Abstract
Among Psychrobacter spp., there are several multireplicon strains, carrying more than two plasmids. Psychrobacter sp. ANT_H3 carries as many as 11 extrachromosomal replicons, which is the highest number in Psychrobacter spp. Plasmids of this strain were subjected to detailed genomic analysis, which enables an insight into the structure and functioning of this multireplicon genome. The replication and conjugal transfer modules of ANT_H3 plasmids were analyzed functionally to discover their potential for being used as building blocks for the construction of novel plasmid-vectors for cold-active bacteria. It was shown that two plasmids have a narrow host range as they were not able to replicate in species other than Psychrobacter, while remaining plasmids had a wider host range and were functional in various Alpha- and Gammaproteobacteria. Moreover, it was confirmed that mobilization modules of seven plasmids were functional, i.e., could be mobilized for conjugal transfer by the RK2 conjugation system. Auxiliary genes were also distinguished in ANT_H3 plasmids, including these encoding putative DNA-protecting protein DprA, multidrug efflux SMR transporter of EmrE family, glycine cleavage system T protein, MscS small-conductance mechanosensitive channel protein, and two type II restriction-modification systems. Finally, all genome-retrieved plasmids of Psychrobacter spp. were subjected to complex genome- and proteome-based comparative analyses showing that Antarctic replicons are significantly different from plasmids from other locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica is known to be one of the coldest regions on Earth, characterized also with increased UV radiation, strong winds, and the low amount of easily accessible nutrients (Martianov and Rakusa-Suszczewski 1990; Cowan et al. 2014). Antarctic bacteria are well-adapted, poly-extremophiles that can survive a variety of harsh physical and chemical conditions (Cowan et al. 2014). In our previous study, we obtained a collection of bacterial strains isolated from soil samples gathered on King George Island. This region is typical for Antarctica and is characterized by extreme conditions, including permanent cold with the average temperature of 2.0 °C, high humidity (78.7% in average), and average wind speed of 4.5 m∙s−1 (Araźny et al. 2013). Analyzed strains differed in their physiological features (including temperature and metals tolerance) and taxonomy, with three dominant genera: Psychrobacter (69 isolates), Pseudomonas (57), and Arthrobacter (47) (Romaniuk et al. 2018).
Psychrobacter spp. are Gram-negative, rod-shaped, non-motile, aerobic, heterotrophic bacteria frequently isolated from various habitats in Arctic and Antarctica. They are usually psychrotolerant, halotolerant, and non-pathogenic (Ayala-del-Rio et al. 2010). This group of bacteria is thoroughly studied, since they are recognized as model psychrotolerant microorganisms. Psychrobacter spp. are sources of cold-active enzymes for biotechnology, e.g., lipase (Esakkiraj et al. 2022), RNase (Wang et al. 2019), and esterase (Wu et al. 2015). Currently, over 250 complete and draft genomes of Psychrobacter spp. were obtained; however, up to date, much less is known about their plasmids. These are mostly small, cryptic replicons; however, in several of them, phenotypic modules were identified and their functionality was experimentally confirmed, including (i) fimbriae synthesis via the chaperone-usher pathway and genes involved in the aerobic and anaerobic metabolism of carnitine—pP32BP2 of Psychrobacter sp. DAB_AL32B (Ciok et al. 2019), (ii) laccase gene—pPspP11F6a of Psychrobacter sp. P11F6 (Moghadam et al. 2016), (iii) β-lactam, streptomycin, and tetracycline resistance genes—pKLH80 (Petrova et al. 2014), and (iv) organic sulfate metabolism operon—pP62BP1 of Psychrobacter sp. DAB_AL62B (Lasek et al. 2012). Only few Psychrobacter plasmids were used for the construction of novel vectors for molecular biology and biotechnology, while they are highly needed since Psychrobacter spp. are reference cold-active bacteria and potentially good candidates for developing effective expression systems active at low temperatures (Lasek et al. 2017; Ciok and Dziewit 2019).
Among Psychrobacter spp., there are several multireplicon strains, including Psychrobacter sp. P11G5 (seven plasmids; 5,511–40,963 bp) (Moghadam et al. 2016), Psychrobacter sp. 4Bb (six plasmids; 2,151–20,934 bp) (GenBank accession number: NZ_PJAS01000000), and Psychrobacter sp. Sarcosine-02u-2 (six plasmids; 1,929–11,462 bp) (GenBank accession number: NZ_PJBX00000000). However, the functioning of these multireplicon genomes was not investigated previously. In this study, we structurally and functionally characterized the plasmidome of a “record holder” Psychrobacter sp. ANT_H3, carrying 11 plasmids (3,124–13,249 bp). Moreover, we analyzed in detail replication and conjugal transfer modules of ANT_H3 plasmids to discover their potential for being used as building blocks for the construction of novel plasmid-vectors.
Materials and methods
Bacterial strains, plasmids, and culture conditions
The following laboratory bacterial strains were used: Achromobacter sp. LM16R (Dziewit et al. 2015), Agrobacterium tumefaciens LBA288 (Hooykaas et al. 1980), Escherichia coli BR825 (Ludtke et al. 1989), E. coli DH5α (Hanahan 1983), E. coli DH5αR (Bartosik et al. 2002), E. coli ER2566 (Zhou et al. 2020), E. coli S17-1 (Simon et al. 1983), Paracoccus alcaliphilus JCM 7364R (Bartosik et al. 2002), Pseudomonas aeruginosa PAO1161 (Bartosik et al. 2014), Pseudomonas sp. LM7R (Dziewit et al. 2013b), and Variovorax paradoxus EPS (Jamieson et al. 2009). The strains were grown on LB medium at 30 °C (EPS, JCM 7364R, LBA288, LM7R and LM16R) or 37 °C (BR825, DH5α, DH5αR, ER2566, and PAO1161). Where necessary, the medium was supplemented with X-gal, IPTG, and antibiotics: kanamycin (50 μg/ml for BR825, DH5α, EPS, JCM 7364R, LBA288, LM7R, or 500 μg/ml for LM16R and PAO1161), rifampicin (50 μg/ml), and streptomycin (50 μg/ml). The following vectors were used in this study: pABW1 (Bartosik et al. 1997)—for the functional analysis of the replication (REP) modules and pBGS18 (Spratt et al. 1986)—for the functional analysis of the mobilization (MOB) for conjugal transfer modules. Plasmids constructed in this study are listed in Supplementary Table S1.
DNA manipulations and introduction of plasmid DNA into bacterial cells
The DNA of the plasmids was isolated using a GeneMATRIX Plasmid Miniprep DNA Purification Kit (EURx, Gdansk, Poland) or the classical alkaline lysis procedure. Routine DNA manipulations (including cloning of fragments of DNA, DNA digestion, and electrophoresis) were carried out using standard molecular biology methods (Sambrook and Russell 2001). DNA was amplified by PCR using a KAPA HiFi PCR Kit (KAPABIOSYSTEMS, Cape Town, South Africa) and appropriate primer pairs (Supplementary Table S2). DNA amplification was performed using a Mastercycler (Eppendorf, Hamburg, Germany). Each amplification started with an initial denaturation at 95 °C for 3 min followed by 30 cycles of denaturation at 98 °C for 20 s, annealing at 65 to 79 °C (depending on the primer pair) for 15 s, extension at 72 °C for 1 min/kb, and finished with a final extension at 72 °C for 1 min/kb. The PCR-amplified DNA fragments were then cloned in pABW1 or pBGS18 vectors.
Plasmids constructed in this study were introduced into Achromobacter sp. LM16R, A. tumefaciens LBA288, P. alcaliphilus JCM 7364, Pseudomonas sp. LM7R and V. paradoxus EPS by triparental mating, and E. coli DH5αR by biparental mating (Sambrook and Russell 2001). Additionally, constructed plasmids were introduced into E. coli BR825, DH5α, ER2566, and S17-1 and P. aeruginosa PAO1161R by chemical transformation (Kushner 1978; Irani and Rowe 1997).
Testing of the host range of ANT_H3 plasmids
Derivatives of pABW1 carrying replication modules (REP) of ANT_H3 plasmids (Supplementary Table 1) were introduced into representatives of Alphaproteobacteria (A. tumefaciens LBA288 and P. alcaliphilus JCM 7364), Betaproteobacteria (V. paradoxus EPS i Achromobacter sp. LM16), and Gammaproteobacteria (E. coli BR825, Pseudomonas sp. LM7R, and P. aeruginosa PAO1161R). The ColE1-type replication system of pABW1 is not functional in any of these recipient strains (E. coli BR825 carries a mutation within the DNA polymerase I gene preventing ColE1-type replication). Thus, maintenance of the shuttle plasmids (pABW1-derivatives with various REP modules) in the tested hosts was fully dependent on the functions encoded within the cloned, predicted replication modules of the analyzed ANT_H3 plasmids. The presence of an introduced shuttle vector within a tested host strain was confirmed by the isolation of plasmid DNA from the recipient strain and subsequent electrophoresis of the isolation products.
Testing the activity of the conjugal transfer systems
Derivatives of pBGS18 carrying conjugal transfer mobilization modules (MOB) of ANT_H3 plasmids (Supplementary Table 1) were introduced into E. coli S17-1 carrying chromosomally integrated tra genes. Biparental mating experiments using E. coli DH5αR as a recipient strain were performed for testing the activity of conjugal transfer systems of analyzed plasmids. The presence of an autonomous form of the introduced plasmid was confirmed by the isolation of plasmid DNA, its electrophoresis in 0.8% agarose gel, and the restriction analysis of isolated plasmid DNA.
Testing of putative DNA methyltransferase activities
The predicted DNA methyltransferase genes identified within plasmids pA3H9 and pA3H10 were amplified using specific primer pairs (Supplementary Table S2). Then, the purified PCR products were digested with appropriate restriction enzymes and ligated with pET30a vector cut with the same enzymes. Resulting recombinant plasmids are listed in Supplementary Table S1. The recombinant methylases were expressed in the E. coli ER2566. Protein expression and restriction enzyme digestion protection assay revealing the sequence specificity of the methylase was performed as previously described (Drozdz et al. 2012).
BIOLOG phenotyping assays
Psychobacter sp. ANT_H3 was tested for their utilization of various carbon and nitrogen sources using BIOLOG Phenotype MicroArrays (plates PM1, PM2A and PM3B) (Biolog, Inc.; Hayward, CA, USA) performed in duplicate. Plates were inoculated with bacteria that had been cultured on LA for 48 h and suspended in GN/GP fluid with Dye Mix G. The results were read after incubation at 20 °C for 96 h.
DNA sequencing
DNA sequencing was performed using an Illumina MiSeq instrument in paired-end mode using a v3 chemistry kit. The obtained sequence reads were filtered for quality and assembled using Newbler v3.0 software (Roche, Basel, Switzerland). Final gap closure and sequencing of the cloned DNA fragments (for checking the constructed plasmids) was performed by the capillary sequencing of PCR products using an ABI3730xl DNA Analyzer (Applied Biosystems, Waltham, USA) applying primer walking technique.
Bioinformatics
Plasmids were manually annotated using the Artemis software (Carver et al. 2012). Similarity searches were performed using the BLAST programs (Altschul et al. 1997) and Pfam tool (Finn et al. 2016) provided by the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The detection of tRNA sequences was performed using tRNAscan-SE program (Lowe and Chan 2016). Helix-turn-helix motifs were identified using the helix-turn-helix DNA-binding motif prediction tool (Dodd and Egan 1990). EC numbers were assigned using the KEGG database (Kanehisa et al. 2017) and UniProt Knowledgebase (UniProtKB) (Pundir et al. 2017). The analyzed draft genome was automatically annotated using RAST (Aziz et al. 2008; Brettin et al. 2015) on the BV-BRC 3.6.8 (Wattam et al. 2017) web service and afterwards manually curated. The final annotation of the genome was performed by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) (Tatusova et al. 2016), including the completeness (99.39%) and contamination (1.88%) verification with CheckM analysis v1.2.0 (Parks et al. 2015). The occurrence of the restriction sites with genetic modules was checked using NEBcutter v3 (Vincze et al. 2003). The ANT_H3 plasmids were delineated and compared with each other using clinker tool v0.0.20 (Gilchrist and Chooi 2021). All of the above analyses were performed using default parameters of each tool. Mobilization proteins, toxin-antitioxon system, and metal metabolism and resistance genes were classified with MOBscan (Garcillán-Barcia et al. 2020), TADB2 (Xie et al. 2018), and METGeneDb (Dziurzynski et al. 2022) databases, respectively. Searches against the TADB2 and METGeneDb databases were performed with a standalone BLASTP v2.12.0 with 1e-2 e-value threshold (Camacho et al. 2009).
Genomic comparison was performed using plasmids recovered from Psychrobacter genomes downloaded from GenBank database on September 12, 2022 using NCBI datasets command line toolkit (the command: datasets download genome taxon Psychrobacter –annotated –assembly-source genbank –include-gbff –filename ncbi_datasets_GenBank_Psychrobacter.zip) and genomes for strains DAB_AL43B, WY6, and KCTC 72,983 with indicated plasmids were downloaded manually (Supplementary Table S3). Plasmids were compared on nucleotide and protein levels. The nucleotide-based comparison was performed with all against all nucleotide BLAST v2.12.0 + searches with 1e-50 e-value threshold and then visualized with Circos v0.69–8 (Krzywinski et al. 2009). Protein-based comparison included all against all using protein BLAST with the following thresholds: e-value of 1e-10 and at least 30% and 90% sequence identity and HSP coverage, respectively. These were then used for the construction of a protein similarity network where a single node represented a protein and edge corresponded to a common reciprocated similarity of two proteins above thresholds. The thickness of the edge reflected the product of sequence alignment identity and coverage. The network was created using self-written Python scripts (available at https://github.com/DEMBresearch/ANT_H3_plasmids_genomics) and visualized in Gephi v0.9.7 using the Fruchterman-Reingold force-directed layout (Fruchterman and Reingold 1991; Bastian et al. 2009).
Results
Characterization of Psychrobacter sp. ANT_H3 and identification of its plasmids
Psychrobacter sp. ANT_H3 originated from petroleum-contaminated soil collected near the petroleum pumping and storage warehouse at the Henryk Arctowski Polish Antarctic Station (GPS coordinates: 62° 09.601′ S, 58° 28.464′ W). This is a psychrotolerant strain, growing in temperatures ranging from 4 to 37 °C, with an optimal temperature of growth at 22 °C. It tolerates a broad range of pH (from 5 to 11) and salinity up to 8%. The ANT_H3 strain exhibits a medium level (MIC = 4 mM) resistance to Cu(II) and low level (MIC = 2 mM) resistance to Ni(II) and Zn(II) (Romaniuk et al. 2018).
Testing of Psychobacter sp. ANT_H3 for its ability to utilize various carbon and nitrogen sources performed using BIOLOG Phenotype MicroArrays revealed that of the 190 carbon sources tested, the strain was able to use 27, including: acetic, glutamic, pyruvic, butyric, β-hydroxybutyric and α-3-methyl-2-oxobutanoic acids, Tween 20, Tween 40, Tween 80, L-glutamine, and L-histidine. In the case of nitrogen sources, activity was demonstrated for 15 compounds out of 95 tested, and the strain coped best with the decomposition of: glutamic acid, L-cysteine, L-glutamine, L-tryptophan, and the dipeptides: Ala-Gln and Ala-Glu.
The plasmid screening (applying alkaline lysis) revealed the presence of multiple plasmids. All plasmids isolated via alkaline lysis were subjected to DNA sequencing. The plasmids assembly revealed the presence of 11 plasmid contigs. After the final gap closure and sequencing of the cloned DNA fragments, it was revealed that plasmids ranged in their sizes between 3,124 and 13,249 bp, and they carried between 2 and 14 genes (Table 1 and Fig. 1).
Linear presentation of the ANT_H3 plasmids. Each horizontal line represents plasmid and each arrow corresponds to the protein-encoding gene. If two arrows are connected with a gray-scale block, they show at least 30% sequence similarity as provided by clinker tool. Proteins belonging to the certain functional module were colored as indicated in the legend
Parallel to targeted isolation and sequencing of the ANT_H3 plasmids, the whole genome of this strain was subjected to sequencing using the Illumina MiSeq platform. This generated 1,485,328 paired reads and 446 Mbp of sequence information. As a result of the assembly of ANT_H3 genome, 65 contigs of a total length of 3,256,779 bp were obtained. The genome sequence was automatically annotated using RAST (Brettin et al. 2015) on the BV-BRC 3.28.9 web service (Olson et al. 2023), and its general features are presented in Table 2. With this genome sequencing, it was ultimately confirmed that the strain carries only 11 abovementioned plasmids and any mega-sized plasmid that could be skipped during alkaline lysis is present.
Replication modules (REP)
Bioinformatic analysis of the ANT_H3 plasmids revealed the presence of three types of REP modules: (i) Rep_3 family (repB-like)—pA3H1, pA3H2, pA3H4, pA3H5, pA3H8, pA3H9, pA3H10, and pA3H11; (ii) repA-like replicase—pA3H6 and pA3H7; (iii) Rep_1 rolling circle replication protein (Rep63 protein)—pA3H3.
The structure of the REP module of plasmids pA3H1, pA3H2, pA3H4, pA3H5, pA3H8, pA3H9, pA3H10, and pA3H11 is typical for the replication systems of many theta-replicating plasmids (Chattoraj 2000; Konieczny et al. 2014), i.e., they carry a gene encoding initiator of plasmid replication (Rep_3 family; RepB-like; pfam01051; COG5527), exhibiting nicking-closing (topoisomerase I-like) activity. The closely related replication protein is encoded by plasmid 1 (GenBank accession number: NC_007968) of the type-strain Psychrobacter cryohalolentis K5. Additionally, each REP module of abovementioned ANT_H3 plasmids contains also a predicted origin of replication (oriV), localized upstream of the rep gene, that is composed of four tandemly placed: (i) 22-bp-long iterons [5′-CATAAAGCTACGTTTAGCGACC-3′ for pA3H1; 5′-CATACCCTTACGTTTAGCGACC-3′ for pA3H2; 5′-CATACCACTACGTCTATCGACC-3′ for pA3H4; 5′-CATATGACGACAAATTCCTACC-3′ for pA3H8; 5′-CT(A/T)(T/G)ATGACTACAAATCCTTAC-3′ for pA3H9; 5′-(G/C)ATA(A/G)(A/G)(C/T)ATACGTTTATACACC-3′ for pA3H10] or (ii) 21-bp-long imperfect iterons [5′-(A/T)(A/T)(A/G)AAGCTAC(A/G)(A/T)(C/T)(A/T)ATCATA(A/G)-3′ for pA3H5] or 20-bp-long iterons [5′-AGAAAAAACAGTAAATGAGC-3′ for pA3H11] that may constitute binding sites for the Rep protein.
The second group of REP modules constitutes these found in pA3H6 and pA3H7 replicons. They contain a single gene encoding a replication protein (RepA-like) with three conserved regions, i.e., (i) the replicase domain (pfam03090), (ii) an alpha helical domain, found in the C-terminal parts of PriCT-1 primases, and (iii) a helix-turn-helix (HTH) motif responsible for interaction of Rep protein with DNA. These replication proteins exhibit amino acid sequence homology with the RepA protein of E. coli plasmid ColE2 (Yasueda et al. 1989). Moreover, in REP modules of plasmids pA3H6 and pA3H7, three DNA regions typical for the origin of replication of the ColE2 plasmid were found. These are (i) two direct repeats (L and R) with a reference consensus sequence 5′-CAGATAA-3′, (ii) sites α and β determining the specificity of the interactions of Rep protein with oriV, and (iii) a short sequence (5′-AGA-3′), where Rep protein synthesizes an unique RNA primer for the subsequent leading-strand DNA synthesis (Yagura et al. 2006).
The last type of the replication system was found in the pA3H3 plasmid, which encodes a Rep_1-type rolling circle replication protein (Rep63 protein). This mechanism of replication relies on generating one double-stranded plasmid and one circular ssDNA “plasmid” (that is later-on used as a matrix for the synthesis of the complementary strand) by a combined interaction of the plasmid-encoded Rep protein and the host DNA replicase (Ruiz-Masó et al. 2015). The Rep protein of pA3H3 plasmid exhibits highest similarity to the replication protein of Psychrobacter alimentarius (GenBank accession number: WP_201510399).
Following sequence analyses, REP modules of each ANT_H3 plasmid were PCR amplified and cloned into the pABW1 vector. This enabled testing their ability to replicate in bacteria belonging to different classes of Proteobacteria. None of the replication systems was functional in Betaproteobacteria. The replication systems of the pA3H3 and pA3H11 plasmids appeared to have a narrow host range as they were not able to replicate in species other than Psychrobacter. The remaining plasmids had a wider host range and, in addition to Psychrobacter, were functional in: (i) pA3H1—P. aeruginosa PAO1161; (ii) pA3H6—A. tumefaciens LBA288 and P. alcaliphilus JCM 7364R; (iii) pA3H2, pA3H5, pA3H7, and pA3H10—E. coli; and (iv) pA3H4, pA3H8, and pA3H9—E. coli and Pseudomonas spp.
ANT-H3-originating REP modules as the building blocks for the construction of novel plasmid-vectors
After revealing the host range of the REP modules, their suitability for being used as the building blocks for the construction of novel plasmid-vectors functional in cold active bacteria was tested. This approach seems to be especially desired in modern biotechnology since psychrotolerant bacteria are becoming the novel type of microbial cell factories to produce cold-active enzymes and secondary metabolites (Santiago et al. 2016; Sonkar and Singh 2020; Styczynski et al. 2022b). What is more, it has been shown that there is a need to develop multi-plasmid systems, which makes cloning of several genes simultaneously (in one host) possible (Lasek et al. 2017; Ciok and Dziewit 2019).
For that purpose, each REP module (i.e., DNA region cloned in pABW1) was in silico checked for the presence of restriction sites most commonly occurring in multiple cloning sites (MCS) used for the construction of plasmid-vectors, i.e., sites recognized by the following 13 endonucleases: BamHI, EcoRI, EcoRV, HindIII, KpnI, NcoI, NheI, PstI, SacI, SalI, SphI, XbaI, and XhoI. Usually, only the modules that are poor in restriction sites are suitable for creation of novel vectors, since it gives the user a better possibility to use more MCS-localized restriction sites. As revealed, in the case of all REP modules of the ANT_H3 plasmids, majority, i.e., between 9 and 12 out of 13 tested restriction sites were available and thus these modules could be successfully used for the construction of the novel plasmid-vectors in the future (Table 3).
Mobilization to conjugal transfer (MOB) modules
In 10 of ANT_H3 plasmids, modules for mobilization to conjugal transfer were identified. Such a module was not present in the smallest identified replicon—pA3H1. Two plasmids (pA3H2 and pA3H4) contained orphan mobC-like gene and possibly oriTs, while remaining plasmids carried classical gene clusters encoding relaxases (MobA). Based on amino acid sequence similarities of the relaxases, they were classified into two distinct families, namely, MOBP and MOBQ (Garcillán-Barcia et al. 2020).
The MOBP family members were found in pA3H6, pA3H7, and pA3H10. All three proteins exhibited the highest similarity to various relaxases of Psychrobacter spp. It was also revealed that enzymes of pA3H6 and pA3H7 showed high reciprocal similarity level (74%), while the relaxase of pA3H10 was more diverse, showing only about 34% of similarity (on the part of the sequence) to the other two.
In five plasmids, namely, pA3H3, pA3H5, pA3H8, pA3H9, and pA3H11, relaxases of the MOBQ family were encoded. This was the most abundant and diverse group of mobilization systems (Francia et al. 2004; Garcillan-Barcia et al. 2009). Analyzed relaxases showed highest similarity to appropriate proteins of Psychrobacter spp. and Moraxella spp. Interestingly, although all these relaxases belonged to the same family, only two of them (i.e., encoded within pA3H8 and pA3H9) showed high level of reciprocal similarity (87%), while others were more diverse, with similarities less than 50%.
The functionality of the MOB modules was examined. Each module was cloned into pBGS18, and then conjugation tests were performed using plasmid pRK2013 (RK2 conjugation transfer system) as the mobilization agent. It was confirmed that MOB modules of seven plasmids: pA3H4, pA3H5, pA3H6, pA3H7, pA3H8, pA3H9, and pA3H11 were functional, i.e., could be mobilized for conjugal transfer by the RK2 conjugation system. The remaining three systems revealed to be inactive, which suggested that they were non-functional or could not be mobilized to conjugal transfer by pRK2013.
Auxiliary genetic information
Within the analyzed plasmids, besides modules comprising conserved plasmid backbone (i.e., REP and MOB), a putative function was predicted for only several other genes. However, their biological activity was mostly based on in silico analyses and would need further investigation for proving.
Among these genes were (i) H5F75_RS00010 of pA3H5 that encoded a predicted DNA-protecting protein DprA (COG0758) that protects incoming foreign DNA and thus is active in many bacteria naturally competent for transformation of DNA, plus it is an accessory factor for RecA-mediated DNA strand exchange (Hovland et al. 2017); (ii) H5F64_RS00010 of pA3H8 encoding predicted multidrug efflux SMR transporter of EmrE family (COG2076) that may be involved in export of a range of toxins, including ethidium bromide and quaternary ammonium compounds (Ovchinnikov et al. 2018); (iii) H5F64_RS00020 of pA3H8 encoding the predicted glycine cleavage system T protein (aminomethyltransferase) (COG0404) that is a part of the glycine cleavage multienzyme complex (GCV) found in bacteria and the mitochondria of eukaryotes (Kikuchi et al. 2008); and (iv) H5F49_RS00035 of pA3H10 encoding a putative MscS small-conductance mechanosensitive, anion- or cation-selective channel protein (COG0668) opening in response to stretch forces in the membrane lipid bilayer (Martinac 2001).
Additionally, in two plasmids (pA3H9 and pA3H10), the putative type II restriction-modification systems were identified. Their function may be linked with cell defense against exogenous DNA or stable maintenance of plasmids in the host cell (Vasu and Nagaraja 2013). The pA3H9 plasmid system was composed of two non-overlapping genes (H5F60_RS00025 and H5F60_RS00030), encoding predicted restriction endonuclease and adenine methyltransferase. In the case of plasmid pA3H10, the potential restriction-modification system was more complex. It consisted of two genes encoding adenine methyltransferases (H5F49_RS00015 and H5F49_RS00020) and a complementary-strand-encoded restriction endonuclease (H5F49_RS00025). The first of these two methyltransferases showed sequence similarity to 5′-GAATTC-3′-specific methylases, e.g., 40% sequence identity with M.EcoRI (GenBank accession number: AAA26372) encoded by the plasmid RI13 from E. coli C600. Functional analysis of the methyltransferases was performed using a restriction enzyme digestion protection assay (Drozdz et al. 2012). The experimental set-up allowed determining the specificity of the methylase of pA3H9, which recognized the 5′-GAWTC-3′ sequence. Additionally, as the DNA of pET-MET10a and pET-MET10b (carrying methyltransferases of pA3H10) were cleaved by all restriction enzymes used in the test known to be insensitive to adenine methylation in their cognate sequences (including EcoRI), we hypothesize that for the full methylation the presence of both enzymes in the cell is needed.
Comparative analyses of the Psychrobacter plasmids
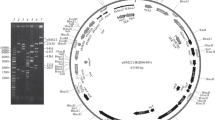
In total, 74 plasmids were distinguished in 24 out of 124 publicly available Psychrobacter spp. genomes (Supplementary Table S3). They ranged in size from 1,839 to 59,859 bp. These plasmids (together with ANT_H3 plasmids) were used for the comparative genomic and proteomic analyses. Ten plasmids (carried by six strains), including pA3H3 and pA3H5, seem to be unique as they did not show any sequence similarities to other analyzed plasmids. Remaining ANT_H3 plasmids showed limited sequence homology (Fig. 2A) with other Psychrobacter plasmids. This can be recognized as a common feature of all Psychrobacter plasmids, since, in general, only short DNA segments of Psychrobacter plasmids were identical or highly similar between various replicons originating from different locations. Interestingly, the most diverse replicons were identified in Antarctic strains.
Comparative genomics of plasmids of Psychrobacter spp. with assembled genomes. Panel A presents nucleotide-based sequence similarity of Psychrobacter plasmids. Their clockwise order corresponds to the order in Supplementary Table S3. Panel B presents a protein-based similarity network where each node corresponds to a single protein and an edge connecting two nodes represents sequence similarity between two proteins above the given thresholds. Four functional groups of proteins were presented. Proteins encoded within the selected plasmids were color-coded leaving other proteins gray
To further explore the common genetic load of these plasmids and to determine the groups of proteins shared among plasmids, a protein-based similarity network based on all against all protein BLAST search was constructed (Fig. 2B). This allowed determining that 68, 9, and 5 of Psychrobacter plasmids carry Rep3, RepA-like, and Rep_1 family replication system, respectively. For three plasmids, namely, unnamed6 (4Bb strain), unnamed1 (4Dc), and unnamed 5 (MES7-P7E), the replication system could not be determined. On the other hand, plasmids unnamed 1 (MES7-P7E), plasmid 1 (K5), and unnamed 1 (FDAARGOS_308) were the only composite replicons carrying two replication systems, here belonging to Rep3 and RepA-like families (Fig. 2B).
In the case of mobilization systems, within 44 plasmids from 18 strains, three groups of MOB families, namely, MOBQ (23 representatives), MOBP (12,) and MOBV (11), were found (Fig. 2B). Plasmid 1 of R10.10B was the only plasmid encoding two nearly identical MOBQ proteins (AOC03_11960 and AOC03_12020; 98% sequence identity), while plasmid 2 of that strain was also the only one with two different mobilization systems, i.e., MOBP (AOC03_12040) and MOBV (AOC03_12075). Moreover, from among other multireplicon strains, besides ANT_H3, only two carried plasmids with different mobilization systems, i.e., Sarcosine-02u-2 (unnamed 2—MOBQ, unnamed 3—MOBV, unnamed 4 and 5—MOBP) and DAB_AL43B (pP43BP1 and pP43BP4—MOBP, pP43BP2 and pP43BP3—MOBV).
Finally, when looking at the additional genetic load of Psychrobacter plasmids, there were two interesting groups of genes (Fig. 2B). The first one consists of 39 metal metabolism and resistance genes (MMRGs), involved in processing zinc and cadmium (ZntA), copper (ActP, CopB, and PcoA), zinc (MdtA), and cobalt, zinc, and cadmium (CzcA, CzcC, and CzcD). They were carried by 11 plasmids from 11 strains and except for the plasmid unnamed 2 (of MES7-P7Y) with the genome size of 6,585 bp, all these replicons were larger than 20 kb. Plasmids unnamed 1 (31,994 bp; WY6), pPsychbacter006 (49,070 bp; KCTC 72,983), unnamed 2 (59,859 bp; 4Dc), and pPspP11F6a (44,793 bp; P11F6) encode the highest number of MMRGs, i.e., 10, 7, 5, and 4, respectively.
Beside MMRGs, 28 plasmids from 17 Psychrobacter spp. encoded type II toxin-antitoxin systems, which could increase replicons’ stability in the host cells. They belonged to four families, i.e., RelBE (11 proteins), Xre-RelE (10), PHD-RelE (2), and RHH-COG2929 (9). The first three rely on RelE cytotoxin targeting ribosome and by that stopping the translational process (Christensen-Dalsgaard et al. 2010), while the RHH-COG2929 antitoxin-toxin pair mechanism remains undetermined.
Discussion
Based on the physiological and metabolic characterization, Antarctic Psychrobacter sp. ANT_H3 turned out to be similar to other species of this genus, including Arctic strains (Dziewit et al. 2013a). The main difference, which was also encouraging for further genomic analyses, was the presence of as many as 11 plasmids in the ANT_H3 genome. This is a true record in Psychrobacter spp. Within the analyzed plasmids, several auxiliary genes of metabolic potential were found; however, it seems that they were inactive, e.g., as judged from the BIOLOG phenotyping assays the ANT-H3 strain could not utilize glycine thus the pA3H8-encoded predicted glycine cleavage system T protein (aminomethyltransferase) was rather non-functional.
It was shown that 14% (17 out of 125) of Psychrobacter strains with sequenced genomes carry at least two plasmids. Majority of these plasmids are small (< 10 kb), cryptic replicons. Therefore, it is highly intriguing what their biological functions are. If they are just the selfish DNAs or may contribute to the host’s evolution as the mobile platforms enabling expression of acquired genetic information or at least act as the vehicles of auxiliary genetic load in horizontal gene transfer. Investigation of the genetic content of these plasmids revealed the dominance (besides the REP and MOB modules) of genes encoding hypothetical proteins for which no function was predicted. This does not preclude the possibility that Psychrobacter plasmids carry genes impacting the host’s phenotype, but rather proofs that annotation of bacterial genomes still remains imperfect and manual data curation and improving the reference databases seems to be the only solution so far, although it is usually laborious and tedious (Petty 2010).
As mentioned above, Psychrobacter spp. represents a group of bacteria with multipartite genomes that carry mostly small, cryptic plasmids. In this group, also other bacteria can be included, e.g., same species of Aeromonas spp. (Attéré et al. 2017) and Paracoccus spp. (Maj et al. 2013). Plasmids of these bacteria are mostly of the narrow host range, but still contain MOB modules, which suggests the possibility of their mobilization for conjugal transfer. As shown previously, such plasmids are efficient carriers of genetic information, since they may act as natural suicide vectors “stimulating” incorporation of genes into hosts’ genomes even among evolutionarily distinct bacterial species (Smorawinska et al. 2012). In the case of the ANT_H3 strain, it was shown that some REP modules are functional in bacteria other than Psychrobacter spp., which suggests that they might be acquired in horizontal transfer of genes and may potentially invade novel hosts, crossing the taxonomic barrier.
From the biotechnological point of view, Psychrobacter spp. are important not only as native producers of various biologically active compounds (Styczynski et al. 2022a; D’Angelo et al. 2022) but also as potential heterological hosts for overexpression of genes of other bacteria, including psychrotolerant ones, like it was previously developed for Pseudoalteromonas haloplanktis TAC125 (Duilio et al. 2004). Using Psychrobacter spp. as cold-active expression systems may potentially improve purification of various proteins, since lowering temperature during overexpression is a common procedure during problematic purification procedures (Sambrook and Russell 2001). However, this is naturally limited by the temperature tolerance of E. coli or other bacteria used in the assays and may be overcome by using naturally psychrotolerant Psychrobacter spp. Planning genetic manipulations in this group of bacteria one has to be aware of the limited number of plasmid-vectors available. Thus, analyses of Psychrobacter plasmids are of high relevance, since this may deliver the building blocks, i.e., functional genetic modules, for the construction of novel vectors. In the case of the ANT_H3 strain, there is a unique situation in which we have as many as 11 plasmids co-occurring in one host, which enables using their compatible REP modules for the construction of multi-plasmid systems functional in Psychrobacter spp., and possibly in other cold-active bacteria.
In this study, a comparative genomic analysis was also performed which revealed the genome size and gene content variability of Psychrobacter plasmids. The most remote were plasmids from Antarctica. That exemplifies the uniqueness of Antarctic ecosystem, most probably caused by its geographic isolation (Doytchinov and Dimov 2022). Nevertheless, it was shown that the similar plasmids often shared short but nearly identical DNA segments, which included not only maintenance systems (replication, mobilization, or stabilization) but also auxiliary genes like MMRGs. It is somehow intriguing to observe that the majority of identified MMRGs were identified in one plasmid even though the given strain carried more plasmids. This suggests that certain replicons could specialize in accumulating this kind of genes. Interestingly, six of 11 MMRG-carrying plasmids were not self-transmissible (lack of MOB module). That raises questions about the evolution of such non-mobile replicons and the source of their auxiliary genes, as these could be acquired from other temporally carried plasmids or the host’s chromosome. It is also possible that non-mobile plasmids simply lost their mobility modules and became “domesticated,” giving their hosts environmental advantage.
Data availability
All data described in the manuscript are available either in Electronic Supplementary Materials associated with this manuscript or in genomic database. The draft genome sequence of Psychrobacter sp. ANT_H3 and complete nucleotide sequences of its plasmids were deposited in the GenBank (NCBI) database with the accession numbers: JAQISM000000000 and MN657079-MN657089, respectively.
Code availability
Not applicable.
References
Altschul SF, Madden TL, Schaffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Araźny A, Kejna M, Sobota I (2013) Ground temperature at the Henryk Arctowski station (King George Island, Antarctic) – case study from the period January 2012 to February 2013. Bull Geogr Phys Geogr Ser 6:59–80. https://doi.org/10.2478/bgeo-2013-0004
Attéré SA, Vincent AT, Paccaud M et al (2017) The role for the small cryptic plasmids as moldable vectors for genetic innovation in Aeromonas salmonicida subsp. salmonicida. Front Genet 8:211. https://doi.org/10.3389/fgene.2017.00211
Ayala-del-Rio HL, Chain PS, Grzymski JJ et al (2010) The genome sequence of Psychrobacter arcticus 273–4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Env Microbiol 76:2304–2312. https://doi.org/10.1128/AEM.02101-09
Aziz RK, Bartels D, Best AA et al (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. https://doi.org/10.1186/1471-2164-9-75
Bartosik D, Bialkowska A, Baj J, Wlodarczyk M (1997) Construction of mobilizable cloning vectors derived from pBGS18 and their application for analysis of replicator region of a pTAV202 mini-derivative of Paracoccus versutus pTAV1 plasmid. Acta Microbiol Pol 46:387–392
Wattam AR, Davis JJ, Assaf R et al (2017) Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res 45:D535–D542. https://doi.org/10.1093/nar/gkw1017
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. Proc Int AAAI Conf Web Soc Media 3:361–362. https://doi.org/10.1609/icwsm.v3i1.13937
Bartosik D, Baj J, Sochacka M et al (2002) Molecular characterization of functional modules of plasmid pWKS1 of Paracoccus pantotrophus DSM 11072. Microbiology 148:2847–2856. https://doi.org/10.1099/00221287-148-9-2847
Bartosik AA, Glabski K, Jecz P et al (2014) Transcriptional profiling of ParA and ParB mutants in actively dividing cells of an opportunistic human pathogen Pseudomonas aeruginosa. PLoS One 9:e87276. https://doi.org/10.1371/journal.pone.0087276
Brettin T, Davis JJ, Disz T et al (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. https://doi.org/10.1038/srep08365
Camacho C, Coulouris G, Avagyan V et al (2009) BLAST+: architecture and applications. BMC Bioinformatics 10:421
Carver T, Harris SR, Berriman M et al (2012) Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28:464–469. https://doi.org/10.1093/bioinformatics/btr703
Chattoraj DK (2000) Control of plasmid DNA replication by iterons: no longer paradoxical. Mol Microbiol 37:467–476. https://doi.org/10.1099/00221287-148-9-2847
Christensen-Dalsgaard M, Jørgensen MG, Gerdes K (2010) Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol Microbiol 75:333–348. https://doi.org/10.1111/j.1365-2958.2009.06969.x
Ciok A, Dziewit L (2019) Exploring the genome of Arctic Psychrobacter sp. DAB_AL32B and construction of novel Psychrobacter-specific cloning vectors of an increased carrying capacity. Arch Microbiol 201:559–569. https://doi.org/10.1007/s00203-018-1595-y
Ciok A, Cegielski A, Bartosik D, Dziewit L (2019) Benefits and drawbacks of harboring plasmid pP32BP2, identified in arctic psychrophilic bacterium Psychrobacter sp. DAB_AL32B. Int J Mol Sci 20:2015. https://doi.org/10.3390/ijms20082015
Cowan DA, Makhalanyane TP, Dennis PG, Hopkins DW (2014) Microbial ecology and biogeochemistry of continental Antarctic soils. Front Microbiol 5:154. https://doi.org/10.3389/fmicb.2014.00154
D’Angelo C, Casillo A, Melchiorre C et al (2022) CATASAN is a new anti-biofilm agent produced by the marine Antarctic bacterium Psychrobacter sp. TAE2020. Mar Drugs 20:747. https://doi.org/10.3390/md20120747
Dodd IB, Egan JB (1990) Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res 18:5019–5026. https://doi.org/10.1093/nar/18.17.5019
Doytchinov VV, Dimov SG (2022) Microbial community composition of the Antarctic ecosystems: review of the bacteria, fungi, and archaea identified through an NGS-based metagenomics approach. Life 12:916. https://doi.org/10.3390/life12060916
Drozdz M, Piekarowicz A, Bujnicki JM, Radlinska M (2012) Novel non-specific DNA adenine methyltransferases. Nucleic Acids Res 40:2119–2130. https://doi.org/10.1093/nar/gkr1039
Duilio A, Tutino ML, Marino G (2004) Recombinant protein production in Antarctic Gram-negative bacteria. Recombinant Gene Expression. Humana Press, New Jersey, pp 225–238
Dziewit L, Cegielski A, Romaniuk K et al (2013a) Plasmid diversity in arctic strains of Psychrobacter spp. Extremophiles 17:433–444. https://doi.org/10.1007/s00792-013-0521-0
Dziewit L, Pyzik A, Matlakowska R et al (2013) Characterization of Halomonas sp. ZM3 isolated from the Zelazny most post-flotation waste reservoir, with a special focus on its mobile DNA. BMC Microbiol 13:59. https://doi.org/10.1186/1471-2180-13-59
Dziewit L, Pyzik A, Szuplewska M et al (2015) Diversity and role of plasmids in adaptation of bacteria inhabiting the Lubin copper mine in Poland, an environment rich in heavy metals. Front Microbiol 6:152. https://doi.org/10.3389/fmicb.2015.00152
Dziurzynski M, Gorecki A, Decewicz P et al (2022) Development of the LCPDb-MET database facilitating selection of PCR primers for the detection of metal metabolism and resistance genes in bacteria. Ecol Indic 145:109606. https://doi.org/10.1016/j.ecolind.2022.109606
Esakkiraj P, Bharathi C, Ayyanna R et al (2022) Functional and molecular characterization of a cold-active lipase from Psychrobacter celer PU3 with potential antibiofilm property. Int J Biol Macromol 211:741–753. https://doi.org/10.1016/j.ijbiomac.2022.04.174
Finn RD, Coggill P, Eberhardt RY et al (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44:D279–D285. https://doi.org/10.1093/nar/gkv1344
Francia MV, Varsaki A, Garcillan-Barcia MP et al (2004) A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol Rev 28:79–100. https://doi.org/10.1016/j.femsre.2003.09.001
Fruchterman TMJ, Reingold EM (1991) Graph drawing by force-directed placement. Softw Pract Exp 21:1129–1164. https://doi.org/10.1002/spe.4380211102
Garcillan-Barcia MP, Francia MV, de la Cruz F (2009) The diversityof conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 33:657–687. https://doi.org/10.1111/j.1574-6976.2009.00168.x
Garcillán-Barcia MP, Redondo-Salvo S, Vielva L, de la Cruz F (2020) MOBscan: automated annotation of MOB relaxases. In: de la Cruz F (ed) Horizontal Gene Transfer. Springer, US, New York, NY, pp 295–308
Gilchrist CLM, Chooi Y-H (2021) clinker & clustermap.js: automatic generation of gene cluster comparison figures. Bioinformatics 37:2473–2475. https://doi.org/10.1093/bioinformatics/btab007
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. https://doi.org/10.1016/S0022-2836(83)80284-8
Hooykaas PJ, den Dulk-Ras H, Schilperoort RA (1980) Molecular mechanism of Ti plasmid mobilization by R plasmids: isolation of Ti plasmids with transposon-insertions in Agrobacterium tumefaciens. Plasmid 4:64–75. https://doi.org/10.1016/0147-619X(80)90083-9
Hovland E, Beyene GT, Frye SA et al (2017) DprA from Neisseria meningitidis: properties and role in natural competence for transformation. Microbiology 163:1016–1029. https://doi.org/10.1099/mic.0.000489
Irani VR, Rowe JJ (1997) Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. Biotechniques 22:54–56. https://doi.org/10.2144/97221bm09
Jamieson WD, Pehl MJ, Gregory GA, Orwin PM (2009) Coordinated surface activities in Variovorax paradoxus EPS. BMC Microbiol 9:124. https://doi.org/10.1186/1471-2180-9-124
Kanehisa M, Furumichi M, Tanabe M et al (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361. https://doi.org/10.1093/nar/gkw1092
Kikuchi G, Motokawa Y, Yoshida T, Hiraga K (2008) Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad Ser B Phys Biol Sci 84:246–263. https://doi.org/10.2183/pjab.84.246
Konieczny I, Bury K, Wawrzycka A, Wegrzyn K (2014) Iteron plasmids. Microbiol Spectr 2. https://doi.org/10.1128/microbiolspec.PLAS-0026-2014
Krzywinski M, Schein J, Birol I et al (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. https://doi.org/10.1101/gr.092759.109
Kushner SR (1978) An improved method for transformation of E. coli with ColE1 derived plasmids. In: Boyer HB, Nicosia S (eds) Genetic engineering. Elsevier/North-Holland, Amsterdam, pp 17–23
Lasek R, Dziewit L, Bartosik D (2012) Plasmid pP62BP1 isolated from an Arctic Psychrobacter sp. strain carries two highly homologous type II restriction-modification systems and a putative organic sulfate metabolism operon. Extremophiles 16:363–376. https://doi.org/10.1007/s00792-012-0435-2
Lasek R, Dziewit L, Ciok A et al (2017) Genome content, metabolic pathways and biotechnological potential of the psychrophilic Arctic bacterium Psychrobacter sp. DAB_AL43B, a source and a host of novel Psychrobacter-specific vectors. J Biotechnol 263:64–74. https://doi.org/10.1016/j.jbiotec.2017.09.011
Lowe TM, Chan PP (2016) tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res 44:W54–W57. https://doi.org/10.1093/nar/gkw413
Ludtke DN, Eichorn BG, Austin SJ (1989) Plasmid-partition functions of the P7 prophage. J Mol Biol 209:393–406. https://doi.org/10.1016/0022-2836(89)90005-3
Maj A, Dziewit L, Czarnecki J et al (2013) Plasmids of carotenoid-producing Paracoccus spp. (Alphaproteobacteria) - structure, diversity and evolution. PLoS One 8:e80258. https://doi.org/10.1371/journal.pone.0080258
Martianov V, Rakusa-Suszczewski S (1989) Ten years of climate observations at the Arctowski and Bellingshausen stations (King George Island, South Shetlands, Antarctia). In: Breymeyer AI (ed) Global change regional research centres: scientific problems and concept developments, IGBP IASA UNESCO PAS seminar. Institute of Geography and Spatial Organization, Polish Academy of Sciences, Warsaw, Jablonna, pp 80–87
Martinac B (2001) Mechanosensitive channels in prokaryotes. Cell Physiol Biochem 11:61–76. https://doi.org/10.1159/000047793
Moghadam MS, Albersmeier A, Winkler A et al (2016) Isolation and genome sequencing of four Arctic marine Psychrobacter strains exhibiting multicopper oxidase activity. BMC Genomics 17:117. https://doi.org/10.1186/s12864-016-2445-4
Olson RD, Assaf R, Brettin T et al (2023) Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res 51:D678–D689. https://doi.org/10.1093/nar/gkac1003
Ovchinnikov V, Stone TA, Deber CM, Karplus M (2018) Structure of the EmrE multidrug transporter and its use for inhibitor peptide design. Proc Natl Acad Sci 115:E7932–E7941. https://doi.org/10.1073/pnas.1802177115
Parks DH, Imelfort M, Skennerton CT et al (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. https://doi.org/10.1101/gr.186072.114
Petrova M, Kurakov A, Shcherbatova N, Mindlin S (2014) Genetic structure and biological properties of the first ancient multiresistance plasmid pKLH80 isolated from a permafrost bacterium. Microbiology 160:2253–2263. https://doi.org/10.1099/mic.0.079335-0
Petty NK (2010) Genome annotation: man versus machine. Nat Rev Microbiol 8:762–762. https://doi.org/10.1038/nrmicro2462
Pundir S, Martin MJ, O’Donovan C (2017) UniProt protein knowledgebase. Methods Mol Biol 1558:41–55. https://doi.org/10.1007/978-1-4939-6783-4_2
Romaniuk K, Ciok A, Decewicz P et al (2018) Insight into heavy metal resistome of soil psychrotolerant bacteria originating from King George Island (Antarctica). Polar Biol 41:1319–1333. https://doi.org/10.1007/s00300-018-2287-4
Ruiz-Masó JA, MachóN C, Bordanaba-Ruiseco L, et al (2015) Plasmid rolling-circle replication. Microbiol Spectr 3:3.1.16. https://doi.org/10.1128/microbiolspec.PLAS-0035-2014
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Santiago M, Ramirez-Sarmiento CA, Zamora RA, Parra LP (2016) Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front Microbiol 7:1408. https://doi.org/10.3389/fmicb.2016.01408
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for invivo genetic-engineering - transposon mutagenesis in gram-negative bacteria. Bio-Technol 1:784–791
Smorawinska M, Szuplewska M, Zaleski P et al (2012) Mobilizable narrow host range plasmids as natural suicide vectors enabling horizontal gene transfer among distantly related bacterial species. FEMS Microbiol Lett 326:76–82. https://doi.org/10.1111/j.1574-6968.2011.02432.x
Sonkar K, Singh DP (2020) Biochemical characterization and thermodynamic study of lipase from psychrotolerant Pseudomonas punonensis. Biocatal Agric Biotechnol 28:101686. https://doi.org/10.1016/j.bcab.2020.101686
Spratt BG, Hedge PJ, te Heesen S et al (1986) Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337–342. https://doi.org/10.1016/0378-1119(86)90117-4
Styczynski M, Biegniewski G, Decewicz P et al (2022) Application of psychrotolerant Antarctic bacteria and their metabolites as efficient plant growth promoting agents. Front Bioeng Biotechnol 10:772891. https://doi.org/10.3389/fbioe.2022.772891
Styczynski M, Rogowska A, Nyabayo C et al (2022) Heterologous production and characterization of a pyomelanin of Antarctic Pseudomonas sp. ANT_H4: a metabolite protecting against UV and free radicals, interacting with iron from minerals and exhibiting priming properties toward plant hairy roots. Microb Cell Factories 21:261. https://doi.org/10.1186/s12934-022-01990-3
Tatusova T, DiCuccio M, Badretdin A et al (2016) NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. https://doi.org/10.1093/nar/gkw569
Vasu K, Nagaraja V (2013) Diverse functions of restriction-modification systems in addition to cellular defense. Microbiol Mol Biol Rev 77:53–72. https://doi.org/10.1128/MMBR.00044-12
Vincze T, Posfai J, Roberts RJ (2003) NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Res 31:3688–3691. https://doi.org/10.1093/nar/gkg526
Wang Y, Hou Y, Nie P et al (2019) A novel cold-adapted and salt-tolerant RNase R from Antarctic sea-ice bacterium Psychrobacter sp. ANT206. Molecules 24:2229. https://doi.org/10.3390/molecules24122229
Wu G, Zhang X, Wei L et al (2015) A cold-adapted, solvent and salt tolerant esterase from marine bacterium Psychrobacter pacificensis. Int J Biol Macromol 81:180–187. https://doi.org/10.1016/j.ijbiomac.2015.07.045
Xie Y, Wei Y, Shen Y et al (2018) TADB 2.0: an updated database of bacterial type II toxin–antitoxin loci. Nucleic Acids Res 46:D749–D753. https://doi.org/10.1093/nar/gkx1033
Yagura M, Nishio S, Kurozumi H et al (2006) Anatomy of the replication origin of plasmid ColE2-P9. J Bacteriol 188:999–1010. https://doi.org/10.1128/JB.188.3.999-1010.2006
Yasueda H, Horii T, Itoh T (1989) Structural and functional organization of ColE2 and ColE3 replicons. Mol Gen Genet MGG 215:209–216. https://doi.org/10.1007/BF00339719
Zhou L, Yu H, Wang K et al (2020) Genome re-sequencing and reannotation of the Escherichia coli ER2566 strain and transcriptome sequencing under overexpression conditions. BMC Genomics 21:407. https://doi.org/10.1186/s12864-020-06818-1
Acknowledgements
We acknowledge access to the infrastructure of the Polish Antarctic Station Arctowski (Antarctica). We thank Jan Gawor from the DNA Sequencing and Oligonucleotide Synthesis Laboratory, IBB Polish Academy of Science for his technical assistance during plasmid sequencing. Library construction and plasmid genome assembly were carried out at the DNA Sequencing and Oligonucleotide Synthesis Laboratory IBB PAN using the CePT infrastructure financed by the European Union – the European Regional Development Fund [Innovative economy 2007–13, Agreement POIG.02.02.00-14-024/08-00].
Funding
This work was supported by the National Science Centre (Poland), grants number 2016/21/N/NZ1/00275 for Krzysztof Romaniuk and 2018/29/N/NZ8/00228 for Przemyslaw Decewicz.
Author information
Authors and Affiliations
Contributions
Conceptualization—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; data curation—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; formal analysis—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; funding acquisition—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; investigation—Przemyslaw Decewicz, Krzysztof Romaniuk, Adrian Gorecki, Maria Dabrowska, Monika Radlinska, and Agnieszka Wyszynska; methodology—Przemyslaw Decewicz, Krzysztof Romaniuk, Maria Dabrowska, Monika Radlinska, and Agnieszka Wyszynska; project administration—Przemyslaw Decewicz, and Krzysztof Romaniuk; resources—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; software—Przemyslaw Decewicz; supervision—Lukasz Dziewit; validation—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; visualization—Przemyslaw Decewicz and Lukasz Dziewit; writing—original draft—Przemyslaw Decewicz, Krzysztof Romaniuk, and Lukasz Dziewit; writing—review and editing—Przemyslaw Decewicz and Lukasz Dziewit.
Corresponding author
Ethics declarations
Ethics approval
This work did not involve the studies of human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
All authors agreed on the publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Agnieszka Szalewska-Palasz
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Decewicz, P., Romaniuk, K., Gorecki, A. et al. Structure and functions of a multireplicon genome of Antarctic Psychrobacter sp. ANT_H3: characterization of the genetic modules suitable for the construction of the plasmid-vectors for cold-active bacteria. J Appl Genetics 64, 545–557 (2023). https://doi.org/10.1007/s13353-023-00759-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-023-00759-7