Summary
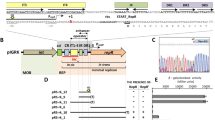
The complete nucleotide sequences of the 1.5 kb regions of ColE2 and ColE3 plasmids containing the segments sufficient for autonomous replication have been determined. They are quite homologous (greater than 90%), indicating that these two plasmids share common mechanisms of initiation of replication and its regulation. An open reading frame with a coding capacity for a protein of about 300 amino acids is present in both ColE2 and ColE3 and it actually specifies the Rep (for replication) protein, which is the plasmid specific trans-acting factor required for autonomous replication. The amino acid sequences of the Rep proteins of ColE2 and ColE3 are quite homologous (greater than 90%). The cis-acting sites (origins) where replication initiates in the presence of the trans-acting factors consist of 32 bp for ColE2 and 33 bp for ColE3. They are the smallest of all the prokaryotic replication origins so far reported. They are nonhomologous only at two positions, one of which, a deletion of a single nucleotide in ColE2 (or an insertion in ColE3), determines the plasmid specificity in interaction of the origins with the Rep proteins. Both plasmids carry a region with an identical nucleotide sequence and the one in ColE2, the IncA region, has been shown to express incompatibility against both ColE2 and ColE3. These results indicate that these plasmids share a common IncA determinant. A possibility that a small antisense RNA is involved in copy number control and incompatibility (IncA function) was suggested.
Similar content being viewed by others
References
Bazaral M, Helinski DR (1968) Circular DNA forms of colicinogenic factors E1, E2, and E3 from Escherichia coli. J Mol Biol 36:185–194
Casadaban MJ, Cohen SN (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol 138:179–207
Cole ST, Saint-Joanis B, Pugsley AP (1985) Molecular characterization of the colicin E2 operon and identification of its products. Mol Gen Genet 198:465–472
Durkacz BW, Kennedy CK, Sherratt DJ (1974) Plasmid replication and the induced synthesis of colicins E1 and E2 in Escherichia coli. J Bacteriol 117:940–946
Easton AM, Sampathkumar P, Rownd RH (1981) Incompatibility of IncFII R plasmid NR1. In: Ray DS (ed) The initiation of DNA replication. Academic Press, New York, pp 125–141
Gerhart E, Wagner H, Nordstrom K (1986) Structural analysis of an RNA molecule involved in replication control of plasmid R1. Nucleic Acids Res 14:2523–2538
Hashimoto-Gotoh T, Timmis KN (1981) Incompatibility properties of ColE1 and pMB1 derivative plasmids: Random replication of multicopy replicons. Cell 23:229–238
Herschman HR, Helinski DR (1967) Comparative study of the events associated with colicin induction. J Bacteriol 94:691–699
Horii T, Itoh T (1988) Replication of ColE2 and ColE3 plasmids: The regions sufficient for autonomous replication. Mol Gen Genet 212:232–240
Inselburg J (1973) Colicin factor DNA: a single non-homologous region in ColE2-E3 heteroduplex molecules. Nature New Biol 241:234–237
Inselburg J (1974) Incompatibility exhibited by colicin plasmids E1, E2, and E3 in Escherichia coli. J Bacteriol 119:478–483
Itoh T, Tomizawa J (1980) Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci USA 77:2450–2454
Kingsbury DT, Helinski DR (1970) DNA polymerase as a requirement for the maintenance of the bacterial plasmid colicinogenic factor E1. Biochem Biophys Res Commun 41:1538–1544
Light J, Molin S (1983) Post-transcriptional control of expression of the repA gene of plasmid R1 mediated by a small RNA molecule. EMBO J 2:93–98
Maxam AH, Gilbert W (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol 65:499–560
Miller J (1972) Assay of β-galactosidase. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 352–355
Rosen J, Ryder T, Ohtsubo H, Ohtsubo E (1981) Role of RNA transcripts in replication incompatibility and copy number control in antibiotic resistance plasmid derivatives. Nature 290:794–799
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Scott JR (1984) Regulation of plasmid replication. Microbiol Rev 48:1–23
Shapira SK, Chou J, Richaud FV, Casadaban MJ (1983) New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene 25:71–82
Som T, Tomizawa J (1982) Origin of replication of Escherichia coli plasmid RSF1030. Mol Gen Genet 187:375–383
Stougaard P, Molin S, Nordstrom K (1981) RNAs involved in copy-number control and incompatibility of plasmid R1. Proc Natl Acad Sci USA 78:6008–6012
Summers DK, Sherratt DJ (1988) Resolution of ColE1 dimers requires a DNA sequence implicated in the three-dimensional organization of the cer site. EMBO J 7:851–858
Summers D, Yaish S, Archer J, Sherratt D (1985) Multimer resolution systems of ColE1 and ColK: localisation of the crossover site. Mol Gen Genet 201:334–338
Tacon W, Sherratt DJ (1976) ColE plasmid replication in DNA polymerase I-deficient strains of Escherichia coli. Mol Gen Genet 147:331–335
Tajima Y, Horii T, Itoh T (1988) Replication of ColE2 and ColE3 plasmids: Two incompatibility functions of ColE2. Mol Gen Genet 214:451–455
Tomizawa J, Itoh T (1981) Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci USA 78:6096–6100
Tsurimoto T, Kouhara H, Matsubara K (1984) Origin and initiation sites of λdv DNA replication in vitro. In: Helinski DR, Cohen SN, Clewell DB (eds) Plasmids in bacteria. Plenum Press, New York, pp 151–172
Watson R, Visentin LP (1980) Restriction endonuclease mapping of ColE2-P9 and ColE3-CA38 plasmids. Gene 10:307–318
Watson RJ, Lau PCK, Vernet T, Visentin LP (1984) Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Gene 29:175–184
Watson RJ, Lau PCK, Vernet T, Visentin LP (1986) Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Corrigenda. Gene 42:351–353
Author information
Authors and Affiliations
Additional information
Communicated by M. Takanami
Rights and permissions
About this article
Cite this article
Yasueda, H., Horii, T. & Itoh, T. Structural and functional organization of ColE2 and ColE3 replicons. Mol Gen Genet 215, 209–216 (1989). https://doi.org/10.1007/BF00339719
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00339719