Abstract
We address the question of ultimate selective advantages of hibernation. Biologists generally seem to accept the notion that multiday torpor is primarily a response to adverse environmental conditions, namely cold climate and low food abundance. We closely examine hibernation, and its summer equivalent estivation, in the edible dormouse, Glis glis. We conclude that in this species, hibernation is not primarily driven by poor conditions. Dormice enter torpor with fat reserves in years that are unfavourable for reproduction but provide ample food supply for animals to sustain themselves and even gain body energy reserves. While staying in hibernacula below ground, hibernators have much higher chances of survival than during the active season. We think that dormice enter prolonged torpor predominantly to avoid predation, mainly nocturnal owls. Because estivation in summer is immediately followed by hibernation, this strategy requires a good body condition in terms of fat reserves. As dormice age, they encounter fewer occasions to reproduce when calorie-rich seeds are available late in the year, and phase advance the hibernation season. By early emergence from hibernation, the best territories can be occupied and the number of mates maximised. However, this advantage comes at the cost of increased predation pressure that is maximal in spring. We argue the predator avoidance is generally one of the primary reasons for hibernation, as increased perceived predation pressure leads to an enhanced torpor use. The edible dormouse may be just an example where this behaviour becomes most obvious, on the population level and across large areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Small animals have a high metabolic rate to begin with, and a further increase may be too expensive when food is scarce or unavailable. The easy way out, and the only logical solution is to give up the struggle to keep warm and let the body temperature drop. This not only eliminates the increased cost of keeping warm, but cold tissues use less fuel and energy reserves last longer. This is, in essence, what hibernation is all about”. This is how Schmidt-Nielsen (Schmidt-Nielsen 1979) in his textbook explains hibernation, and indeed the temporal escape from cold and the scarcity of food are at the centre of arguments for hibernation and estivation of mammals. Why is it then, that edible dormice in certain years enter prolonged torpor (estivation) during early summer when the vegetation, that provides ample food, is still growing? To make matters even more confusing: why is it that only the heaviest, fattest animal begin to estivate then while dormice In poor condition stay above ground (Hoelzl et al. 2015)? This behaviour seems to contradict the common rationales for hibernation.
In short, dormice enter hibernation in early summer when they skip reproduction, which is the case on average every three years (Fig. 2 in Ruf and Bieber 2020a). Dormice estivate only when they are in good body condition, because estivation is immediately followed by hibernation. Thus, it requires staying below ground and living on body energy reserves, particularly fat, for almost 1 year (Dark 2005; Hoelzl et al. 2015). The main reason why dormice avoid activity in the canopy is predator avoidance, mainly that of owls. Low predation and high survival rates generally seem one of the major advantages of hibernation (Turbill et al. 2011). There is also experimental evidence showing that small mammals use torpor to lower their predation risk (Turbill and Stojanovski 2018).
In edible dormice, hibernation is an integral part of their reproductive strategy. Although dormice can live in areas with a very low seed-tree abundance, they respond strongly to year-to-year fluctuations in the mast seeding of deciduous trees. Reproduction is tightly coupled to the availability of high-caloric seeds, particularly of beech trees (Bieber 1998; Schlund et al. 2002; Fietz et al. 2005; Ruf et al. 2006; Vekhnik 2019). The proportion of successfully reproducing females is high in full mast years with virtually all trees seeding, average in intermediate mast years, when only a fraction of trees produce seeds, and entire populations of dormice can skip reproduction in mast failure years (Fietz et al. 2005; Ruf et al. 2006). However, that species skip a year of reproduction and strongly respond to pulsed resources is not restricted to hibernators (Yang et al. 2010; Bergeron et al. 2011).
In dormice, certain years are unfavourable for reproduction but fully sufficient to gain mass on foliage, berries, or arthropods (Santini 1978; Koppmann-Rumpf et al. 2003; Fietz et al. 2005; Ruf et al. 2006). In those years, dormice may enter estivation after just 2–3 weeks of activity in late spring (Hoelzl et al. 2015). Here, we review the biology of hibernation in this species both as a regular component of its life history and a possible response to environmental conditions, such as predator abundance.
Fattening and growth
In the Vienna woods, adult and yearling dormice emerge in spring with a mean mass of 87 g. The last group to be encountered in traps or nest boxes are adult females, which is a proxy of emergence, are seen up to early July. This pattern is very similar to a site in Germany with a similar climate (Bieber and Ruf 2004). Dormice enter hibernation in autumn (after October 1) with an average mass of 154 g (Fig. 1). This corresponds to a 77% mass gain, and there is no notable size dimorphism between sexes. Similar mass gains over summer, sometimes with intermediate losses during reproduction, have been also observed at different sites in Germany (Bieber 1998; Schlund et al. 2002). Outside the distribution range of beech, e.g., in Lithuania, the seasonal weight gains are somewhat smaller (98–128 g, Juškaitis and Augutė, 2015). In England, on the other hand, a substantial fraction of animals even reach a pre-hibernation mass of > 300 g (Morris and Morris 2010). This fat gain is not unusual among hibernators, which often double their body mass during summer. Increases in body mass represent a programmed increase in fat deposition and, in certain species, of fat-free dry mass (Dark 2005). In the edible dormouse, a greater body mass seems to be entirely due to white adipose tissue deposition (Schaefer et al. 1976), and autopsies indicate the fat deposition is mostly subcutaneous and intraperitoneal. However, ultrasonography imaging revealed that the content of fat in the liver, distributed in an unexpected pattern of discrete focal areas, visibly increased at the end of the active season. This was accompanied by a significant increase in the transverse liver diameter of edible dormice (Bieber et al. 2011).
Body mass development over the active season in edible dormice. Body masses of adults, yearlings and juveniles in the Vienna woods at ~ 400 m a.s.l. In winter, animals hibernate below ground. Data from a capture-recapture study 2006–2020. Body masses are from both sexes, as no noticeable sex differences were found. Data from Bieber and Ruf (unpublished)
Experimental food self-selection trials showed that this body mass increase is predominantly caused by hyperphagia, first causing a large (up to tenfold) increase in carbohydrate uptake followed by a surge in lipid uptake (Jastroch et al. 2016). In natural environments, beech seeds or acorn would be a natural food source rich in lipids (Bieber and Ruf 2004). Like in other hibernators, the concentration of the gut-produced orexigenic hormone ghrelin probably gradually increases in dormice during summer to reach high values at the autumnal-hyperphagic period (Florant and Healy 2012). Peripheral injections of ghrelin cause the increase in food intake at all seasons, even in aphagic hibernators at the start of hibernation (Healy et al. 2011). However, food intake in hibernators is in fact controlled by a multitude of signals. Besides ghrelin, changes in circulating leptin and insulin, as well as in nutrients (glucose and free fatty acids), and cellular enzymes such as AMP-activated protein kinase (AMPK) determine the activity of neurons involved in the food intake pathway. The complexity of this system is underlined by the fact that during hyperphagia more than 900 genes can be differentially expressed in white adipose tissue (Jansen et al. 2019). Importantly, during the fattening phase, hibernators become temporarily insensitive towards leptin, the hormone that normally signals white fat content and limits lipid uptake and deposition. In hibernators, leptin can be temporarily disassociated from adiposity (Kronfeld-Schor et al. 2000; Florant and Healy 2012; Jastroch et al. 2016).
However, changes of body mass are not necessarily a consequence of increased food intake alone. In certain hibernators, but not in others, resting metabolic rate at euthermia can decrease in late summer, several weeks before food intake declines, thereby enhancing late lipid deposition by shifting the energy balance (Dark 2005; Sheriff et al. 2013). It remains to be seen if edible dormice also use this mechanism to aid pre-hibernation fattening.
Dormice give birth late in the active season, mostly in August. Juvenile dormice increase body mass extremely fast, which includes both structural growth and fattening (Fig. 1). At the Austrian study site, juveniles grow from ~ 30 g prior to September 1, around weaning, to an average of 86 g right before hibernation, i.e., after October 1. This is equal to a mass gain of 280% in 2–3 months. Data from other species with one litter per year suggest that in hibernators birth weights are at, or slightly below, the values expected from the allometric curve for mammals (Peters 1993; Bieber and Ruf 2004). Weaning weights, however, are typically higher than expected for mammals of that size (Millar 1977), indicating that growth rates in hibernators may be generally high. In the closely related species, Eliomys quercinus, a hibernator that may have several litters per year, growth and fatting of juveniles are supported by their use of torpor during summer. G. glis is also capable of short torpor, but it still has to be evaluated if this behaviour helps to gain mass in this species.
A humoral pathway that can facilitate growth are high glucocorticoid levels. It has been demonstrated that female red squirrels provisioned with cortisol have faster growing pups, and that the growth of young is sped up by maternal effects of high glucocorticoids, especially when they experienced a high population density (Dantzer et al. 2013). High amounts of cortisol metabolites were indeed found in reproducing dormice, and only in reproductive years (Cornils et al. 2018; Havenstein et al. 2021). Partly, high cortisol levels in dormice may reflect their response to stressors caused by reproductive activity, such as competition for mates and exposure to predators during high foraging periods (Cornils et al. 2018; Havenstein et al. 2021). However, a major cause for high glucocorticoid levels, which are more than twice as high in females than in male dormice (Havenstein et al. 2021), their beneficial effect is probably increased growth of the young, although the exact energetic source of which still has to be determined (Dantzer et al. 2013). The mass gain of both young and adults seems to be a prerequisite for hibernation, since it is accompanied, as in many hibernators, by the complete cessation of foraging, no matter what the environmental food situation.
Hibernation and estivation timing
Dormice normally commence hibernation in September to November, first are males followed by females. In both sexes, hibernation onset is delayed by reproductive investment by 2–3 weeks (Ruf and Bieber 2020a). These delays occur among females that actually give birth to young and males that develop large testes and become sexually competent. The last class to enter hibernation is juveniles (Fig. 1). Dormice hibernate close to their summer nests, 90% of the hibernacula are less than 400 m from the “home” nest box (Trout et al. 2018). G. glis usually hibernates below ground in self-dug underground chambers, typically alone or accompanied by one or two conspecifics (Morris and Hoodless 1992; Jurczyszyn 2007; Trout et al. 2018).
Hibernation, i.e., multiday torpor in edible dormice in Austria normally starts after one or two initial short deep torpor bouts in September/October (Fig. 2a). It lasts on average until June, so mean hibernation duration is slightly more than 8 months in this population. This duration of 7–8 months seems to be quite typical for the central and northern edge of its distribution (Lebl et al. 2011; Juškaitis and Augutė, 2015). The shortest active season and longest hibernation of dormice may be that in the Kazan region of Russia (Rossolimo et al. 2001). On the southern edge of the range, in central Italy and Sicily, the hibernation season of dormice may last just 6 months (from November/December until late April to May, Santini 1978; Milazzo et al. 2003). It seems, however, that most estimates of hibernation duration are based on the long-term absence of animals from nest boxes (which generally is a reliable measurement of occupancy; Bieber and Ruf 2009), while the only long-term body temperature records in free-living dormice are those from the Austrian population (e.g., Hoelzl et al. 2015).
a Hibernation as well as b estivation and hibernation in non-reproductive years in edible dormice. Prior to hibernation the animal in Fig. 2a (#73,028) displays many bouts of short torpor, particularly early in the summer season (July to early August). The dormouse in Fig. 2a also shows two episodes of multiday torpor in July. The dormouse in Fig. 2b (#33,261) enters estivation (hibernation in summer) already in June, data from Hoelzl et al. (2015). Light blue lines show soil temperature in the middle of the study area at a depth of 60 cm, the approximate depth of burrows that are relatively shallow (30–80 cm: Ruf and Bieber 2020a). Apparently, the soil temperature was measured closer to the hibernaculum in Fig. 2a than in 2b. Negative gradients between body and environment in Fig. 2b imply a well-insulated hibernaculum. The red lines show body temperature
These records show that, in most cases, animals display numerous short torpor bouts during the summer season in non-reproductive years in nest boxes, typically rewarming to euthermia (~ 37–40 °C) and activity during the night (Fig. 2a). Dormice may also show prolonged episodes of torpor in underground burrows (Hoelzl et al. 2015). This behaviour is most prominent in the early active season. In years of sexual activity, hibernation onset is also only in autumn, but short torpor early in the year is virtually absent (Bieber and Ruf 2009; Bieber et al. 2017). As mentioned above, in non-reproductive years, dormice alternatively may re-enter hibernation, also called estivation as it occurs in summer, only a few weeks after their emergence from the previous hibernation. This way, dormice may spent up to 11.4 months in continuous hibernation (Hoelzl et al. 2015). Estivation is restricted to the ~ 50% heaviest animals with large body fat reserves, albeit this dichotomy is not absolute (Hoelzl et al. 2015).
Entry into hibernation in G. glis is characterised by a rapid decline of heat production and metabolic rate, followed by a much slower decrease in body temperature (Wyss 1932; Wilz and Heldmaier 2000). The average metabolic rate reached in hibernation is 0.017 ml O2 g−1. This is a reduction to ~ 2% of basal metabolic rate. The minimum body temperature observed was 0.7 °C, and the temperature difference between body and environment was only 0.2 °C, on average, in deep hibernation (Wilz and Heldmaier 2000). Like most hibernators, dormice enter hibernation in a curled, ball-like position that minimises heat loss, and thermal conductance stays minimal and constant in torpor (Wilz and Heldmaier 2000). This underlines that metabolic reductions by hibernation are mainly achieved by lowering the heat loss gradient to the environment (Heldmaier and Ruf 1992). Metabolic decreases are not much supported by facilitated cooling, which would be expected if pure tissue temperature (Q10) effects were important. It seems that pure temperature (Q10) effects play only a negligible role and that the lowering of metabolic rate in hibernation is mainly due to an active downregulation (Nogueira de Sá and Chaui-Berlinck 2022). In this context, it should be noted the reducing respiration and metabolism in hibernation has several effects: not only does it minimise energy expenditure, it also helps the animals to remain motion, and largely odourless, which makes them difficult to detect for predators (Turbill et al. 2011; Ruf et al. 2012).
Both hibernation and estivation (Fig. 2a, b) are interrupted by periodic rewarming, also called arousals, to interbout euthermia. These arousals are typical for the vast majority of hibernators and were first described by Hall (1832) for bats, hedgehogs, and common dormice. It has been argued thar periodic arousals are beneficial for sleep, the immune system, or heart function. However, it still remains unclear why hibernators periodically rewarm from torpor, especially since these episodes are responsible for at least 70% of the energy expenditure during winter (Wang 1979; Strijkstra 1999). For a more thorough discussion of this topic, see Ruf et al. (2022). Both a comparison between hibernators and data from within a species (E. quercinus) show that the duration of torpor bouts is shortened if the metabolic rate during torpor is increased. This strongly points to an increasing metabolic imbalance during torpor, such as the depletion of a crucial substance, which is restored during interbout euthermia at high body temperature (Ruf and Geiser 2015; Ruf et al. 2021). The duration of torpor bouts in G. glis is the longest among hibernating rodents, only surpassed by a few species of bats. The maximum torpor bout duration in dormice is often above 6 weeks (Fig. 2a), the absolute maximum is 1278 h (7.6 weeks), and the average duration of interbout euthermia is 6.7 h (Hoelzl et al. 2015; Ruf and Geiser 2015).
The duration of torpor bouts is subject to evolution among hibernators. Torpor bout duration increases, and hence the frequency of costly arousals decreases, with increasing distance of the species distribution centre from the equator (Ruf and Geiser 2015). As mentioned above, the same tendency can be found within G. glis, i.e., a gradient in torpor bout duration between Lithuania and the Mediterranean (Rossolimo et al. 2001; Milazzo et al. 2003).
There are only two known environmental factors that affect torpor bout duration in hibernation. The first is dietary fatty acids. Increased dietary uptake of n-6 polyunsaturated fatty acids, particularly of linoleic acid (LA, C18:2 n-6), enables animals to reach lower body temperatures, lengthens torpor bout duration, and results in lower energy expenditure during hibernation (Arnold et al. 2015; Giroud et al. 2018). In fact, providing LA in the diet can more than double torpor bout duration (Munro and Thomas 2004; Ruf and Arnold 2008). Dormice of the sister clade E. quercinus even delay hibernation and remodel their membranes if fed a LA deficient diet (Giroud et al. 2018). However, LA apparently is no limiting environmental factor for edible dormice, which over the summer season increasingly digest seeds very rich in LA, and deposit it in white fat (Fietz et al. 2005). It would be interesting to study torpor bout duration and hibernation energy expenditure in areas that lack LA-rich seeds.
The second extrinsic factor that is known to influence torpor bout duration is ambient temperature. Increasing environmental temperatures shorten torpor bout length and increase arousal frequency both in hibernators in general and in edible dormice (Bieber and Ruf 2009; Geiser 2021). There is a single linear relationship between soil temperature and bout duration, which approximately doubles as ambient temperature decreases by 10 °C (Bieber and Ruf 2009). Hibernators respond even to the < 3 °C fluctuations of a cooling chamber (Ruf et al. 2021). Thus, rapidly globally rising temperatures, if the rise is too fast to allow evolutionary adaptation (Hoffmann and Sgrò 2011), expectedly will lead to an increase in rewarming frequency and energy depletion during hibernation. Hence, climate change poses a severe threat for G. glis and other hibernators.
Estivation in G. glis occurs in animals without severe cold load during summer (Fig. 2b). In fact, a good body condition in terms of ample fat reserves is even a prerequisite for this behaviour (Bieber and Ruf 2009; Hoelzl et al. 2015). Hence, at least in this species, energy savings seem not the primary goal of retreating to underground burrows during summer.
In this regard, G. glis appears to differ from other mammals that undergo estivation mainly to endure periods of severe heat and drought (Geiser 2010). Well-known examples are north American and Australian species that may enter torpor in summer (Bartholomew and Cade 1957; Bartholomew and MacMillen 1961; Bartholomew and Hudson 1962; Brice et al. 2002; Turner et al. 2012). Data on edible dormouse estivation underline the functional uniformity of hibernation and estivation. The frequency and duration, for example, of arousals follow identical dependencies on burrow temperature in summer and winter (Bieber and Ruf 2009). The motives for employing estivation may however differ between species.
Arguably, edible dormice and several other hibernators use hibernation below ground in non-reproductive years to escape their predators, which include both mammals and nocturnal birds of prey (Kryštufek 2010). A comprehensive comparative study on hibernators in general revealed that monthly survival is significantly higher during hibernation than during the active season (Turbill et al. 2011; Fig. 3). Since predation is the main cause of mortality in small rodents, this is likely the main source of these differences.
Monthly survival rates in 19 species of hibernators are significantly higher (P < 0.05) during hibernation vs. the remainder of the year. N = 38 populations (adults) and N = 8 populations (juveniles), data from Turbill et al. (2011)
As mentioned before, predator avoidance is augmented by the fact that cold, torpid mammals hardly emit any odours. In fact, Brown (1970) introduced hungry weasels on several occasions into a room containing hibernating jumping mice, and the predators were unable to locate the hidden, but accessible mice. Thus, reports of predators, such as American badgers, specialising on hunting hibernators seem to be the exception, rather than the rule (Michener 2004). Hibernation as an escape from predators is particularly pronounced in the edible dormouse. Comparing dormouse populations from five European countries, Lebl et al. (2011) found that survival rates were close to 100% in hibernation, and were lowest in early summer in all populations (monthly survival ~ 80% or below). It is at this time of the year in spring that avian and mammalian predators reproduce themselves and show increased foraging activity. This work, especially the seminal paper by Turbill and colleagues (2011) has led to a new view: Hibernation is no longer regarded as merely a response to harsh environmental conditions but also serves to minimise predation. In edible dormice, entire summer seasons are spent in estivation in years of reproduction skipping, and long-term torpor has become an integral part of their life history. Hibernators are generally long-lived, and have long generation times (Turbill et al. 2011). The maximum lifespan of edible dormice in England, Germany, and Austria is 13–14 years (Trout et al. 2015; Havenstein et al. 2021, and C. Bieber pers. comm.). An important prerequisite for this high longevity in a 100-g rodent is low extrinsic mortality, for instance by a low predation rate on younger adults (Kirkwood 1977). Only if extrinsic mortality is low, it pays to invest in cellular maintenance and high longevity. Apparently, one important factor for this decreased mortality is the repeated absence from aboveground, and estivation instead, of G. glis.
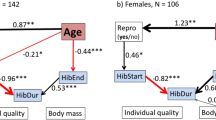
Age has a profound effect on hibernation, primarily because in dormice there is an intricate relationship between hibernation and reproduction. As dormice grow older, the probability to reproduce increases as there are diminishing chances for future reproduction (Bieber et al. 2018; Ruf and Bieber 2020a). In other words, old dormice cannot afford to ‘sit tight’ forever until environmental conditions are optimal for reproduction. With increasing age, edible dormice therefore phase advance the entire hibernation season, with a most pronounced forward-shift of spring emergence. By coming out of hibernation, early females will have access to the best territories (Bieber et al. 2018). Despite their use of huddling, mainly with related individuals, dormice defend foraging territories (e.g. Cornils et al. 2017; Ruf and Bieber 2020b). An earlier onset of reproduction generally gives offspring a better chance to survive the following winter, which is also true for dormice (Pilastro et al. 1994). The timing of emergence from hibernation in spring is also sex-specific (Vietinghoff-Riesch 1960; Bieber 1998; Schlund et al. 2002). In edible dormice, as in many hibernators, males emerge before females (Michener 1983; Körtner and Geiser 1998; Blumstein 2009; Lane et al. 2011). An early emergence of males is thought to enhance their reproductive success by maximising the number of potential mates available. However, the early termination of hibernation will expose both sexes to additional predation pressure, which is particularly high in spring (Lebl et al. 2011). Together, this evidence shows that hibernation is a flexible life history trait, with its duration and timing depending on an animals’ age, body condition, and residual reproductive value.
Trade-offs and seasonal clocks
The lowering of body temperature to save energy as well as the escape from predation during torpor comes at a cost. This can be most easily seen by the fact that short torpor is almost completely restricted to non-mast, non-reproductive years (Bieber et al. 2017). It has long been known that torpor is largely incompatible with reproduction in rodents (Fietz et al. 2004;; but see McAllan and Geiser 2014). In males, high testosterone levels prevent torpor and high body temperatures are required for spermatogenesis (e.g., Barnes et al. 1987). Only in reproductive failure years dormice may save energy by lowering body temperature during the day and return to activity during the night (Hoelzl et al. 2015; Bieber et al. 2017). Occasionally, dormice show torpor episodes lasting several days during cold spells early in the summer season (Hoelzl et al. 2015). Dormice use short torpor predominantly in the early active season but hardly in its second half, just prior to hibernation (Fig. 2). This period of pre-hibernation fattening is associated with intense, energy-consuming foraging leading to body temperatures above 40 °C for several hours per night (Bieber et al. 2017). Possibly, torpor is avoided during this time because high rates of digestion of food and fat deposition require high body temperatures. It has been suggested that the use of torpor generally has a negative impact on activity because the reduction of energy expenditure during torpor allows animals to minimise foraging (Ruf and Heldmaier 2000; Turbill and Stojanovski 2018). When reproductive activity prevents torpor, but also in non-reproductive years, dormice use an alternative avenue of energy savings: they utilise huddling and communal nesting of up to 16 animals at a time (Ruf and Bieber 2020b). Social thermoregulation is particularly used at low ambient temperatures during summer and is employed mainly by small dormice, often by yearlings, and frequently by related individuals (Ruf and Bieber 2020b).
An indication of negative, potentially risky effects of deep torpor comes from a closer analysis of hibernation (Bieber et al. 2014). This analysis shows that fat dormice with large white adipose tissue depots avoid deep torpor have shorter mean torpor bout length and rewarm more frequently (Bieber et al. 2014; Fig. 4). Torpor avoidance, if possible, may be a general phenomenon since almost identical effects of increasing body mass were obtained from woodchucks (Zervanos et al. 2014). Also, chipmunks avoid torpor when ample food is available (Humphries et al. 2003). The maintenance, for example, of a continued cardiac function at a body temperature around 0 °C may be particularly challenging and is avoided whenever possible (Ruf and Arnold 2008; Bieber et al. 2014; Giroud et al. 2018).
The mean duration of torpor bouts during hibernation as a function of body mass prior to hibernation inedible dormice. Particularly, fat animals minimise hibernation and show relatively short torpor bouts, data from Bieber et al. (2014). Animals were kept in large outdoor enclosures and hibernated in the soil
Another example for trade-offs involved in hibernation is the accompanying shortening of telomeres, a marker of somatic maintenance and damage. Together with the telomere-associated protein, telomeres prevent the degradation of linear DNA, but shorten with every cell division—because of the end replication problem in mitosis (Blackburn 1991; Blasco 2007). In addition to the shortening during cell proliferation, oxidative stress has a strong effect on telomere erosion (Proctor and Kirkwood 2002; von Zglinicki 2002). High oxidative stress during rewarming from deep torpor (Buzadžić et al. 1990; Carey et al. 2000; Orr et al. 2009) is probably the reason why telomeres in dormice are shortened more with an increasing number of arousals (Hoelzl et al. 2016a). This negative effect of hibernation is surprising, because hibernators in general, and dormice in particular invest in somatic maintenance and are very long-lived. It turns out that G. glis is highly unusual because animals older than ~ 5 years actually prolong their telomeres again (Hoelzl et al. 2016b), perhaps to prepare for telomere loss due to oxidative stress during reproduction. The net effect on telomeres is shortening in younger and elongation in older dormice (Hoelzl et al. 2016b).
Telomere elongation takes place not only in spring after hibernation (Hoelzl et al. 2016a) but even the last third of the hibernation season itself (Nowack et al. 2019). This finding points to an endogenous seasonal rhythm of telomere control, just like of hibernation itself, which for example is terminated without any photoperiod or temperature cues (see Fig. 1 in Hoelzl et al. 2015). In many species, hibernation is controlled by an endogenous circannual clock that is normally entrained by photoperiod (Pengelley and Fisher 1957; e.g., Fisher 1964). Ground squirrels kept in constant conditions showed a cycle of about 11 months (Davis 1976). We know now that the seasonal control of hibernation involves a cell population called tanycytes adjacent to the third ventricle of the brain. Tanycytes have been identified as major players in the seasonal control of energetic states in mammals; however, the exact mechanism of hibernation control is currently unknown (review in Jastroch et al. 2016).
It has been suggested that G. glis requires normal seasonal fluctuations of temperature to show a circannual cycle of hibernation, which is why this species has been called “thermoperiodic” (Jallageas et al. 1989). Mrosovsky (1977) found that body mass and hibernation cycles had a mean length of only 53 days (range 22–85 days) at 22 °C, whereas dormice in a cold room at 5 °C had a mean cycle length of 162 days (range 28–425 days). Hence, it seems that ambient temperature indeed affects annual cycles, but the mechanism causing circannual hibernation rhythms in G. glis is less than clear. However, the above conclusions were based on experiments in the laboratory with animals kept in standard cages. Notably, edible dormice are extremely reluctant to display normal torpor if they do not have access to self-dug hibernacula in the soil that may convey relative safety from predation. Thus, our understanding of genuine hibernation cycles in G. glis may require experiments that combine constant photoperiod or temperature with conditions of simulated natural hibernacula.
Conclusions
Dormice clearly use estivation and hibernation not primarily to avoid cold environments and a scarcity of food, but to avoid predation by remaining in hibernacula whenever possible. This becomes evident when they enter long torpor during a time when there is ample food to survive but predators are active. As a consequence, edible dormice spend up to three quarters of their life in hibernation, hidden below ground. In dormice, this extreme hibernation is linked to the pattern of reproduction, and absent in areas where reliably fruiting beech are not the dominating tree (Vekhnik 2017, 2019). This raises the question whether G. glis is unique in this respect or reflects a widespread pattern. Among hibernators, a close link to pulsed resources is only known from Eastern chipmunks in North America. Strikingly, chipmunks were found to interrupt aboveground activity for 9–11 months when mast was not available (Munro et al. 2008). However, while hibernation in response to pulsed good availability seems rare, it is quite possible that this link only becomes obvious on a population level, when all or most individuals remain absent in certain years. Given that increasing the perceived predation pressure enhances the use of torpor (Turbill and Stojanovski 2018), it is likely that other hibernators individually, and locally, trade reproduction against safety from predators. Possibly, this trade-off is as important in causing hibernation as the harshness of environments.
References
Arnold W, Giroud S, Valencak TG, Ruf T (2015) Ecophysiology of omega fatty acids: a lid for every jar. Physiology 30:232–240. https://doi.org/10.1152/physiol.00047.2014
Barnes BM, Licht P, Zucker I (1987) Temperature dependence of invitro androgen production in testes from hibernating ground squirrels, Spermophilus lateralis. Can J Zool 65:3020–3023. https://doi.org/10.1139/z87-457
Bartholomew GA, Hudson JW (1962) Hibernation, estivation, temperature regulation, evaporative water loss, and heart rate of the pigmy possum, Cercaertus nanus. Physiol Zool 35:94–107. http://www.jstor.org/stable/30152716
Bartholomew GA, Cade TJ (1957) Temperature regulation, hibernation, and aestivation in the little pocket mouse, Perognathus longimembris. J Mammal 38:60–72. https://doi.org/10.2307/1376476
Bartholomew GA, MacMillen RE (1961) Oxygen consumption, estivation, and hibernation in the kangaroo mouse, Microdipodops pallidus. Physiol Zool 34:177–183. https://doi.org/10.2307/30152696
Bergeron P, Réale D, Humphries MM, Garant D (2011) Anticipation and tracking of pulsed resources drive population dynamics in eastern chipmunks. Ecology 92:2027–2034. https://doi.org/10.1890/11-0766.1
Bieber C (1998) Population dynamics, sexual activity, and reproduction failure in the fat dormouse (Myoxus glis). J Zool (lond) 244:223–229. https://doi.org/10.1111/j.1469-7998.1998.tb00027.x
Bieber C, Ruf T (2004) Seasonal timing of reproduction and hibernation in the edible dormouse (Glis glis). In: Barnes BM, Carey HV (eds) Life in the cold V: evolution, mechanism, adaptation, and application Twelfth International Hibernation Symposium. University of Alaska, Fairbanks, Alaska, USA, Institute of Arctic Biology, pp 113–125
Bieber C, Ruf T (2009) Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften 96:165–171. https://doi.org/10.1007/s00114-008-0471-z
Bieber C, Außerlechner K, Skerget C, Walzer C, Ruf T (2011) Seasonal changes in liver size in edible dormice (Glis glis): non-invasive measurements using ultrasonography. Eur J Wildl Res 57:657–662. https://doi.org/10.1007/s10344-010-0476-8
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T (2014) Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol 28:167–177. https://doi.org/10.1111/1365-2435.12173
Bieber C, Cornils JS, Hoelzl F, Giroud S, Ruf T (2017) The costs of locomotor activity? Maximum body temperatures and the use of torpor during the active season in edible dormice. J Comp Physiol B 187:803–814. https://doi.org/10.1007/s00360-017-1080-y
Bieber C, Turbill C, Ruf T (2018) Effects of aging on timing of hibernation and reproduction. Sci Rep 8:13881. https://doi.org/10.1038/s41598-018-32311-7
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573. https://doi.org/10.1038/350569a0
Blasco MA (2007) Telomere length, stem cells and aging. Nat Chem Biol 3:640–649. https://doi.org/10.1038/nchembio.2007.38
Blumstein DT (2009) Social effects on emergence from hibernation in yellow-bellied marmots. J Mammal 90:1184–1187. https://doi.org/10.1644/08-MAMM-A-344.1
Brice P, Grigg G, Beard L, Donovan JA (2002) Patterns of activity and inactivity in echidnas (Tachyglossus aculeatus) free-ranging in a hot dry climate: correlates with ambient temperature, time of day and season. Aust J Zool 50:461–475. https://doi.org/10.1071/ZO01080
Brown LN (1970) Population dynamics of the western jumping mouse (Zapus princeps) during a four-year study. J Mammal 51:651–658. http://www.jstor.org/stable/1378291
Buzadžić B, Spasić M, Saičić ZS, Radojičić R, Petrović VM, Halliwell B (1990) Antioxidant defenses in the ground squirrel Citellus citellus 2. The effect of hibernation. Free Radical Bio Med 9:407–413. https://doi.org/10.1016/0891-5849(90)90017-D
Carey HV, Frank CL, Seifert JP (2000) Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. J Comp Physiol [b] 170:551–559. https://doi.org/10.1007/s003600000135
Cornils JS, Hoelzl F, Rotter B, Bieber C, Ruf T (2017) Edible dormice (Glis glis) avoid areas with a high density of their preferred food plant - the European beech. Front Zool 14:23. https://doi.org/10.1186/s12983-017-0206-0
Cornils JS, Hoelzl F, Huber N, Zink R, Gerritsmann H, Bieber C et al (2018) The insensitive dormouse: reproduction skipping is not caused by chronic stress in Glis glis. J Exp Biol 221:jeb183558. https://doi.org/10.1242/jeb.183558
Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM et al (2013) Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340:1215–1217. https://doi.org/10.1126/science.1235765
Dark J (2005) Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr 25:469–497. https://doi.org/10.1146/annurev.nutr.25.050304.092514
Davis DE (1976) Hibernation and circannual rhythms of food consumption in marmots and ground squirrels. Q Rev Biol 51:477–514. https://doi.org/10.1086/409594
Fietz J, Schlund W, Dausmann KH, Regelmann M, Heldmaier G (2004) Energetic constraints on sexual activity in the male edible dormouse (Glis glis). Oecologia 138:202–209. https://doi.org/10.1007/s00442-003-1423-0
Fietz J, Pflug M, Schlund W, Tataruch F (2005) Influences of the feeding ecology on body mass and possible implications for reproduction in the edible dormouse (Glis glis). J Comp Physiol [b] 175:45–55. https://doi.org/10.1007/s00360-004-0461-1
Fisher KC (1964) On the mechanism of periodic arousal in the hibernating ground squirrel. In: Suomalainen P (ed.) Annales Academiae Scientiarum Fennicae Ser A, Helsinki, Suomalainen Tiedeakatemia, pp 143–156.
Florant GL, Healy J (2012) The regulation of food intake in mammalian hibernators: a review. J Comp Physiol [b] 182:451–467. https://doi.org/10.1007/s00360-011-0630-y
Geiser F (2010) Aestivation in Mammals and Birds. In: Navas CA, Carvalho JE (eds) Aestivation molecular and physiological aspects. Springer, Berlin Heidelberg, pp 95–111
Geiser F (2021) Ecological physiology of daily torpor and hibernation. Springer
Giroud S, Stalder G, Gerritsmann H, Kübber-Heiss A, Kwak J, Arnold W et al (2018) Dietary lipids affect the onset of hibernation in the garden dormouse (Eliomys quercinus): Implications for Cardiac Function. Front Physiol 9:1235. https://doi.org/10.3389/fphys.2018.01235
Hall M (1832) On hybernation. Phil Trans R Soc Lond 122:335–360. https://doi.org/10.1098/rstl.1832.0017
Havenstein N, Langer F, Weiler U, Stefanski V, Fietz J (2021) Bridging environment, physiology and life history: stress hormones in a small hibernator. Mol Cell Endocrinol 533:111315. https://doi.org/10.1016/j.mce.2021.111315
Healy JE, Bateman JL, Ostrom CE, Florant GL (2011) Peripheral ghrelin stimulates feeding behavior and positive energy balance in a sciurid hibernator. Horm Behav 59:512–519. https://doi.org/10.1016/j.yhbeh.2011.01.013
Heldmaier G, Ruf T (1992) Body temperature and metabolic rate during natural hypothermia in endotherms. J Comp Physiol [b] 162:696–706. https://doi.org/10.1007/BF00301619
Hoelzl F, Bieber C, Cornils JS, Gerritsmann H, Stalder GL, Walzer C et al (2015) How to spend the summer? Free-living dormice (Glis glis) can hibernate for 11 months in non-reproductive years. J Comp Physiol B 185:931–939. https://doi.org/10.1007/s00360-015-0929-1
Hoelzl F, Cornils JS, Smith S, Moodley Y, Ruf T (2016) Telomere dynamics in free-living edible dormice (Glis glis): the impact of hibernation and food supply. J Exp Biol 219:2469–2474. https://doi.org/10.1242/jeb.140871
Hoelzl F, Smith S, Cornils JS, Aydinonat D, Bieber C, Ruf T (2016) Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Sci Rep 6:36856. https://doi.org/10.1038/srep36856
Hoffmann AA, Sgrò CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485. https://doi.org/10.1038/nature09670
Humphries MM, Kramer DL, Thomas DW (2003) The role of energy availability in mammalian hibernation: an experimental test in free-ranging eastern chipmunks. Physiol Biochem Zool 76:180–186. https://doi.org/10.1086/367949
Jallageas M, Mas N, Assenmacher I (1989) Further demonstration of the ambient temperature dependence of the annual biological cycles in the edible dormouse, Glis glis. J Comp Physiol B Biochem Syst Environ Physiol 159:333–338. https://doi.org/10.1007/BF00691513
Jansen HT, Trojahn S, Saxton MW, Quackenbush CR, Evans Hutzenbiler BD, Nelson OL et al (2019) Hibernation induces widespread transcriptional remodeling in metabolic tissues of the grizzly bear. Commun Biol 2:336. https://doi.org/10.1038/s42003-019-0574-4
Jastroch M, Giroud S, Barrett P, Geiser F, Heldmaier G, Herwig A (2016) Seasonal control of mammalian energy balance: recent advances in the understanding of daily torpor and hibernation. J Neuroendocrinol 28. https://doi.org/10.1111/jne.12437
Jurczyszyn M (2007) Hibernation cavities used by the edible dormouse, Glis glis (Gliridae, Rodentia). Folia Zool 56:162–168. https://www.ivb.cz/wp-content/uploads/56_162-168.pdf
Juškaitis R, Augutė V (2015) The fat dormouse, Glis glis, in Lithuania: living outside the range of the European beech, Fagus sylvatica. Folia Zool 64:310–315. https://doi.org/10.25225/fozo.v64.i4.a3.2015
Kirkwood TBL (1977) Evolution of ageing. Nature 270:301–304. https://doi.org/10.1038/270301a0
Koppmann-Rumpf B, Heberer C, Schmidt K-H (2003) Long term study of the reaction of the edible dormouse Glis glis (Rodentia: Gliridae) to climatic changes and its interaction with hole-breeding passerines. Acta Zool Acad Sci Hung 49 (Suppl. 1):69–76. http://publication.nhmus.hu/actazool/cikkreszletes.php?idhoz=3672
Körtner G, Geiser F (1998) Ecology of natural hibernation in the marsupial mountain pygmy-possum (Burramys parvus). Oecologia 113:170–178. https://doi.org/10.1007/s004420050365
Kronfeld-Schor N, Richardson C, Silvia BA, Kunz TH, Widmaier EP (2000) Dissociation of leptin secretion and adiposity during perhibernatory fattening in little brown bats. Am J Physiol Reg Int Comp Physiol 279:R1277–R1281. https://doi.org/10.1152/ajpregu.2000.279.4.R1277
Kryštufek B (2010) Glis glis (Rodentia: Gliridae). Mamm Species 42:195–206. https://doi.org/10.1644/865.1
Lane JE, Kruuk LEB, Charmantier A, Murie JO, Coltman DW, Buoro M et al (2011) A quantitative genetic analysis of hibernation emergence date in a wild population of Columbian ground squirrels. J Evol Biol 24:1949–1959. https://doi.org/10.1111/j.1420-9101.2011.02334.x
Lebl K, Bieber C, Adamík P, Fietz J, Morris P, Pilastro A et al (2011) Survival rates in a small hibernator, the edible dormouse: a comparison across Europe. Ecography 34:683–692. https://doi.org/10.1111/j.1600-0587.2010.06691.x
McAllan BM, Geiser F (2014) Torpor during reproduction in mammals and birds: dealing with an energetic conundrum. Integr Comp Biol 54:516–532. https://doi.org/10.1093/icb/icu093
Michener GR (1983) Spring emergence schedules and vernal behavior of Richardson’s ground squirrels: why do males emerge from hibernation before females? Behav Ecol Sociobiol 14:29–38. https://doi.org/10.2307/4599648
Michener GR (2004) Hunting techniques and tool use by north American badgers preying on Richardson’s ground squirrels. J Mammal 85:1019–1027. https://doi.org/10.1644/Bns-102
Milazzo A, Falletta W, Sara M (2003) Habitat selection of fat dormouse (Glis glis italicus) in deciduous woodlands of sicily. Acta Zool Acad Sci Hung 49:117–124. http://publication.nhmus.hu/pdf/actazool/ActaZH_2003_Vol_49_Suppl1_117.pdf.
Millar JS (1977) Adaptive features of mammalian reproduction. Evolution 31:370–386. https://doi.org/10.2307/2407759
Morris PA, Hoodless A (1992) Movements and hibernaculum site in the fat dormouse (Glis glis). J Zool (lond) 228:685–687. https://doi.org/10.1111/j.1469-7998.1992.tb04468.x
Morris PA, Morris MJ (2010) A 13-year population study of the edible dormouse Glis glis in Britain. Acta Theriol 55:279–288. https://doi.org/10.4098/j.at.0001-7051.066.2009
Mrosovsky N (1977) Hibernation and body weight in dormice: a new type of endogenous cycle. Science 196:902–903. https://doi.org/10.1126/science.860123
Munro D, Thomas DW (2004) The role of polyunsaturated fatty acids in the expression of torpor by mammals: a review. Zoology 107:29–48. https://doi.org/10.1016/j.zool.2003.12.001
Munro D, Thomas DW, Humphries MM (2008) Extreme suppression of aboveground activity by a food-storing hibernator, the eastern chipmunk (Tamias striatus). Can J Zool 86:364–370. https://doi.org/10.1139/Z08-008
Nogueira de Sá PG, Chaui-Berlinck JG (2022) A thermodynamic-based approach to model the entry into metabolic depression by mammals and birds. J Comp Physiol B. https://doi.org/10.1007/s00360-022-01442-9
Nowack J, Tarmann I, Hoelzl F, Smith S, Giroud S, Ruf T (2019) Always a price to pay: hibernation at low temperatures comes with a trade-off between energy savings and telomere damage. Biol Lett 15:20190466. https://doi.org/10.1098/rsbl.2019.0466
Orr AL, Lohse LA, Drew KL, Hermes-Lima M (2009) Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol 153:213–221. https://doi.org/10.1016/j.cbpa.2009.02.016
Pengelley ET, Fisher KC (1957) Onset and cessation of hibernation under constant temperature and light in the golden mantled ground squirrel, Citellus lateralis. Nature 180:1371–1372. https://doi.org/10.1038/1801371b0
Peters RH (1993) The ecological implications of body size. Cambridge University Press, Cambridge
Pilastro A, Gomiero T, Marin G (1994) Factors affecting body mass of young fat dormice (Glis glis) at weaning and by hibernation. J Zool 234:13–23. https://doi.org/10.1111/j.1469-7998.1994.tb06053.x
Proctor CJ, Kirkwood TBL (2002) Modelling telomere shortening and the role of oxidative stress. Mech Ageing Dev 123:351–363. https://doi.org/10.1016/S0047-6374(01)00380-3
Rossolimo OL, Potapova E, Pavlinov I, Kruskop S, Voltzit O (2001) Dormice (Myoxidae) of the world. Moscow University Publisher, Moscow
Ruf T, Arnold W (2008) Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am J Physiol Reg Int Comp Physiol 294:R1044–R1052. https://doi.org/10.1152/ajpregu.00688.2007
Ruf T, Bieber C (2020) Physiological, behavioral, and life-history adaptations to environmental fluctuations in the edible dormouse. Front Physiol 11:423. https://doi.org/10.3389/fphys.2020.00423
Ruf T, Bieber C (2020) Use of social thermoregulation fluctuates with mast seeding and reproduction in a pulsed resource consumer. Oecologia 192:919–928. https://doi.org/10.1007/s00442-020-04627-7
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. https://doi.org/10.1111/brv.12137
Ruf T, Heldmaier G (2000) Djungarian hamsters - small graminivores with daily torpor. In: Stenseth NC, Halle S (eds) Activity patterns in small mammals. Springer Verlag, Berlin, pp 217–234
Ruf T, Fietz J, Schlund W, Bieber C (2006) High survival in poor years: life history tactics adapted to mast seeding in the edible dormouse. Ecology 87:372–381. https://doi.org/10.1890/05-0672
Ruf T, Bieber C, Turbill C (2012) Survival, aging, and life-history tactics in Mammalian Hibernators. In: Ruf T, Bieber C, Arnold W, Millesi E (eds) Living in a Seasonal World Thermoregulatory and Metabolic Adaptations, Heidelberg, New York, pp 123–132
Ruf T, Gasch K, Stalder G, Gerritsmann H, Giroud S (2021) An hourglass mechanism controls torpor bout length in hibernating garden dormice. J Exp Biol 224:jeb243456. https://doi.org/10.1242/jeb.243456
Ruf T, Giroud S, Geiser F (2022) Hypothesis and theory: a two-process model of torpor-arousal regulation in hibernators. Front Physiol 13:901270. https://doi.org/10.3389/fphys.2022.901270
Santini L (1978) Biology, damage and control of the edible dormouse (Glis glis L.) in central Italy. In: Proceedings of the 8th Vertebrate Pest Conference, pp. 78–84.
Schaefer A, Piquard F, Haberey P (1976) Food self-selection during spontaneous body weight variations in the dormouse (Glis glis L.). Comp Biochem Physiol A 55:115–118. https://doi.org/10.1016/0300-9629(76)90077-3
Schlund W, Scharfe F, Ganzhorn JU (2002) Long-term comparison of food availability and reproduction in the edible dormouse (Glis glis). Mamm Biol 67:219–223. https://doi.org/10.1078/1616-5047-00033
Schmidt-Nielsen K (1979) Animal physiology: adaptation and environment. Cambridge University Press, New York
Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527. https://doi.org/10.1086/673092
Strijkstra AM (1999). Periodic euthermy during hibernation in the European ground squirrel: causes and consequences. (Thesis). Rijksuniversiteit te Groningen.
Trout RC, Brooks S, Morris P (2015) Nest box usage by old edible dormice (Glis glis) in breeding and non-breeding years. Folia Zool 64:320–324. https://doi.org/10.25225/fozo.v64.i4.a5.2015
Trout RC, Brooks S, Lim J, Rozycka D, Grimsey P, Grimsey M et al (2018) Movements by edible dormice (Glis glis) to their hibernation site and implications for population control. Folia Zool 67:91–97. https://doi.org/10.25225/fozo.v67.i2.a4.2018
Turbill C, Stojanovski L (2018) Torpor reduces predation risk by compensating for the energetic cost of antipredator foraging behaviours. Proc R Soc B 285:20182370. https://doi.org/10.1098/rspb.2018.2370
Turbill C, Bieber C, Ruf T (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc B 278:3355–3363. https://doi.org/10.1098/rspb.2011.0190
Turner JM, Körtner G, Warnecke L, Geiser F (2012) Summer and winter torpor use by a free-ranging marsupial. Comp Biochem Physiol A 162:274–280. https://doi.org/10.1016/j.cbpa.2012.03.017
Vekhnik VA (2017) The edible dormouse (Glis glis, Gliridae, Rodentia) in the periphery of its distribution range: body size and life history parameters. Biol Bull 44:1123–1133. https://doi.org/10.1134/S1062359017090163
Vekhnik VA (2019) Effect of food availability on the reproduction in edible dormice (Glis glis L., 1766) on the eastern periphery of the range. Mamm Res 64:423–434. https://doi.org/10.1007/s13364-019-00425-6
Vietinghoff-Riesch AFV (1960) Der Siebenschläfer. Gustav Fischer Verlag, Jena
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344. https://doi.org/10.1016/S0968-0004(02)02110-2
Wang LCH (1979) Time patterns and metabolic rates of natural torpor in the Richardson’s ground squirrel. Can J Zool 57:149–155. https://doi.org/10.1139/Z79-012
Wilz M, Heldmaier G (2000) Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. J Comp Physiol [b] 170:511–521. https://doi.org/10.1007/s003600000129
Wyss OAM (1932) Winterschlaf und Wärmehaushalt, untersucht am Siebenschläfer (Myoxus glis). Pflügers Arch Ges Physiol 229:599–635. https://doi.org/10.1007/BF01754494
Yang LH, Edwards KF, Byrnes JE, Bastow JL, Wright AN, Spence KO (2010) A meta-analysis of resource pulse-consumer interactions. Ecol Monogr 80:125–151. https://doi.org/10.1890/08-1996.1
Zervanos SM, Maher CR, Florant GL (2014) Effect of body mass on hibernation strategies of woodchucks (Marmota monax). Integr Comp Biol 54:443–451. https://doi.org/10.1093/icb/ict100
Acknowledgements
We thank the Österreichische Bundesforste AG and the City of Vienna for their continued support of our research on dormice. Thanks to Renate Hengsberger for her help with the literature search and formatting of the manuscript. We also thank two anonymous reviewers for their thoughtful comments.
Funding
Open access funding provided by University of Veterinary Medicine Vienna Open Access funding by the Veterinary University if Vienna. This study was funded by the Austrian Science Foundation, grant numbers P 25023 and P 25034, and the City of Vienna.
Author information
Authors and Affiliations
Contributions
TR and CB both conceived and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Facundo Luna
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruf, T., Bieber, C. Why hibernate? Predator avoidance in the edible dormouse. Mamm Res 68, 1–11 (2023). https://doi.org/10.1007/s13364-022-00652-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00652-4