Abstract
ASM deficiency in Niemann-Pick disease type A results in aberrant cellular accumulation of sphingomyelin, neuroinflammation, neurodegeneration, and early death. There is no available treatment because enzyme replacement therapy cannot surmount the blood–brain barrier (BBB). Nanocarriers (NCs) targeted across the BBB via transcytosis might help; yet, whether ASM deficiency alters transcytosis remains poorly characterized. We investigated this using model NCs targeted to intracellular adhesion molecule-1 (ICAM-1), transferrin receptor (TfR), or plasmalemma vesicle-associated protein-1 (PV1) in ASM-normal vs. ASM-deficient BBB models. Disease differentially changed the expression of all three targets, with ICAM-1 becoming the highest. Apical binding and uptake of anti-TfR NCs and anti-PV1 NCs were unaffected by disease, while anti-ICAM-1 NCs had increased apical binding and decreased uptake rate, resulting in unchanged intracellular NCs. Additionally, anti-ICAM-1 NCs underwent basolateral reuptake after transcytosis, whose rate was decreased by disease, as for apical uptake. Consequently, disease increased the effective transcytosis rate for anti-ICAM-1 NCs. Increased transcytosis was also observed for anti-PV1 NCs, while anti-TfR NCs remained unaffected. A fraction of each formulation trafficked to endothelial lysosomes. This was decreased in disease for anti-ICAM-1 NCs and anti-PV1 NCs, agreeing with opposite transcytosis changes, while it increased for anti-TfR NCs. Overall, these variations in receptor expression and NC transport resulted in anti-ICAM-1 NCs displaying the highest absolute transcytosis in the disease condition. Furthermore, these results revealed that ASM deficiency can differently alter these processes depending on the particular target, for which this type of study is key to guide the design of therapeutic NCs.
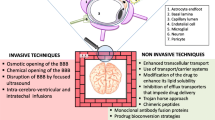
Graphical Abstract











Similar content being viewed by others
Availability of data and materials
Data will be provided upon request. Unique materials can be shared upon reasonable request and at recipient cost, respecting protection of any active confidentiality and intellectual property provisions.
References
OMIM Entry - # 257200 - NIEMANN-PICK DISEASE, TYPE A.
OMIM Entry - # 607616 - NIEMANN-PICK DISEASE, TYPE B.
Vanier MT. Niemann-Pick diseases. Handb Clin Neurol. 2013;113:1717–21.
Schuchman EH. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J Inherit Metab Dis. 2007;30:654–63.
Quinn PJ. Sphingolipid symmetry governs membrane lipid raft structure. Biochim Biophys Acta. 2014;1838:1922–30.
Abe A, Shayman JA. Sphingolipid catabolism. Encycl Biol Chem Second Ed. 2013;287–292.
Breiden B, Sandhoff K. Acid sphingomyelinase, a lysosomal and secretory phospholipase C, is key for cellular phospholipid catabolism. Int J Mol Sci. 2021;22:9001.
Wasserstein MP, et al. The natural history of type B Niemann-Pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114:e672–7.
Hollak CEM, et al. Acid sphingomyelinase (Asm) deficiency patients in The Netherlands and Belgium: disease spectrum and natural course in attenuated patients. Mol Genet Metab. 2012;107:526–33.
McGovern MM, et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 2008;122:e341–9.
Marín T, et al. c-Abl activation linked to autophagy-lysosomal dysfunction contributes to neurological impairment in Niemann-Pick type A disease. Front Cell Dev Biol. 2022;10:844297.
Carsana EV, et al. Massive accumulation of sphingomyelin affects the lysosomal and mitochondria compartments and promotes apoptosis in Niemann-Pick disease type A. J Mol Neurosci. 2022;72:1482–99.
Gabandé‐Rodríguez E, et al. Lipid-induced lysosomal damage after demyelination corrupts microglia protective function in lysosomal storage disorders. EMBO J. 2019;38:e99553.
McGovern MM, Aron A, Brodie SE, Desnick RJ, Wasserstein MP. Natural history of Type A Niemann-Pick disease: possible endpoints for therapeutic trials. Neurology. 2006;66:228–32.
Keam SJ. Olipudase Alfa: first approval. Drugs. 2022;82:941–7.
He X, et al. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim Biophys Acta. 1999;1432:251–64.
Diaz GA, et al. One-year results of a clinical trial of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency. Genet Med. 2021;23:1543–50.
Muro S. Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders. Drug Deliv Transl Res. 2012;2:169–86.
Miranda SRP, et al. Infusion of recombinant human acid sphingomyelinase into niemann-pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14:1988–95.
Tomsen-Melero J, et al. Liposomal formulations for treating lysosomal storage disorders. Adv Drug Deliv Rev. 2022;190:114531.
Solomon M, Muro S. Lysosomal enzyme replacement therapies: historical development, clinical outcomes, and future perspectives. Adv Drug Deliv Rev. 2017;118:109–34.
Del Grosso A, Parlanti G, Mezzena R, Cecchini M. Current treatment options and novel nanotechnology-driven enzyme replacement strategies for lysosomal storage disorders. Adv Drug Deliv Rev. 2022;188:114464.
Seras-Franzoso J, et al. Extracellular vesicles from recombinant cell factories improve the activity and efficacy of enzymes defective in lysosomal storage disorders. J Extracell Vesicles. 2021;10:e12058.
Schuster T, et al. Potential of surfactant-coated nanoparticles to improve brain delivery of arylsulfatase A. J Control Release. 2017;253:1–10.
Mayer FQ, et al. Laronidase-functionalized multiple-wall lipid-core nanocapsules: promising formulation for a more effective treatment of mucopolysaccharidosis type I. Pharm Res. 2015;32:941–54.
Salvalaio M, et al. Targeted polymeric nanoparticles for brain delivery of high molecular weight molecules in lysosomal storage disorders. PLoS ONE. 2016;11:1–17.
Rigon L, et al. Targeting brain disease in MPSII: preclinical evaluation of IDS-loaded PLGA nanoparticles. Int J Mol Sci. 2019;20:1–15.
Del Grosso A, et al. Brain-targeted enzyme-loaded nanoparticles: a breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci Adv. 2019;5:eaax7462.
Tian X, et al. LRP-1-mediated intracellular antibody delivery to the central nervous system. Sci Rep. 2015;5:11990.
Manthe RL, et al. Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme. J Control Release. 2020;324:181–93.
Muntimadugu E, et al. Comparison between nanoparticle encapsulation and surface loading for lysosomal enzyme replacement therapy. Int J Mol Sci. 2022;23:4034.
Muro S. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. in Endothelial Biomed. (ed. Aird, W.) 2007;1058–1070 (Cambridge University Press).
Muro S, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–609.
Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13:135–41.
Garnacho C, Muro S. ICAM-1 targeting, intracellular trafficking, and functional activity of polymer nanocarriers coated with a fibrinogen-derived peptide for lysosomal enzyme replacement. J Drug Target. 2017;25:786–95.
Garnacho C, Dhami R, Solomon M, Schuchman EH, Muro S. Enhanced delivery and effects of acid sphingomyelinase by ICAM-1-targeted nanocarriers in type B Niemann-Pick disease mice. Mol Ther. 2017;25:1686–96.
Papademetriou J, et al. Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor. J Inherit Metab Dis. 2013;36:467–77.
Garnacho C, et al. Delivery of acid sphingomyelinase in normal and Niemann-Pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325:400–8.
Muro S, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16:1450–8.
Papademetriou IT, Garnacho C, Schuchman EH, Muro S. In vivo performance of polymer nanocarriers dually-targeted to epitopes of the same or different receptors. Biomaterials. 2013;34:3459–66.
Hsu J, Rappaport J, Muro S. Specific binding, uptake, and transport of ICAM-1-targeted nanocarriers across endothelial and subendothelial cell components of the blood-brain barrier. Pharm Res. 2014;31:1855–66.
Marcos-Contreras OA, et al. Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain. J Control Release. 2019;301:54–61.
Glassman PM, et al. Targeted nanocarriers coopting pulmonary leukocytes for drug delivery to the injured brain. bioRxiv. 2022;479150.
Moos T, Nielsen TR, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. 2007;103:1730–40.
Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61.
Pardridge WM. Blood-brain barrier delivery for lysosomal storage disorders with IgG-lysosomal enzyme fusion proteins. Adv Drug Deliv Rev. 2022;184:114234.
Wu L, Gonias SL. The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J Cell Biochem. 2005;96:1021–33.
Taylor DR, Hooper NM. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem J. 2007;402:17–23.
Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49:265–80.
Tosi G, et al. Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier. Nanomedicine (Lond). 2011;6:423–36.
Stan RV, et al. The diaphragms of fenestrated endothelia—gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203.
Shuvaev VV, et al. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J Control Release. 2018;272:1–8.
Schuchman EH, Desnick RJ. Types A and B Niemann-Pick disease. Mol Genet Metab. 2017;120:27.
Gabandé-Rodríguez E, Boya P, Labrador V, Dotti CG, Ledesma MD. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014;21:864–75.
Breiden B, Sandhoff K. Mechanism of secondary ganglioside and lipid accumulation in lysosomal disease. Int J Mol Sci. 2020;21.
Breilyn MS, Zhang W, Yu C, Wasserstein MP. Plasma lyso-sphingomyelin levels are positively associated with clinical severity in acid sphingomyelinase deficiency. Mol Genet Metab Reports. 2021;28: 100780.
Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;92:9, 112–124.
Stieger B, Steiger J, Locher KP. Membrane lipids and transporter function. Biochim Biophys Acta - Mol Basis Dis. 2021;1867, 166079.
Casares D, Escribá PV, Rosselló CA. Membrane Lipid composition: effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int J Mol Sci. 2019;20:2167.
Simons K, Gruenberg J. Jamming the endosomal system: lipid rafts and lysosomal storage diseases. Trends Cell Biol. 2000;10:459–62.
Fraldi A, et al. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010;29:3607.
Rappaport J, Garnacho C, Muro S. Clathrin-mediated endocytosis is impaired in type A-B Niemann-Pick disease model cells and can be restored by ICAM-1-mediated enzyme replacement. Mol Pharm. 2014;11:2887–95.
Rappaport J, Manthe RL, Solomon M, Garnacho C, Muro S. A comparative study on the alterations of endocytic pathways in multiple lysosomal storage disorders. Mol Pharm. 2016;13:357–68.
Dhami R, Schuchman EH. Mannose 6-phosphate receptor-mediated uptake is defective in acid sphingomyelinase-deficient macrophages: implications for Niemann-Pick disease enzyme replacement therapy. J Biol Chem. 2004;279:1526–32.
Rappaport J, Manthe RL, Garnacho C, Muro S. Altered clathrin-independent endocytosis in type A Niemann-Pick disease cells and rescue by ICAM-1-targeted enzyme delivery. Mol Pharm. 2015;12:1366–76.
Roki N, et al. A method to improve quantitative radiotracing‐based analysis of the in vivo biodistribution of drug carriers. Bioeng Transl Med. 2021;6:e10208.
Wiseman ME, Frank CW. Antibody adsorption and orientation on hydrophobic surfaces. Langmuir. 2012;28:1765–74.
Beckmann N, Sharma D, Gulbins E, Becker KA, Edelmann B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front Physiol. 2014;5:331.
Solomon M, et al. Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases. J Control Release. 2022;349:1031–44.
Muro S, Muzykantov VR, Murciano JC. Characterization of endothelial internalization and targeting of antibody-enzyme conjugates in cell cultures and in laboratory animals. Methods Mol Biol. 2004;283:21–36.
Bui TM, Wiesolek HL, Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–99.
Muro S, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther. 2006;317:1161–9.
Schuchman EH. Acid sphingomyelinase, cell membranes and human disease: lessons from Niemann-Pick disease. FEBS Lett. 2010;584:1895–900.
Brenner JS, et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine. 2017;13:1495–506.
Myerson JW, et al. Flexible nanoparticles reach sterically obscured endothelial targets inaccessible to rigid nanoparticles. Adv Mater. 2018;30:1802373.
Myerson JW, et al. Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv Drug Deliv Rev. 2016;99:97–112.
Hsu J, Hoenicka J, Muro S. Targeting, endocytosis, and lysosomal delivery of active enzymes to model human neurons by ICAM-1-targeted nanocarriers. Pharm Res. 2015;32:1264–78.
Serrano D, Bhowmick T, Chadha R, Garnacho C, Muro S. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler Thromb Vasc Biol. 2012;32:1178–85.
Yu YJ, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44.
Daniele R, et al. Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes. Pharmaceutics. 2023;15:864.
Prachayasittikul V, Worachartcheewan A, Shoombuatong W, Prachayasittikul V, Nantasenamat C. Classification of p-glycoprotein-interacting compounds using machine learning methods. EXCLI J. 2015;14:958–70.
Muro S, et al. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Physiol. 2003;285:C1339–47.
Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:692.
Bien-Ly N, et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med. 2014;211:233–44.
Acknowledgements
BioRender.com was used for the creation of the graphical abstract and Fig. 10.
Funding
SM: RTI2018-101034-B-I00 from the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) and “ERDF A way of making Europe,” and CERCA Program (Generalitat de Catalunya). ML: INPhINIT Predoctoral Fellowship (LCF/BQ/DI18/11660018) funded by Fundación La Caixa and Horizon 2020 Marie Sklodowska-Curie (grant 713673). MP: FPI PRE2021-098133 funded by the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) and “ESF Investing in your future.”
Author information
Authors and Affiliations
Contributions
M.L. performed and analyzed majority of the experiments, drafted the manuscript, and prepared figures. M.P. performed and analyzed the flow cytometry experiment, prepared respective figure, and helped edit the manuscript. S.M conceptualized and directed the investigation, secured funding, helped plan experiments and interpret results, and helped write and edit the text and figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study did not involve human subjects. The human cell model used is a commercial cell line with no personal information.
Consent for publication
All authors have read and approved this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loeck, M., Placci, M. & Muro, S. Effect of acid sphingomyelinase deficiency in type A Niemann-Pick disease on the transport of therapeutic nanocarriers across the blood–brain barrier. Drug Deliv. and Transl. Res. 13, 3077–3093 (2023). https://doi.org/10.1007/s13346-023-01374-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01374-z