present study align with the general conclusion that clade C is neurovirulent.
In conclusion, this is the first rs-fMRI to investigate RSFC in relation to neurocognitive deficits in treatment-naïve clade C HIV. We found that reduced RSFC was associated with increased global cognitive deficits in the attention and sensorimotor networks, the DMN, and the thalamus. Neurocognitive functions controlled by these networks are involved in the clinical expression of HAND. Future studies using longitudinal designs are needed to better establish whether RSFC is a dynamic biomarker of neuronal well-being and function in HIV.
References
Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA (2006) Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology 66(6):862–866. https://doi.org/10.1212/01.wnl.0000203524.57993.e2
Ann HW, Jun S, Shin NY, Han S, Ahn JY, Ahn MY, Jeon YD, Jung IY, Kim MH, Jeong WY, Ku NS (2016) Characteristics of resting-state functional connectivity in HIV-associated neurocognitive disorder. PLoS One 11(4):e0153493. https://doi.org/10.1371/journal.pone.0153493
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslén M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799 http://dx.doi.org.ezproxy.uct.ac.za/10.1212/01.WNL.0000287431.88658.8b
Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD (1993) Reduced basal ganglia volume in HIV-1-associated dementia results from quantitative neuroimaging. Neurology 43(10):2099–2099. https://doi.org/10.1212/WNL.43.10.2099
Beckmann CF, Mackay CE, Filippini N, Smith SM (2009) Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage 47(Suppl 1):S148
Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B (1996) Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess 8(2):145
Berger JR, Arendt G (2000) HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14(3):214–221. https://doi.org/10.1177/026988110001400304
Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM (2010) Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107(10):4734–4739. https://doi.org/10.1073/pnas.0911855107
Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC (1998) Variable progression of HIV-associated dementia. Neurology 50(6):1814–1820 http://dx.doi.org.ezproxy.uct.ac.za/10.1212/WNL.50.6.1814
Brandt J, Benedict RHB (2001) Hopkins Verbal Learning Test, Revised: Professional manual. Psychological Assessment Resource, Lutz
Cancelliere AE, Kertesz A (1990) Lesion localization in acquired deficits of emotional expression and comprehension. Brain Cogn 13(2):133–147 https://doi-org.ezproxy.uct.ac.za/10.1016/0278-2626(90)90046-Q
Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26(3):307–319 http://dx.doi.org.ezproxy.uct.ac.za/10.1080/13803390490510031
Corrigan JD, Hinkeldey NS (1987) Relationships between parts A and B of the Trail Making Test. J Clin Psychol 43(4):402–409. https://doi.org/10.1002/1097-4679(198707)43:4<402::AID-JCLP2270430411>3.0.CO;2-E
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173. https://doi.org/10.1006/cbmr.1996.0014
Cysique LA, Brew BJ (2009) Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev 19(2):169–185. https://doi.org/10.1007/s11065-009-9092-3
D’Elia L, Satz P (1996) Color trails test. Psychological Assessment Resources, Inc., Odessa
Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC (1992) Patterns of cerebral atrophy in HIV–1–infected individuals results of a quantitative MRI analysis. Neurology 42(11):2125. https://doi.org/10.1212/WNL.42.11.2125
De Francesco D, Underwood J, Post FA, Vera JH, Williams I, Boffito M, Sachikonye M, Anderson J, Mallon PW, Winston A, Sabin CA (2016) Defining cognitive impairment in people-living-with-HIV: the POPPY study. BMC Infect Dis 16(1):617. https://doi.org/10.1186/s12879-016-1970-8
Gartner S (2000) HIV infection and dementia. Science 287(5453):602–604. https://doi.org/10.1126/science.287.5453.602
Gisslén M, Price RW, Nilsson S (2011) The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 11(1):356. https://doi.org/10.1186/1471-2334-11-356
Golden CJ (1978) Stroop color and word test: a manual for clinical and experimental uses. Stoelting, Chicago
Grant DA, Berg E (1948) A behavioral analysis of degree of reinforcement and ease of shifting to new responses in Weigl-type card-sorting problem. J Exp Psychol 38:404–411. https://doi.org/10.1037/h0059831
Graybiel AM (2000) The basal ganglia. Curr Biol 10(14):R509–R511. https://doi.org/10.1016/S0960-9822(00)00593-5
Graybiel AM (2005) The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 15(6):638–644. https://doi.org/10.1016/j.conb.2005.10.006
Groenewegen HJ (2003) The basal ganglia and motor control. Neural Plast 10(1–2):107–120. https://doi.org/10.1155/NP.2003.107
Heaps JM, Joska J, Hoare J, Ortega M, Agrawal A, Seedat S, Ances BM, Stein DJ, Paul R (2012) Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neuro-Oncol 18(3):151–156. https://doi.org/10.1007/s13365-012-0090-5
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10(03):317–331. https://doi.org/10.1017/S1355617704102130
Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology 75(23):2087–2096 http://dx.doi.org.ezproxy.uct.ac.za/10.1212/WNL.0b013e318200d727
Juengst SB, Aizenstein HJ, Figurski J, Lopez OL, Becker JT (2007) Alterations in the hemodynamic response function in cognitively impaired HIV/AIDS subjects. J Neurosci Methods 163(2):208–212 https://doi-org.ezproxy.uct.ac.za/10.1016/j.jneumeth.2007.03.004
Knight JA, Kaplan E (2003) The handbook of Rey-Osterrieth Complex Figure usage: Clinical and research applications. Psychological Assessment Resources, Inc., Lutz
Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar GC (1997) The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 12(5):224–231. https://doi.org/10.1016/S0924-9338(97)83296-8
McArthur JC (2004) HIV dementia: an evolving disease. J Neuroimmunol 157(1):3–10 https://doi-org.ezproxy.uct.ac.za/10.1016/j.jneuroim.2004.08.042
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425 https://doi-org.ezproxy.uct.ac.za/10.1016/S0301-0082(96)00042-1
Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, Mcarthur J (2008) Evolution of HIV dementia with HIV infection. Int Rev Psychiatry 20(1):25–31 http://dx.doi.org.ezproxy.uct.ac.za/10.1080/09540260701861930
Ortega M, Heaps JM, Joska J, Vaida F, Seedat S, Stein DJ, Paul R, Ances BM (2013) HIV clades B and C are associated with reduced brain volumetrics. J Neuro-Oncol 19(5):479–487. https://doi.org/10.1007/s13365-013-0202-x
Ortega M, Brier MR, Ances BM (2015) Effects of HIV and combination antiretroviral therapy (cART) on cortico-striatal functional connectivity. AIDS 29(6):703. https://doi.org/10.1097/QAD.0000000000000611
Packard MG, Knowlton BJ (2002) Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25(1):563–593. https://doi.org/10.1146/annurev.neuro.25.112701.142937
Paul R, Cohen R, Navia B, Tashima K (2002) Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 26(3):353–359. https://doi.org/10.1016/S0149-7634(02)00006-4
Paul RH, Joska JA, Woods C, Seedat S, Engelbrecht S, Hoare J, Heaps J, Valcour V, Ances B, Baker LM, Salminen LE (2014) Impact of the HIV Tat C30C31S dicysteine substitution on neuropsychological function in patients with clade C disease. J Neuro-Oncol 20(6):627–635 https://doi-org.ezproxy.uct.ac.za/10.1007/s13365-014-0293-z
Paulmann S, Pell MD, Kotz SA (2008) Functional contributions of the basal ganglia to emotional prosody: evidence from ERPs. Brain Res 1217:171–178. https://doi.org/10.1016/j.brainres.2008.04.032
Qiu W, Yan B, Li J, Tong L, Wang L, Shi D (2011) A resting-state fMRI study of patients with HIV infection based on regional homogeneity method. In: 2011 seventh international conference on Natural computation (ICNC 2011) 2:997–1000. IEEE. https://doi.org/10.1109/ICNC.2011.6022180
Rotta I, Almeida SMD (2011) Genotypical diversity of HIV clades and central nervous system impairment. Arq Neuropsiquiatr 69(6):964–972. https://doi.org/10.1590/S0004-282X2011000700023
Salib E, McCarthy J (2002) Mental Alternation Test (MAT): a rapid and valid screening tool for dementia in primary care. Int J Geriatr Psychiatry 17(12):1157–1161. https://doi.org/10.1002/gps.738
Selnes OA (2005) Memory loss in persons with HIV/AIDS: assessment and strategies for coping. AIDS Read 15(6):289–294
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:S208–S219 https://doi-org.ezproxy.uct.ac.za/10.1016/j.neuroimage.2004.07.051
Taylor PA, Saad ZS (2013) FATCAT:(an efficient) functional and tractographic connectivity analysis toolbox. Brain Connect 3(5):523–535. https://doi.org/10.1089/brain.2013.0154
Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM (2013) Pathways to neurodegeneration effects of HIV and aging on resting-state functional connectivity. Neurology 80(13):1186–1193 http://dx.doi.org.ezproxy.uct.ac.za/10.1212/WNL.0b013e318288792b
Van Den Heuvel MP, Pol HEH (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20(8):519–534 https://doi-org.ezproxy.uct.ac.za/10.1016/j.euroneuro.2010.03.008
Wang X, Foryt P, Ochs R, Chung JH, Wu Y, Parrish T, Ragin AB (2011) Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect 1(3):207–217. https://doi.org/10.1089/brain.2011.0016
Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, Jia J, Han Y, He Y (2013) Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry 73(5):472–481 https://doi-org.ezproxy.uct.ac.za/10.1016/j.biopsych.2012.03.026
Watkins CC, Treisman GJ (2015) Cognitive impairment in patients with AIDS—prevalence and severity. HIV/AIDS (Auckl) 7:35–47. https://doi.org/10.2147/HIV.S39665
Wechsler D (1997) Wechsler memory scale (WMS-III). Psychological Corporation, San Antonio
Wechsler D (1999) Manual for the Wechsler abbreviated intelligence scale (WASI). The Psychological Corporation, San Antonio
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. NeuroImage 92:381–397 https://doi-org.ezproxy.uct.ac.za/10.1016/j.neuroimage.2014.01.060
Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P, Wang L, Xi Z, Jiang T (2013) Impaired functional connectivity of the thalamus in Alzheimer’s disease and mild cognitive impairment: a resting-state fMRI study. Curr Alzheimer Res 10(7):754–766
Acknowledgements
The authors would like to thank the National Institute of Mental Health for supporting this research. We also want to acknowledge the South African Research Chairs Initiative of the Department of Science and Technology, National Research Foundation of South Africa, National Institute of Health, Medical Research Council of South Africa, and NIMH and NINDS Intramural Research Programs of the NIH for further partial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
du Plessis, L., Paul, R.H., Hoare, J. et al. Resting-state functional magnetic resonance imaging in clade C HIV: within-group association with neurocognitive function. J. Neurovirol. 23, 875–885 (2017). https://doi.org/10.1007/s13365-017-0581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-017-0581-5