Abstract
Caspian white fish (Rutilus frisii kutum) is a fish of the family Cyprinidae, which is commercially harvested from the Caspian Sea. Experimental infection with Spring viraemia of carp virus (SVCV) was conducted in order to examine susceptibility of caspian White Fish and clinical impacts of infection. Fingerling fish were injected intra-peritoneally or waterborne-exposed with SVCV and were monitored daily for 7 weeks. Dead fish and those survived at the end of experimental period were collected for virus isolation and reverse transcriptase polymerase chain reaction analysis. Epithelioma papulosum cyprini cell line was used to re-isolate the virus and indirect fluorescent antibody test was conducted to identify the isolated virus. Infection trials showed that SVCV was highly pathogenic for the Caspian White Fish with mortality rate ranging from 75 to 85 %, depending on the viral challenge model. SVCV genome was detected from dead and apparently healthy fish tissues of both virus exposure models, which showed Caspian White Fish not only can be regarded as a susceptible host, but also serve as a vector of the virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caspian white fish, Rutilus frisii kutum, is a fish of the Cyprinidae Family living in Caspian Sea and its freshwater tributaries [1]. It is typically a medium sized fish, which is harvested commercially, forming up to 60 % bony fish products in Iranian costal water of the Caspian Sea in Iran and culture of the mentioned species is a recent phenomenon. This fish is highly appreciated by Iranian consumers and is cultured in Iran since its population reported to have reduced due to overfishing, increased marine pollution and over exploitation of sands and sediments of the Caspian Sea [2, 3].
Spring viraemia of carp virus (SVCV) is classified as a member of Rhabdoviridae Family, belonging to the genus of vesiculovirus, [4] is a bullet-shaped virus associated with an acute haemorrhagic and contagious viraemia in cyprinids including common carp (Cyprinus carpio) [5], Grass carp (Ctenopharyngodon idella), Silver carp (Hypophthalmichthys molitrix), Big head carp (Aristichthys nobilis), goldfish carp (Carassius auratus) and European catfish (Silurus glanis) [6–9] as well as other cyprinids such as zebra fish (Danio rerio) [10] and Roach (Rutilus rutilus) [11]. The virus genome is composed of a linear, non-segmented, negative-sense and single strand of RNA containing five genes in the order 3′-N-P-M-G-L-5′ which encodes viral nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and RNA-dependent RNA polymerase (L), respectively [4].
SVCV-infected carps in ponds tend to gather at the water inlet or sides of a pond. Reactions to sensory stimulation, swimming speed and the respiration rate are slowed down progressively; lethargy, resting and leaning mark the terminal stage of disease. External signs of the disease under natural conditions are darkening of the skin, distended abdomen, exophthalmia, petechial hemorrhages in the skin, gills and eyes, inflamed and edematous vent, and pale gills. Mortality rate of young carps due to SVCV infection fluctuates reaching to 70 % during spring time outbreaks [4]. Thus the disease has great economic impact in pond culture of cyprinids.
Spring Viraemia of Carp (SVC) occurs during spring at water temperatures between 10 and 17 °C, affecting fish of all age categories independent of their health status, virulence of the infectious agents, the environment and fish density [12, 13]. The disease was initially identified in European countries and has been reported in the Middle East area, China and America [4, 14–18]. Recently SVCV infection has been reported in the North of Iran [19].
The aim of the present work was to evaluate susceptibility of Rutilus frisii kutum to SVCV for the first time using immersion and intra-peritoneal (i.p.) injection challenge models and to associate pathogenicity of the SVCV with different routes of transmission using virus isolation, IFAT and also RT-PCR tests.
Materials and methods
Experimental fish
Caspian White Fish used in this study collected from “Shahid Ansari Reconstruction and Proliferation Center of bony fish stocks” (Rasht city, Iran). Fingerling fish with the mean weight of 3 g transferred alive to the laboratory and stocked at a density of 10 fish per 10 L aquarium. Prior to infection experiments, 10 fish were randomly selected and examined for the absence of SVCV infection by viral isolation and RT-PCR assays. Fish were acclimated to desired temperature at 20 °C for 20 days where they were maintained in aerated aquaria and fed with commercial food (BioMar®).
In-vitro virus amplification
SVCV reference strain (isolate 56/70, Accession No. Z37505.1) [16] was used in the infection experiments. Epithelioma Papulosum Cyprini (EPC) cell line was used for propagation, titration and infectivity assay of this virus. EPC cells were grown at 25 °C in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10 % Fetal Bovine Serum (FBS) (Gibco), 100 IU mL−1 penicillin and 0.1 mg mL−1 streptomycin. After virus inoculation, serum content of the medium was reduced to 2 % and the temperature to 15 °C. When complete cytopathic effect (CPE) was observed, the medium containing virus was harvested and centrifuged at 2,000×g for 10 min at 4 °C, and the supernatants were stored at -80 °C [11]. In order to quantify the virus, harvested virus was titrated and expressed as TCID50 [20, 21] following a single freeze–thaw cycle.
Virus challenge
Prior to challenge, fish were starved for 24 h and water temperature was maintained at 17 °C during experimental infection. Two virus inoculation methods, immersion and i.p. injection were conducted. Each treatment was carried out in duplicates with 20 fish per treatment and 10 fish as negative control groups were set also up for each treatment. Inoculated fish were clinically examined daily for 7 weeks. During exposure time, mortality and morbidity of fish were monitored.
For i.p. injection, fish were injected with 6.5 × 104 TCID50/fish by micro-syringes and negative control groups were injected similarly with EMEM containing no virus. In order to conduct immersion challenge, fish were exposed to virus at concentration of 6.5 × 105 TCID50/mL in a total volume of 10 L for 4 h. An equal volume of EMEM medium containing no virus was added to the related negative control aquarium. After 4 h virus containing water was removed and replaced with fresh water gradually. Clinical signs and cumulative mortality of both challenge models were recorded daily for 7 weeks.
Virology sampling
Gills, liver, spleen, intestines and kidney from dead fish and those surviving at the end of the challenges were sampled and frozen immediately at −80 °C for virus isolation.
Virus isolation and titration
Tissue samples of inoculated fish were homogenized and diluted 1:10 (w/v) in EMEM medium containing antibiotics (1,000 IU/mL penicillin, 1,000 μg/mL streptomycin, 500 μg/mL gentamycin). Following centrifugation of homogenates at 2,000×g for 15 min at 4 °C, the supernatants were passed through a 0.45 μm syringe membrane filter and inoculated onto a monolayer of EPC cells flat-bottom 24-well culturing plates (150 μL per well). The plates were incubated at 15 °C for 7 days and CPE formation was monitored. CPE negative plates were subjected to a second passage and monitored for CPE for a further 7 days. Virus titration of each sample was performed using Reed and Muench method [20] in 96-well flat bottom plates.
Indirect fluorescence antibody test (IFAT)
Re-isolated virus was identified with IFAT. Monolayers of EPC cells were prepared in flat-bottom 24-well culturing plates and incubated with virus suspensions for 24 h at 15 °C. After incubation, culture medium was removed and plates were rinsed with 0.01 M phosphate-buffered saline (PBS). Fixation was performed using 80 % cold acetone for 15 min. Then the cell monolayers dried in air and incubated with 30 μL of a rabbit anti SVCV antibody solution (diluted 1:100 in PBS), received from European Union Reference Laboratory for Fish Diseases (Aarhus, Denmark), at 37 °C for 30 min. After rinsing with PBS, 30 μL of anti rabbit IgG, FITC conjugate (Razi Biotech), diluted 1:20 in PBS, was added to each well and incubated for 30 min at 37 °C. Then plates were rinsed with PBS and monolayers were examined under fluorescence microscope and compared with positive and negative control wells.
RNA extraction and RT-PCR assay
Reverse-transcription polymerase chain reaction (RT-PCR) was performed on dead and survived fish tissue extracts in order to detect viral genome in fish treated tissues. Total RNA extraction was done by RNA extraction kit following the manufacturer’s protocol (Qiagen®). Briefly, 600 μL RLT buffer was added to 30 mg of fish tissues in 1.5 mL sterile microtubes. Tissue cell lyses was performed using rotor–stator homogenizer. The cell lysates were centrifuged at 8,000×g for 3 min at 4 °C. Total RNA was precipitated by ethanol and purified using RNeasy spin columns.
The RT-PCR assay was performed using One-Step RT-PCR kit (Qiagen®) according to the manufacturer’s instruction. Following mixture was used for RT-PCR assay: 5 μL of 5× RT-PCR buffer, 1 μL dNTPs mix, 1 μL one-step RT-PCR enzyme mix, 13 μL RNase free distilled water, 2 μL sense and antisense primers as well as 3 μL RNA samples, to make a final reaction volume of 25 μL in each case. The primer pairs for RT-PCR (sense: 5′-GCC TAA ATG TGT TGA TGG AAC G-3′; antisense: 5′-GGA TAA TAT CGG CTT GGA AAG C-3′), was derived from nucleotides 814–835 and 1262–1283 of the G gene, respectively [22]. The reaction mix incubated for 30 min at 50 °C followed by 3 min at 94 °C in order to inactivate the reverse transcriptase. Then, 35 amplification cycles including denaturation for 30 s at 94 °C, annealing for 30 s at 50 °C and extension for 1 min at 72 °C, with a final extension period of 10 min at 72 °C was conducted. Finally 470 bp RT-PCR product was visualized under UV transillumination following electrophoresis on 1 % agarose gel containing ethidium bromide.
Results
Clinical signs and cumulative mortality
Our results revealed that i.p. injection of SVCV to the Caspian white fish resulted in mortality of 75 %. The onset mortality associated with this challenge model observed on the 14 days post inoculation (dpi) and continued until 23 dpi. The cumulative mortality in the fish inoculated with SVCV via water-born exposure model reached 85 %. Mortalities associated with this infection model began on the 14 dpi and continued through 24 dpi. Cumulative mortalities and mortality kinetics of both experimental infection models are shown in Fig. 1.
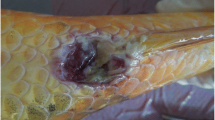
Clinical signs and gross pathologic changes associated with i.p. injection of SVCV to the Caspian White Fish included partial to complete anorexia, erratic swimming, exophthalmia, pale gills, haemorrhages on the skin and darker body. Similar signs were also observed in the fish of immersion challenge model (Fig. 2).
Virus isolation and confirmation
Inoculation of EPC cell line using the homogenates of the SVCV i.p. injected fish induced CPE similar to the original SVCV strain 72 h post-inoculation which was characterized by rounding of cells and focal lysis of the monolayer. When homogenates of the SVCV infected fish from immersion treatment model were inoculated onto the EPC cell line, a well-defined CPE as described for the original SVCV strain occurred 72 h post-inoculation. EPC cells inoculated with fish homogenates from negative control groups showed no CPE (Fig. 3).
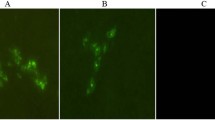
In addition, IFAT analysis was performed on CPE positive EPC cells of both challenge routes in order to identify re-isolated virus. IFAT analysis on CPE positive EPC cells confirmed identity of re-isolated virus as SVCV (Fig. 4).
To verify if there is any change in the infectivity of the recovered virus, the infectivity of the infected cell suspension was measured using TCID50. The results indicated that TCID50 of the virus harvested from experimentally infected fish remained identical.
RT-PCR analysis
In order to evaluate presence of viral nucleic acid, all fish including dead and survived fish were analyzed by RT-PCR using primers derived from the G gene of SVCV. The 470 bp product of RT-PCR was observed in fishes challenged with the virus through intraperitoneal route. In addition, SVCV nucleic acid was detected in apparently healthy fish of this treatment group that survived from SVCV i.p. injection. RT-PCR analysis was also performed on tissues from fish that exposed to water-born SVCV infection. The result confirmed SVCV infection in mortalities of this treatment group and viral nucleic acid was also detected in apparently healthy fish that survived from water-born exposure of SVCV (Fig. 5).
Discussion
The present work describes kinetics and pathology of experimental infection of the Caspian White Fish with SVCV. SVCV seems to be highly adapted to fish of the Cyprinidae Family, as it was pathogenic for many cyprinids such as common carp, grass carp, roach and zebra fish [11, 23, 24]. Fingerling Caspian White Fish were exposed to SVCV infection via immersion and i.p. injection. Our results demonstrated that SVCV was highly infective and pathogenic for Caspian White Fish which can result in high mortality rate in fish population. However, there is variability in the degree of susceptibility to SVCV depending on routes of infection. It was revealed that water-borne exposure caused higher mortality reaching to 85 % whereas 75 % mortality observed in the i.p. injected fish. Our results showed that, immersion as a natural route of infection, caused the higher mortality, thus water could be regarded as the major abiotic factor of virus transmission in nature [25]. During waterborne exposure the entire body surface is potentially in contact with the virus, allowing transmission through gills, skin and fin bases simultaneously. Moreover, there are reports on entry and progression of SVCV in susceptible fish. Ahne [12] suggested that gills are portal of entry and primary multiplication site of SVCV. Intra-peritoneal injection caused fewer mortality compared to immersion which indicates that viral exposure is more restricted in this viral transmission model than immersion. Statistically significant difference (one-way ANOVA, p < 0.05) was observed between the control and both treatment groups.
First mortalities of both treatment groups were observed on day 14 post inoculation. This was earlier than experimental infection of fingerling common carps at similar water temperature reported by Ahne [12]. This difference may be due to different kinetic of SVCV infection in common carps and Caspian White Fish. Diseased fish from both treatment groups had clinical signs consistent with SVC, including dark coloration, erratic swimming, exophthalmia and haemorrhages on the skin.
In our study, SVCV recovered from experimentally infected fish of both treatment groups induced CPE similar to the original viral strain when grown in EPC cells suggesting that SVCV remained live during the experimental period in Caspian White Fish and indicates possibility of the development of carrier state in this fish in aquatic environments. In addition, titration of SVCV recovered from experimentally infected fish and the original strain in EPC cells exhibited identical TCID50 which suggests that SVCV did not lose infectivity potential during passage in Caspian White Fish. However, possibility of change of infectivity and pathogenicity of SVCV following several passages in Caspian White Fish need more investigations.
SVCV nucleic acid was confirmed in dead and survived fish of both challenge routes by RT-PCR. Thus, it can be concluded that Caspian White Fish not only could be regarded as susceptible host of SVCV, but also apparently healthy fish similar to other cyprinids may serve as a reservoir of the virus and transmit infection to healthy population of susceptible fish [26].
In conclusion, the present work demonstrated the high susceptibility of the Caspian White Fish to SVCV and effect of routes of infection on mortalities. This study has established infection models for SVCV in Caspian White Fish for the first time that is essential in refining future experimental studies with the purpose of testing the efficacy of preventive and protective strategies against SVCV.
References
Kazancheev EN. The fishes of Caspian Sea. Moscow, 166 pages. Translated by Abolghasem Shariaty, Iranian Fisheries, Tehran, Iran. 1981.
Azari Takami G. Fecundity of Rutilus frisii kutum. J Vet Fac Univ Tehran. 1979;35:66–78.
Emadi H. The state of the fishing and reproduction of the Kutum, Rutilus frisii kutum, in the Caspian sea of Iran. J Ichthyol. 1979;19(4):151–4.
Ahne W, Bjorklund HV, Essbauer S, Fijan N, Kurath G, Winton JR. Spring viraemia of carp (SVC). Dis Aquat Org. 2002;52:261–72.
Garver KA, Dwilow AG, Richard J, Booth TF, Beniac DR, Souter BW. First detection and confirmation of spring viraemia of carp virus in common carp, Cyprinus carpio L., from Hamilton Harbour, Lake Ontario, Canada. J Fish Dis. 2007;30:665–71.
Fijan N. Vaccination of fish in European pond culture: prospects and constraints. Symposium Biologica Hungarica. 1984;23:233–41.
Jorgensen PEV, Olesen NJ, Ahne W, Lorenzen N. SVCV and PFR viruses: serological examination of 22 isolates indicates close relationship between the two fish rhabdoviruses. In: Ahne W, Kursted E, editors. Viruses of lower vertebrates. Berlin: Springer Verlag; 1989. p. 349–66.
Shchelkunov IS, Shchelkunova TI. Rhabdovirus carpio in herbivorous fishes: isolation, pathology and comparative susceptibility of fishes. In: Ahne W, Kurstak E, editors. Viruses of lower vertebrates. Berlin: Springer Verlag; 1989. p. 333–48.
Ahne W, Kurath G, Winton JR. A ribonuclease protection assay can distinguish SVCV from PFR. Bull Eur Assoc Fish Pathol. 1998;6:220–4.
Sanders GE, Batts WN, Winton JR. Susceptibility of Zebra fish (Danio rerio) to a Model Pathogen, Spring Viraemia of Carp Virus. Comp Med. 2003;53(5):514–21.
Haenen LM, Davidse A. Comparative pathogenicity of two strains of pike fry rhabdovirus and spring viraemia of carp virus for young roach, common carp, grass carp and rainbow trout. Dis Aquat Org. 1993;15:87–92.
Ahne W. Uptake and multiplication of spring viraemia of carp virus in carp, Cyprinus carpio L. J Fish Dis. 1978;1:265–8.
Rodak L, Pospisil Z, Tomanek J, Vesely T, Obr T, Valicek L. Enzyme-linked immunosorbent assay (ELISA) for the detection of spring viraemia of carp virus (SVCV) in tissue homogenates of the carp, Cyprinus carpio L. J Fish Dis. 1993;16:101–11.
Fijan N, Petrinec Z, Sulimanovi CD, Zwillenberg LO. Isolation of the viral causative agent from the acute form of infectious dropsy of carp. Veterinarski Arhiv. 1971;41:125–38.
Hoffmann B, Schutze H, Mettenleiter TC. Determination of the complete genomic sequence and analysis of the gene products of the virus of Spring Viraemia of Carp, a fish rhabdovirus. Virus Res. 2002;84:89–100.
Stone DM, Ahne W, Denham KL, Dixon PF, Liu CTY, Sheppard AM, Taylor GR, Way K. Nucleotide sequence analysis of the glycoprotein gene of putative spring viraemia of carp virus and pike fry rhabdovirus isolates reveals four genogroups. Dis Aquat Org. 2003;53:203–10.
Liu H, Gao L, Shi X, Gu T, Jiang Y, Chen H. Isolation of spring viraemia of carp virus (SVCV) from cultured Koi (Cyprinus carpio koi) and common carp (Cyprinus carpio carpio) in PR China. Bull Eur Assoc Fish Pathol. 2004;24:192–202.
Teng Y, Liu H, Lv JQ, Fan WH, Zhang QY, Qin QW. Characterization complete genome sequence of spring viraemia of carp virus isolated from common carp (Cyprinus carpio) in China. Arch Virol. 2007;152:1457–65.
Haghighi A, Sharifnia Z, Bandehpoor M, Kazemi B. The first report of spring viraemia of carp in some rainbow trout propagation and breeding by pathology and molecular techniques in Iran. Asian J Anim Vet Adv. 2008;3:263–8.
Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7.
Burleson FG, Chambers TM, Wiedbrauk DL. Virology, a laboratory manual. London: Academic Press; 1992.
Koutna M, Vesely T, Psikal I, Hulova J. Identification of spring viraemia of carp virus (SVCV) by combined RT-PCR and nested PCR. Dis Aquat Org. 2003;55:229–35.
Basic A, Schachner O, Bilic I, Hess M. Phylogenetic analysis of spring viraemia of carp virus isolates from Austria indicates the existence of at least two subgroups within genogroup Id. Dis Aquat Org. 2009;85:31–40.
Dixon PF. Virus diseases of cyprinids. In: Eiras JC, Segner H, Wahli T, Kapoor BG, editors. Fish diseases, vol. 1. Enfield: Science Publishers; 2008. p. 87–184.
Fijan N. Vaccination against spring viraemia of carp. In: Fish vaccination, Ellis AE, Eds,. Academic Press, London 1988; 204–215.
Office International des Epizooties (OIE). Manual of diagnostic tests for aquatic animals. Spring viraemia of carp. 2012. Chapter 2.3.8: 257–273.
Acknowledgments
The authors would like to appreciate Iranian Fisheries Research Organization (IFRO) for providing facilities to carry out this study. We thank Niels Jørgen Olesen and his colleagues from European Union Reference Laboratory of fish Diseases (Aarhus, Denmark) for providing SVCV reference strain used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamani, H., Ghasemi, M., Hosseini, S.M. et al. Experimental susceptibility of Caspian white fish, Rutilus frisii kutum to Spring viraemia of carp virus . VirusDis. 25, 57–62 (2014). https://doi.org/10.1007/s13337-013-0179-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-013-0179-3