Abstract
Helium direct analysis in real time (He-DART) mass spectrometry (MS) of some compounds, polysaccharides, for example, usually tends to be challenging because of the occurrence of prominent in-source decay (ISD), which was considered as an undesired side reaction, as it complicated the resulting mass spectra. Our approach is to take advantage of an efficient and practical method termed the temperature-dependent ISD (TDISD) technique combined with fragmentation of the dehydrated dimers using DART Q-TOF tandem mass spectrometry for differentiation of disaccharide isomers. In this study, cross-ring cleavages and non-ovalent complexes were detected in the spectra of the saccharides. It was observed that the gas heater temperature had a significant effect on the absence or presence of signal in DART spectra. At high gas temperature, ions in high mass region began to appear. Based on the types of cross-ring cleavages and noncovalent complexes, disaccharide isomers with different linkage positions can be differentiated in both positive and negative ion modes at a lower DART gas temperature. Additionally, anomeric configurations were assigned on the basis of the relative abundance ratio of m/z 198:342 obtained by the comparison of the positive ion mode tandem mass spectrum of an α isomer dimer generated at higher DART gas temperature and that of the corresponding β one. In general, this method is easy, fast, effective, and robust for identifying disaccharide isomers.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few years, direct analysis in real time (DART) has represented one of the numerous desorption/ionization techniques that allows the rapid analysis of samples or objects in their native state in the open environment. Compared with conventional mass spectrometry (MS) techniques, for instance, electrospray ionization (ESI), DART has clear advantages, such as requiring minimal or no sample preparation, high throughput, lack of memory effects, and a relatively low tendency toward ion suppression [1–3]. DART employs an atmospheric pressure glow discharge for the ionization. Metastable helium or nitrogen atoms, originating in the plasma, react with ambient water, oxygen, or other atmospheric components to ionize analytes [4–6]. The DART ionization mechanisms are not yet fully understood, but the widely accepted mechanism is Penning ionization [7]. During the gas-phase ionization processes, protonation, deprotonation, and adduct ion formation will occur in DART [8, 9].
Numerous DART MS methods have been developed to rapidly analyze various samples in the past few years [10–14]. For instance, Liu’s research team reported its application to the rapid determination of triazine herbicides in water [10]. Chernetsova and collaborators used DART MS to identify phenolic compounds in propolis [14]. Although many types of analytes have been studied, information on analyzing monosaccharides and disaccharides using DART MS is limited [15]. The structures of the oligosaccharides are in many cases related to their multiple important biological functions, while structural analysis of carbohydrates is still challenging because of the enormous structural diversity and microheterogeneity [16–18]. With the development of MS, MS-based technology has become a pivotal methodology for structural elucidation of carbohydrates [19–28]. In our recent reports [22–24], oligosaccharide isomers were successfully distinguished by laser-enhanced in-source decay (LEISD) of matrix-assisted laser desorption/ionization (MALDI) MS or 1-phenyl-3-methyl-5-pyrazolone labeling technique in conjunction with electrospray ionization (ESI) tandem mass spectrometry. The monosaccharide structural isomers could exhibit different mobility drift times in both drift tube and traveling wave ion mobility mass spectrometry, depending on differences in their anomeric and stereochemical configurations [26]. For disaccharide isomers, they can be distinguished by traveling wave ion mobility mass spectrometry using CO2 as drift gas [27] or data mining techniques in conjunction with variable wavelength infrared multiple photon dissociation mass spectrometry [28].
Even if DART is generally considered as a soft ionization process, a significant degree of in-source decay (ISD) fragmentation can occur during the ionization event [6, 29, 30], especially when using He as DART gas. Interestingly, in-source adducts after fragmentation were observed during analysis of nucleotides by DART MS [30]. In the present paper, high numbers of ISD fragments and complexes produced from monosaccharides and disaccharides were observed for the first time during our investigation of the feasibility of detecting oligosaccharides using DART MS. The extent of fragmentation and generation of the complexes is dependent on the DART gas temperature. The application of the identification of the isomeric disaccharides is presented to demonstrate the effectiveness of the temperature-dependent ISD (TDISD) of DART quadrupole time-of-flight (Q-TOF) MS. The interesting phenomena described in this paper will also provide new insights into the complicated processes present in the DART ion source.
Experimental
Chemicals and Reagents
Glucose and lactose were obtained from Beihua Fine Chemicals Co., Ltd. (Beijing, China). Sophorose, maltose, isomaltose, and nigerose were purchased from Sigma (St. Louis, MO, USA). Cellobiose and gentiobiose were bought from J&K Chemical Ltd. (Beijing, China). Laminaribiose was acquired from Megazyme (Wicklow, Ireland). Kojibiose was acquired from Carbosynth (Berkshire, UK). Methanol (HPLC grade) was obtained from Fisher Chemical Company (Fair Lawn, NJ, USA). High-purity helium and nitrogen (99.999%) were supplied by Changchun Juyang Gas Co., Ltd. (Changchun, China). Ultrapure water (specific conductivity, 18.2 MΩ/cm) was produced by a MilliQ device (Millipore, Milford, MA, USA).
DART MS Analysis
Analysis was performed on a fourth generation DART source with standard voltage, pressure, and angling capability (SVPA) (IonSense, Inc., Saugus, MA, USA) interfaced to a 6520 Q-TOF mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The DART ion source was operated with helium for analysis and nitrogen in the standby mode. The DART gas temperature was varied between 200 and 450°C, and gas flow rates were set to 2 L/min. Grid electrode voltages were set to 350 V (positive ion mode) and –300 V (negative ion mode), respectively. Automated acquisition of mass spectra was executed by Agilent Mass Hunter Qualitative Analysis software.
The analytes were introduced into the DART sample gap using the closed end of a melting point capillary tube that was directly dipped into the sample vial. For each sample, the capillary tube was held close to the DART cap for about 30 s, which was positioned 1 mm below and 1 mm in front of the tapered ceramic exit cap on the DART-SVPA source.
The settings for the Q-TOF mass spectrometer were as follows: gas temperature, 250°C; drying gas, 2 L/min; fragmentor voltage, 10-400 V; and capillary voltage, 3500 V. Selected precursor ions were fragmented with collision-induced dissociation (CID) at collision energy ranging from 1 to 20 V with ultra-high purity nitrogen gas (99.999%) to preserve the signal intensity of the precursor ion in the range of 5%–20%.
Results and Discussion
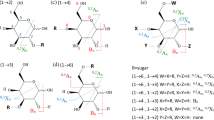
In this study, the DART ion source was interfaced with a time-of-flight (TOF) mass spectrometer enabling accurate mass measurements and providing elemental compositions. The Domon and Costello nomenclature [31] has been employed throughout this work to define the fragment ions from the monosaccharides and disaccharides. According to this nomenclature, the ions retaining the charge at the nonreducing terminus are designated as Ai for cross-ring cleavages, and Bi and Ci for glycosidic bond cleavages. The subscript I represents the number of the glycosidic bond cleavage, counted from the nonreducing end. Those retaining the charge at the reducing terminus are designated as Xj for cross-ring cleavages, and Yj and Zj for glycosidic bond cleavages. The subscript j represents the number of the glycosidic bond cleavage, counted from the reducing end. In the case of ring cleavages, superscript numbers are given to show the ruptured bonds. Oligosaccharides predominately produced ammonium-adducted peaks in the positive ion mode. The structures of the nine disaccharide isomers are shown in Figure 1.
Recognition of the Linkage Types of Disaccharide Isomers
The observation of cross-ring fragmentation ions and noncovalent complexes in the DART mass spectra of glucose (Figure S-1 of Supporting Information) is hoped to be helpful for distinguishing disaccharide isomers. With this aim, the eight glucose-containing disaccharides were determined. As anticipated, disaccharide isomers with different linkage positions were differentiated based on the types of cross-ring cleavages. Figure 2 presents the DART mass spectra of the four α-linked disaccharides at heated helium gas temperature of 250°C in positive ion mode. Besides B- and Y-type ions, valuable A-ions marked in bold are detected in Figure 2. The mass spectrum in Figure 2a shows the base peak, m/z 240.1082, which corresponds to 1,3A2 ion, whereas no cross-ring cleavage ion is observed in Figure 2b. In comparison with Figure 2a, besides the ion at m/z 240.1078, another cross-ring cleavage ion at m/z 300.1292 is detected in Figure 2c. As for the α1→6 linked disaccharides, three noticeable A-ions at m/z 240.1080, 270.1187, and 300.1295 were generated (Figure 2d). In contrast, considerable noncovalent dimers such as the ions at m/z 225.0578 and 269.0881 were represented in the spectra of the four α-linked disaccharide isomers in negative ion mode (Figure 3). The characteristic fragment ions are also marked in bold in Figure 3.
In an effort to determine whether the anomeric configurations can be discriminated, the four β-linked disaccharides were analyzed again in positive and negative ion modes under the same experimental conditions (Figures S-2 and S-3 of Supporting Information). The characteristic ions were similar to those of α-linked disaccharides. Thus, the anomeric configurations cannot be distinguished under these conditions. In addition, to confirm the generality of the method, lactose (Galβ(1→4)Glc) was also determined (Figure S-4 of Supporting Information). No obvious difference was found between lactose and cellobiose (β1→4), which indicated that the monomer units in a disaccharide did not affect the observed absence/presence of diagnostic ions.
In short, according to the fragmentation characteristic, the disaccharide isomers with different linkage positions can be differentiated in either positive or negative ion mode. The characteristic fragments in positive ion mode are as follows: 1→2, m/z 240.1082 (1,3A2); 1→3, no cross-ring cleavage ion; 1→4, m/z 240.1078 (2,4A2) and m/z 300.1292 (0,2A2); 1→6, m/z 240.1080 (0,4A2), m/z 270.1187 (0,3A2), and m/z 300.1295 (0,2A2). In negative ion mode, the characteristic fragment ions are as follows: 1→2, m/z 221.0620 (1,3A2), m/z 239.0721 (Y1 + C2H4O2), and m/z 311.0918 (0,1A2); 1→3, no characteristic fragment ions; 1→4, m/z 239.0721 (Y1 + C2H4O2) and m/z 299.0922 (Y1 + C4H8O4); 1→6, m/z 239.0721 (Y1 + C2H4O2) and m/z 311.0925 (0,1A2).
Influence of DART Gas Temperature and Fragmentor Voltage on Mass Spectrometric Analysis of Disaccharides
Among several parameters of the DART ion source affecting the analyte signal, helium gas temperature [32, 33] is a key factor for fragmentation and cluster ion formation. The temperature effect was investigated in both positive and negative ion modes. By raising the temperature in increments of 50°C from 200 to 450°C, the greatest signal intensity was observed at a temperature of 450°C for both positive and negative ion modes. The representative results with gas temperature of 450°C in positive and negative ion modes are illustrated in Figure 4a and b, respectively, which is not-so-easily explainable. The high degree of ISD fragmentation and cluster of the analyte, which are not typically observed in DART MS, were detected in our investigation. They were different from those with a lower temperature, and abundant ions in the higher mass region (m/z > 400) of the spectrum appeared, as displayed in Figure 4a and b. In positive ion mode, the complexes consisting of 1,3A2 ion at m/z 240.1067 are observed in Figure 4a. Alathough actual signal intensity using DART-MS in the negative ion mode was less compared with that in positive ion mode, fragmentation ion and cluster ion formation were still readily apparent. Most of the complexes detected were Y1-related cluster ions such as the ions at m/z 269.1013, 405.1428, and 449.1704 (Figure 4b).
In theory, lower source temperature is in favor of the formation of noncovalent complexes since their thermal dissociation occurs at higher temperature [34]. Conversely, herein, a higher helium gas temperature formed more complexes. A reasonable explanation is that the final ion internal energy is not high attributable to the energy expenditure during the loss of water and in-source fragmentation in the gas phase at higher temperatures. On the other hand, the ions in high mass range can just be desorbed at the above mentioned conditions. Thus, more complexes were observed in our study.
The influence of fragmentor voltage on the mass spectrometric analysis of saccharides was also investigated. Fragmentor voltage was adjusted from 10 to 400 V, but no significant difference was found in the results except that the absolute abundances were higher at higher fragmentor voltage. This provided some evidence that the fragmentation occurred in the ion source.
Identification of Anomeric Configurations by the TDISD Method
In an effort to identify anomeric configurations, tandem mass spectrometric analysis was performed. As expected, the anomeric configurations can be differentiated by the comparison of the tandem mass spectra of the ions at m/z 684.2507 in the mass spectra of α- and β-linked disaccharide isomers at higher DART gas temperature in positive ion mode. To clarify this question, we took the tandem mass spectra of the ions at m/z 684.2506 from α1→2- and β1→2-linked disaccharides as examples. From Figure 5a and b, it can be seen that the dominant noncovalent product ions at m/z 378.1575 corresponding to 2Y1 are generated, which allowed us to draw a conclusion that the precursor ions at m/z 684.2506 must be noncovalent. Additionally, the fragment ions with the m/z values at 504.1914 and 522.1973 were determined to be formed from the neutral losses of C6H12O6 (180 Da) and C6H10O5 (162 Da), respectively. Therefore, it was deduced that these product ions must be formed directly from the dissociation of the dimer without the breakdown of the noncovalent bonds. In Figure 5a, the relative abundance ratio of m/z 198:342 is far greater than 1. Inversely, the ratio value is much less than 1 in Figure 5b. It is speculated that the results are due to the fact that β glycosidic bonds were more stable than the corresponding α-linked ones [22, 35].
In addition, the other eight disaccharides were also studied under the same conditions. Table 1 displays the relative abundance ratios of m/z 198:342 in the tandem mass spectra of the nine analytes. The relative abundance ratios of m/z 198:342 in α-linked disaccharides are far greater than 1. Inversely, the ratio values of β-linked disaccharide are less than 1 in Table 1. In brief, anomeric configurations were distinguished on the basis of the relative abundance ratios of m/z 198:342 obtained by the comparison of the positive-ion-mode tandem mass spectrum of an α isomer dimer generated at higher DART gas temperature and that of the corresponding β one.
In short, the TDISD method is a fast and efficient technique for differentiation of disaccharide isomers with potential analytical applications such as for food quality control [36]. Disaccharides are often the products of enzymatic activity; hence, our method can also be applied to this field.
Conclusions
A convenient method named as TDISD is developed to identify disaccharide isomers using DART source coupled to the Q-TOF mass spectrometer. To our knowledge, abundant ISD ions, including both glycosidic bond and cross-ring cleavage ions, and complexes arising from glucose and disaccharides, have never been observed in DART mass spectrometry before. We observed a remarkable effect of the temperature of the helium gas flow into the DART ionization source on the intensity and types of signals in the gaseous phase. Prominent ions in the higher mass region (m/z > 400) of the spectra, which were not detected at lower gas temperature, only appeared at higher gas temperature.
On the basis of types of cross-ring cleavage ions and noncovalent complexes, the disaccharide isomers with different linkage positions can be differentiated with lower heated gas temperature in either positive or negative ion mode. In addition, anomeric configurations were distinguished according to the relative abundance ratios of m/z 198:342 obtained by the comparison of the tandem mass spectra of the ions at m/z 684.2507 in the full mass spectra of α- and β-linked disaccharide isomers at higher DART gas temperature in positive ion mode. The work presented here suggests that the chemistry associated with oligosaccharides in DART is complicated, providing new insights into DART. The simplicity of the method makes it an attractive option for unequivocal identification of disaccharide isomers.
References
Weston, D.J.: Ambient ionization mass spectrometry: current understanding of mechanistic theory; analytical performance and application areas. Analyst 135, 661–668 (2010)
Cody, R.B., Laramée, J.A., Durst, H.D.: Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 77, 2297–2302 (2005)
Lesiak, A.D., Shepard, J.R.: Recent advances in forensic drug analysis by DART-MS. Bioanalysis 6, 819–842 (2014)
Harris, G.A., Nyadong, L., Fernandez, F.M.: Recent developments in ambient ionization techniques for analytical mass spectrometry. Analyst 133, 1297–1301 (2008)
Song, L., Gibson, S.C., Bhandari, D., Kelsey, D., Cook, K.D., Bartmess, J.E.: Ionization mechanism of positive-ion direct analysis in real time: a transient microenvironment concept. Anal. Chem. 81, 10080–10088 (2009)
Yang, H., Wan, D., Song, F., Liu, Z., Liu, S.: Argon direct analysis in real time mass spectrometry in conjunction with makeup solvents: a method for analysis of labile compounds. Anal. Chem. 85, 1305–1309 (2013)
Venter, A.R., Douglass, K.A., Shelley Jr., J.T., Hasman, G., Honarvar, E.: Mechanisms of real-time, proximal sample processing during ambient ionization mass spectrometry. Anal. Chem. 86, 233–249 (2014)
Saang’onyo, D.S., Smith, D.L.: Optimization of direct analysis in real time (DART) linear ion trap parameters for the detection and quantitation of glucose. Rapid Commun. Mass Spectrom. 26, 385–391 (2012)
Mazzotta, M.G., Pace, R.B., Wallgren, B.N., Morton III, S.A., Miller, K.M., Smith, D.L.: Direct analysis in real time mass spectrometry (DART-MS) of ionic liquids. J. Am. Soc. Mass Spectrom. 24, 1616–1619 (2013)
Wang, X., Li, X., Li, Z., Zhang, Y., Bai, Y., Liu, H.: Online coupling of in-tube solid-phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith. Anal. Chem. 86, 4739–4747 (2014)
Zhou, Z., Zhang, J., Zhang, W., Bai, Y., Liu, H.: Rapid screening for synthetic antidiabetic drug adulteration in herbal dietary supplements using direct analysis in real time mass spectrometry. Analyst 136, 2613–2618 (2011)
Gu, H., Pan, Z., Xi, B., Asiago, V., Musselman, B., Raftery, D.: Principal component directed partial least squares analysis for combining nuclear magnetic resonance and mass spectrometry data in metabolomics: application to the detection of breast cancer. Anal. Chim. Acta 686, 57–63 (2011)
Domin, M.A., Steinberg, B.D., Quimby, J.M., Smith, N.J., Greene, A.K., Scott, L.T.: Routine analysis and characterization of highly insoluble polycyclic aromatic compounds by direct analysis in real time mass spectrometry (DART). Analyst 135, 700–704 (2010)
Chernetsova, E.S., Bromirski, M., Scheibner, O., Morlock, G.E.: DART-Orbitrap MS: a novel mass spectrometric approach for the identification of phenolic compounds in propolis. Anal. Bioanal. Chem. 403, 2859–2867 (2012)
Wang, Y., Liu, L., Ma, L., Liu, S.: Identification of saccharides by using direct analysis in real time (DART) mass spectrometry. Int. J. Mass Spectrom. 357, 51–57 (2014)
Zaia, J.: On-line separations combined with MS for analysis of glycosaminoglycans. Mass Spectrom. Rev. 28, 254–272 (2009)
Mechref, Y., Novotny, M.V.: Structural investigations of glycoconjugates at high sensitivity. Chem. Rev. 102, 321–369 (2002)
Harvey, D.J.: Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2003–2004. Mass Spectrom. Rev. 28, 273–361 (2009)
Zaia, J.: Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 23, 161–227 (2004)
Park, Y., Lebrilla, C.B.: Application of Fourier transform ion cyclotron resonance mass spectrometry to oligosaccharides. Mass Spectrom. Rev. 24, 232–264 (2005)
Harvey, D.J., Dwek, R.A., Rudd, P.M.: Determining the structure of glycan moieties by mass spectrometry. Curr. Protoc. Protein Sci. 12, unit 12.7.1–12.7.18 (2006)
Yang, H., Yu, Y., Song, F., Liu, S.: Structural characterization of neutral oligosaccharides by laser-enhanced in-source decay of MALDI-FTICR MS. J. Am. Soc. Mass Spectrom. 22, 845–855 (2011)
Yang, H., Li, M., Li, Z., Liu, S.: Gas-phase fragmentation of oligosaccharides in MALDI laser-enhanced in-source decay induced by thermal hydrogen radicals. Analyst 137, 3624–3626 (2012)
Wan, D., Yang, H., Song, F., Liu, Z., Liu, S.: Identification of isomeric disaccharides in mixture by the 1-phenyl-3-methyl-5-pyrazolone labeling technique in conjunction with electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 780, 36–45 (2013)
da Costa, E.V., Moreira, A.S., Nunes, F.M., Coimbra, M.A., Evtuguin, D.V., Domingues, M.R.: Differentiation of isomeric pentose disaccharides by electrospray ionization tandem mass spectrometry and discriminant analysis. Rapid Commun. Mass Spectrom. 26, 2897–2904 (2012)
Li, H., Giles, K., Bendiak, B., Kaplan, K., Siems, W.F., Hill Jr., H.H.: Resolving structural isomers of monosaccharide methyl glycosides using drift tube and traveling wave ion mobility mass spectrometry. Anal. Chem. 84, 3231–3239 (2012)
Fasciotti, M., Sanvido, G.B., Santos, V.G., Lalli, P.M., McCullagh, M., de Sá, G.F., Daroda, R.J., Peter, M.G., Eberlin, M.N.: Separation of isomeric disaccharides by traveling wave ion mobility mass spectrometry using CO2 as drift gas. J. Mass Spectrom. 47, 1643–1647 (2012)
Stefan, S.E., Ehsan, M., Pearson, W.L., Aksenov, A., Boginski, V., Bendiak, B., Eyler, J.R.: Differentiation of closely related isomers: application of data mining techniques in conjunction with variable wavelength infrared multiple photon dissociation mass spectrometry for identification of glucose-containing disaccharide ions. Anal. Chem. 83, 8468–8476 (2011)
Shelley, J.T., Hieftje, G.M.: Ionization matrix effects in plasma-based ambient mass spectrometry sources. J. Anal. At. Spectrom. 25, 345–350 (2010)
Curtis, M., Minier, M.A., Chitranshi, P., Sparkman, O.D., Jones, P.R., Xue, L.: Direct analysis in real time (DART) mass spectrometry of nucleotides and nucleosides: elucidation of a novel fragment [C5H5O]+ and its in-source adducts. J. Am. Soc. Mass Spectrom. 21, 1371–1381 (2010)
Domon, B., Costello, C.E.: A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5, 397–409 (1988)
Nilles, J.M., Connell, T.R., Durst, H.D.: Thermal separation to facilitate direct analysis in real time (DART) of mixtures. Analyst 135, 883–886 (2010)
Cody, R.B.: Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal. Chem. 81, 1101–1107 (2009)
Barylyuk, K., Balabin, R.M., Grünstein, D., Kikkeri, R., Frankevich, V., Seeberger, P.H., Zenobi, R.: What happens to hydrophobic interactions during transfer from the solution to the gas phase? The case of electrospray-based soft ionization methods. J. Am. Soc. Mass Spectrom. 22, 1167–1177 (2011)
Guan, B., Cole, R.B.: MALDI linear-field reflectron TOF post-source decay analysis of underivatized oligosaccharides: determination of glycosidic linkages and anomeric configurations using anion attachment. J. Am. Soc. Mass Spectrom. 19, 1119–1131 (2008)
Ferrão, M.F., Mello, C., Borin, A., Maretto, D.A., Poppi, R.J.: LSSVM: a new chemometric tool for multivariate regression. Comparison of LS-SVM and PLS regression for determination of common adulterants in powdered milk by NIR spectroscopy. Quim. Nova 30, 852–859 (2007)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 21305135, 21175127, and 21475012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hongmei Yang and Lei Shi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 923 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Shi, L., Yao, W. et al. Differentiation of Disaccharide Isomers by Temperature-Dependent In-Source Decay (TDISD) and DART-Q-TOF MS/MS. J. Am. Soc. Mass Spectrom. 26, 1599–1605 (2015). https://doi.org/10.1007/s13361-015-1192-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1192-0