Abstract
Background and Objectives
Viloxazine extended-release (viloxazine ER) capsules (QelbreeTM) is a novel nonstimulant recently approved as a treatment for attention-deficit/hyperactivity disorder in children and adolescents. Here, we determined whether the pharmacokinetics of viloxazine are impacted by consuming the capsule contents sprinkled on applesauce rather than an intact capsule, and the effect of a high-fat meal on the pharmacokinetics of viloxazine ER.
Methods
This was a randomized, open-label, crossover, three-treatment, three-period study in healthy adults using orally administered single-dose viloxazine ER 200 mg capsules. Subjects consumed: (1) an intact capsule after a 10-h fast (control condition); (2) the capsule contents sprinkled on one tablespoon of applesauce; and (3) an intact capsule with a standard high-fat meal. Blood samples were collected for 48 h post-dosing. Relative bioavailability analyses were performed to assess the impact of each test condition against the control condition (intact capsule, fasting). The absence of an impact was indicated if the 90% confidence interval (CI) for the least-squares geometric mean ratio (LSGMR) of maximal concentration (Cmax), the area under the concentration–time curve from time 0 to the last measurable concentration time (AUClast), and the area under the concentration–time curve from time 0 to infinity (AUCinf) were within the predetermined no-difference limits of 80–125%.
Results
Out of 27 enrolled subjects, 25 were included in the pharmacokinetic analysis. The LSGMR (90% CI) for viloxazine ER sprinkled vs. intact were 90.10% (83.35–97.40) for Cmax, 93.71% (89.09–98.57) for AUClast, and 95.37% (89.80–101.28) for AUCinf. The LSGMR (90% CI) for viloxazine ER consumed in the fed state vs. fasting state were 90.86% (84.05–98.21) for Cmax, 89.68% (85.26–94.33) for AUClast, and 92.35% (86.96–98.07) for AUCinf. The 90% CIs of the LSGMRs were within the predetermined no-difference limits of 80–125%. Viloxazine ER was well tolerated, with most adverse events reported as mild.
Conclusions
These data suggest that viloxazine ER can be consumed sprinkled on applesauce or as intact capsules with or without meals without significantly changing its pharmacokinetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Viloxazine extended-release is a novel nonstimulant for the treatment of attention-deficit/hyperactivity disorder in children and adolescents. |
Viloxazine extended-release can be consumed by sprinkling the capsule contents on one tablespoon of applesauce or with a high-fat meal with no impact on systemic exposure. |
1 Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a lifelong neurodevelopmental disorder that typically begins in childhood and can persist into adulthood [1]. Thought to occur in up to 10% of children [2] and 4.4–5.0% of adults [3, 4], ADHD is among the most common psychiatric disorders [5,6,7]. A major treatment challenge for many children (and, to a lesser extent, adults) is the inability to swallow pills or an aversion to doing so, particularly with persistent disorders such as ADHD, for which treatment may last months or years [8,9,10]. While some new drug formulations have resulted in liquids, sublinguals, or transdermal patches, the majority of ADHD medications (including the Food and Drug Administration [FDA]-approved nonstimulants atomoxetine [11] and guanfacine extended-release [XR] [12]) are available only in tablet or capsule form [13]. Particularly in the case of extended, delayed, or controlled-release formulations, modifying drug delivery by sprinkling, chewing, or crushing pills to facilitate consumption can change a medication’s pharmacokinetic profile, with potentially serious clinical effects [14,15,16].
Similarly, since the dissolution, absorption, and metabolism of some drug preparations can be significantly impacted by the presence of food [17], some medications require special consideration with respect to meals, adding further inconvenience to patients [10, 18]. Among nonstimulant ADHD medications, food has been shown to decrease the maximal concentration (Cmax) of atomoxetine by up to 37% (with a high-fat breakfast) and to delay the time to Cmax (Tmax) by 3 h [11, 19]. However, these changes are not thought to be clinically significant, and atomoxetine consumption is not restricted with regard to meals [11]. By comparison, a typical high-fat meal has been shown to increase guanfacine XR plasma Cmax and total exposure (area under the curve; AUC) by 75% and 40%, respectively [20]. Accordingly, it is recommended that guanfacine XR not be taken concurrently with a high-fat meal [12].
A common barrier to medication adherence is convenience; medications that must be taken multiple times a day, are unpleasant to consume, or have strict procedures (e.g., must be taken with a meal) can result in missed or late doses as individuals adjust their lives to the treatment guidelines [10, 18, 21, 22]. Variable dosing can, in turn, result in an altered pharmacokinetic profile, with potential downstream consequences for the drug’s efficacy or safety [14, 15]. Because ADHD is characterized in part by disorganization, distractibility, and forgetfulness [1], complex medication guidelines can be particularly burdensome for this patient population. Medications with flexible guidelines with respect to meals or ease of administration are more likely to see improved treatment adherence [10, 18] and minimize variability associated with drug exposure [14, 15], which consequently may impact the efficacy and safety of the administered drug.
Viloxazine extended-release (viloxazine ER) capsules is a novel nonstimulant recently approved by the US FDA under the trade name QelbreeTM as a once-daily treatment for ADHD in children and adolescents [23,24,25,26], with phase 3 trials in adults currently underway [27]. Its primary metabolic route in humans is through 5-hydroxylation followed by glucuronidation [28], with P450 (CYP) 2D6 contributing to the majority of 5-hydroxyviloxazine formation, and minor involvement of CYP1A2, 2B6, 2C9, 2C19, and 3A4. Subsequent glucuronidation to 5-hydroxyviloxazine glucuronide is mediated by uridine 5'-diphospho-glucuronosyltransferase 1A9 and 2B15 [28].
The purpose of the current study was to evaluate whether viloxazine pharmacokinetics are impacted by (1) consuming the viloxazine ER 200 mg capsule contents sprinkled on applesauce and (2) consuming the viloxazine ER 200 mg capsule with a high-fat meal, as compared to consuming a viloxazine ER 200 mg capsule intact while fasting. Sprinkling on applesauce was selected due to its favorable in vitro dissolution results and because it is a commonly used medium for medication administration. Further, both applesauce and the high-fat meal were selected as test conditions in accordance with the FDA’s guidance on food effect bioavailability [29]. Safety was also evaluated.
2 Methods
2.1 Study Design
The trial conduct was reviewed and approved by IntegReview Institutional Review Board (Austin, TX, USA) and conducted in accordance with the Helsinki Declaration and the International Council for Harmonisation Note for Guidance on Good Clinical Practice. The trial was conducted by Worldwide Clinical Trials, Early Phase Services, LLC (San Antonio, TX, USA).
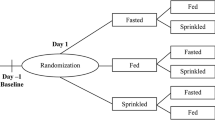
This was a single-center, randomized, open-label, crossover, single-dose, three-treatment, three-period, three-sequence study in healthy adults 18–55 years of age. This study evaluated the effects of sprinkling on applesauce and a high-fat meal on the relative bioavailability of viloxazine after a single dose of viloxazine ER capsule 200 mg. Subjects received each of the three treatments in a randomized three-sequence design (Fig. 1). Within 28 days of screening, subjects were admitted to the clinic on Day −1 (entry day) to confirm eligibility prior to enrollment and randomization on Day 1 of Period 1. Subjects began one continuous 11-day clinic residency, where they received viloxazine ER 200 mg on Day 1 of each of the three treatment periods separated by a 4-day washout period.
Study schematic. a Study design with three treatment periods. A single dose of viloxazine ER 200 mg was orally administered on Day 1 of each treatment period. Plasma samples for viloxazine PK were collected for 48 h beginning pre-dose at time 0. b Treatment order assignments were evenly balanced with nine subjects per group. n number of subjects, PK pharmacokinetic, viloxazine ER viloxazine extended-release
Each treatment was preceded by an overnight fast of at least 10 h (only water was permitted). In Treatment A, subjects swallowed one intact capsule of viloxazine ER under fasted conditions. In Treatment B, the contents of a viloxazine ER capsule were sprinkled on one tablespoon of applesauce (approximately 15 mL), which subjects swallowed without chewing under fasted conditions. In Treatment C, subjects swallowed one intact capsule of viloxazine ER after consuming a high-calorie (800–1000 calories), high-fat (~ 50% of the calories) meal. The high-fat meal consumed in Treatment C consisted of two eggs fried in butter, two strips of bacon, two slices of white bread toast, two pats of butter, four ounces of hash brown potatoes, and eight ounces of whole milk; condiments were not allowed. Subjects had 30 min or less to consume the meal following a minimum 10-h fast, and viloxazine ER was administered approximately 30 min after the start of the meal. For all treatments, subjects fasted for 4 h after dosing, and fluid intake was restricted from 1 h before until 1 h after morning dosing to minimize absorption variability, with the exception of 240 mL water at the time of oral dosing.
During each of the three treatment periods, blood samples were collected at regular intervals from pre-dose (0 h) through 48 h post-viloxazine ER dosing (see “Sample Collection and Bioanalytical Methods” below). Vital signs were collected 12 h post-dose. On study Day 11 (Day 3 of Period 3) or at early termination (collectively described as end of study; EOS), subjects underwent final safety assessments and provided a final blood sample for pharmacokinetic analysis (see “Safety Monitoring and Assessments” below).
Based on the expected inter- and intrasubject variability of viloxazine, other similar studies [30,31,32], and FDA guidance [29], a sample size of 27 was deemed sufficient to assess the study endpoints.
2.2 Study Subjects
Healthy adults (18–55 years of age) were recruited for the trial. Standard inclusion criteria were used, which required subjects to have a body mass index (BMI) of 18–30 kg/m2 (inclusive), be nonsmokers, and be able and willing to swallow whole capsules. Females of childbearing potential had to be abstinent or using acceptable birth control. Criteria for exclusion included a history or presence of significant diseases, a history of seizures, clinically significant safety laboratory or electrocardiogram (ECG) abnormalities, infection with human immunodeficiency virus or hepatitis B or C, alcohol or drug abuse, a need for prescription medication (other than hormonal agents), recent use (within 30 days of screening) of drugs known to notably induce or inhibit hepatic drug metabolism/pregnancy/lactation, allergy to viloxazine ER, or any other qualifier that had the potential to interfere with participation in the trial as determined by the investigator.
2.3 Sample Collection and Bioanalytical Methods
Blood samples (~ 3 mL each) for viloxazine pharmacokinetic analysis were collected pre-dose (baseline, time 0) on Day 1 of each treatment period relative to viloxazine ER administration and at 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 18, 24, 36, and 48 h post-dose. A total of 42 pharmacokinetic samples were collected throughout the study; the maximum total volume of blood drawn for pharmacokinetic analysis was approximately 126 mL per subject. Each blood sample for pharmacokinetic analysis was collected in a K2-EDTA vacutainer tube. Blood samples were then centrifuged at 3000 rpm for 10 min at 4 °C. The resulting plasma was aliquoted in approximately equal amounts into two appropriately labeled polypropylene screw-cap tubes. Within 60 min of collection, samples were frozen in an upright position at approximately − 20 °C pending analysis.
Plasma concentrations of viloxazine were quantified using a validated ultra-performance liquid chromatographic/tandem mass spectrometry detection method with a lower limit of quantification of 0.0100 µg/mL. Bioanalytical methods for viloxazine have been previously published [33, 34].
2.4 Pharmacokinetic Analyses
The safety population included any subject who received at least one dose of study medication and had at least one post-baseline safety assessment. The pharmacokinetic population was defined as all subjects who received at least one dose of study medication and who had an adequate pharmacokinetic profile for viloxazine for Treatment A and either Treatment B or C without any major protocol deviation. The concentration–time data for viloxazine were analyzed using standard noncompartmental methods in Phoenix™ WinNonlin® (Version 6.3, Pharsight Corporation). During the pharmacokinetic analysis, viloxazine plasma concentrations that were below the limit of quantification (BLQ) were treated as zero from time 0 up to the time at which the first quantifiable concentration was observed; embedded and terminal BLQ were treated as missing. Actual sample times were used for pharmacokinetic and statistical analyses. Pharmacokinetic results are summarized by period and time using descriptive statistics including arithmetic mean, standard deviation (SD), median, minimum, maximum, and coefficient of variation (CV); geometric means were used for calculations of bioavailability.
The potential for sprinkling on applesauce or consumption of a high-fat meal to impact the metabolism and pharmacokinetics of viloxazine ER was evaluated using analysis of variance (ANOVA) on the log-transformed pharmacokinetic parameters Cmax, area under the concentration–time curve from time 0 to the last measurable concentration time (AUClast), and area under the concentration–time curve from time 0 to infinity (AUCinf). Two comparisons were conducted: (1) the effect of sprinkling on applesauce (Treatment A versus Treatment B) and (2) the effect of a high-fat meal (Treatment A versus Treatment C). The ANOVA model included fixed-effect terms for sequence, period, and treatment, with subject nested within sequence as a random effect.
For each comparison, the least-squares (LS) geometric means, the difference in LS means, and the two-sided 90% confidence intervals (CIs) were calculated. The results from the log-transformed data were transformed back to the original scale by exponentiation to obtain the 90% CI for the LS geometric mean ratio (GMR), calculated as Treatment B/Treatment A (for the sprinkle effect) and Treatment C/Treatment A (for the food effect). The absence of an effect was indicated if the 90% CIs for the treatment ratios were within the predefined no-difference limits of 80–125% for Cmax, AUClast, and AUCinf.
2.5 Safety Monitoring and Assessments
Baseline measurements included medical history, physical examination, vital signs, and clinical laboratory tests (i.e., hematology, biochemistry, and urinalysis). Vital signs (diastolic/systolic blood pressure, pulse rate, respiratory rate, and temperature) were assessed at screening, Day −1 (study entry), Day 1 of each treatment period, and EOS (Day 11). Blood pressure and heart rate were taken after the subject had been sitting or supine for a minimum of 5 min. Clinical laboratory tests occurred at screening, Day −1, and EOS. A 12-lead ECG was conducted at Day −1 and at EOS. Assessments were performed as scheduled or at any time deemed necessary by the investigator. Adverse events (AEs) were monitored over the course of treatment and classified into standardized terminology from the verbatim description (investigator term) according to the Medical Dictionary for Regulatory Activities (MedDRA) Coding Dictionary version 19.0. AEs were summarized by system organ class and MedDRA preferred term. Safety and tolerability data are summarized using descriptive statistics.
3 Results
3.1 Subject Demographics
Out of the 27 subjects (14 males, 13 females) enrolled in the study, all received at least one treatment and were included in the safety population, and 25 subjects had sufficient pharmacokinetic data to be included in the pharmacokinetic population. One subject received Treatment A only and discontinued prior to dosing in Period 2 (see below), and another had no detectable viloxazine levels following Treatments A and C (despite a thorough investigation, no explanation was found for these anomalous results). Thus, data for these two subjects were excluded from the pharmacokinetic parameter summary statistics and the statistical analyses.
Two subjects discontinued early: one subject was terminated prior to dosing in Period 2 for protocol deviation (failure to consume the entire breakfast within the required timeframe), and another was terminated after dosing in Period 3 for failure to follow clinic rules (note that this subject’s pharmacokinetic data were included in the analysis). Subjects were 36.1 ± 9.18 years old (mean ± SD; range 21–51) and had a BMI of 26.45 ± 2.68 kg/m2 (mean ± SD). Subjects were 51.9% Black or African American, 37.0% White, and 7.4% Asian.
3.2 Effect of Sprinkling on Viloxazine Pharmacokinetics and Bioavailability
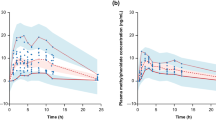
Pharmacokinetic parameters for viloxazine are listed in Table 1, and plasma concentrations over time are shown in Fig. 2. Under fasting conditions, sprinkling the contents of a viloxazine ER capsule on one tablespoon of applesauce (Treatment B) did not appreciably alter the pharmacokinetic profile of viloxazine relative to consuming the intact capsule (Treatment A). The median time to maximal concentration was relatively unchanged (Tmax = 5.0 h for both Treatment A and B), as were mean AUClast and AUCinf. Sprinkling (Treatment B) slightly lowered the mean maximal concentration (Cmax = 1.24 µg/mL ± 0.25 SD) relative to the intact capsule (Treatment A, Cmax = 1.39 µg/mL ± 0.37 SD) and slightly prolonged the mean elimination half-life (Treatment B, t1/2 = 9.52 h ± 3.21 SD; Treatment A, t1/2 = 6.75 h ± 3.38 SD), but these differences were within normal variability.
Viloxazine concentration–time profiles after viloxazine ER 200 mg consumption during each of three treatments, plotted on linear (a) and semi-logarithmic (b) scales. In Treatment A (open circles, solid line), subjects consumed a single intact capsule of viloxazine ER after a minimum 10-h fast. In Treatment B (red diamonds, broken line), subjects consumed viloxazine ER sprinkled on a tablespoon of applesauce after a minimum 10-h fast. In Treatment C (blue triangles, dotted line), subjects consumed a single intact capsule of viloxazine ER after a high-fat meal. Mean ± 95% confidence interval are shown. viloxazine ER viloxazine extended-release
The GMR (90% CI) for viloxazine sprinkled vs. intact capsule were Cmax = 90.10% (83.35–97.40), AUClast = 93.71% (89.09–98.57) for and AUCinf = 95.37% (89.80–101.28) (Table 2, Fig. 3). The 90% CIs for each pharmacokinetic parameter were fully contained within the predetermined no-difference limits of 80–125%, suggesting no impact of sprinkling on overall viloxazine relative bioavailability.
Relative bioavailability of viloxazine under fasted conditions after administration of intact versus sprinkled viloxazine ER capsules. Relative bioavailability of viloxazine in plasma after Treatment B (sprinkled) relative to Treatment A (intact capsule) after a minimum 10-h fast. Fold change ± 90% confidence interval (log scale) are shown. AUCinf area under the concentration–time curve from time 0 to infinity, AUClast area under the concentration–time curve from time 0 to the last quantifiable concentration, Cmax maximum measured plasma concentration, viloxazine ER viloxazine extended-release
3.3 Effect of Food on Viloxazine Pharmacokinetics and Bioavailability
Pharmacokinetic parameters for viloxazine are listed in Table 1 and plasma concentrations over time are shown in Fig. 2. Consuming an intact capsule of viloxazine ER with a high-fat meal (Treatment C) did not appreciably alter the pharmacokinetic profile of viloxazine relative to consuming viloxazine ER after a 10-h fast (Treatment A). The mean AUClast and AUCinf were relatively unchanged after the high-fat meal, although median time to maximal concentration was slightly increased by 2 h after the meal (Treatment C, Tmax = 7.0 h) relative to after fasting (Treatment A, Tmax = 5 h). Consuming viloxazine ER with a high-fat meal (Treatment C) slightly lowered the mean maximal concentration (Cmax = 1.25 µg/mL ± 0.29 SD) relative to the fasted condition (Treatment A Cmax = 1.39 µg/mL ± 0.37 SD) and slightly prolonged the mean elimination half-life (Treatment C, t1/2 = 8.78 h ± 3.69 SD; Treatment A t1/2 = 6.75 h ± 3.38 SD), but these differences were within normal variability and did not appear to be significant.
The GMR (90% CI) for viloxazine in fed state vs. fasted state were Cmax = 90.86% (84.05–98.21), AUClast = 89.68% (85.26–94.33), and AUCinf = 92.35% (86.96–98.07) (Table 3, Fig. 4). The 90% CIs for each pharmacokinetic parameter were fully contained within the predetermined no-difference limits of 80–125%, suggesting no impact of food on overall viloxazine relative bioavailability.
Relative Bioavailability of Viloxazine After Administration of Intact Viloxazine ER Capsules Under Fasted versus Fed Conditions. Relative bioavailability of viloxazine in plasma after Treatment C ( high-fat meal) relative to Treatment A (10-h fast). Fold change ± 90% confidence interval (log scale) are shown. AUCinf area under the concentration–time curve from time 0 to infinity, AUClast area under the concentration–time curve from time 0 to the last quantifiable concentration, Cmax maximum measured plasma concentration, viloxazine ER viloxazine extended-release
3.4 Safety
All subjects that received at least one dose of the study drug were included in the safety population (N = 27). Most of the reported AEs were mild; there were no serious AEs, AEs that led to subject withdrawal, or deaths. The only AEs considered moderate were constipation and fatigue, reported by one subject each. A total of nine (33%) subjects reported AEs over the course of the study. Seven (26%) subjects reported AEs that were considered to be treatment related by the investigator (Table 4). There were no clinically significant abnormal results for clinical laboratory tests, ECGs, vital signs, or physical exams. There were no AEs related to clinical laboratory test results.
4 Discussion
This study was designed to test the feasibility of different oral viloxazine ER administrations by comparing the pharmacokinetics of viloxazine after consuming an intact or sprinkled capsule, and under fed or fasted conditions. We report no significant impact of sprinkling or a high-fat meal on viloxazine pharmacokinetics after a single dose of viloxazine ER 200 mg capsule in healthy adults. Specifically, although the viloxazine Cmax, AUClast, and AUCinf after sprinkling or a high-fat meal was 5–10% lower than that following the consumption of an intact capsule in the fasted state, these differences were not significant. For all three pharmacokinetic parameters, the 90% CIs were fully contained within the accepted 80–125% limits, suggesting no appreciable effect of either condition. Further, for all three conditions, the viloxazine ER 200 mg dose appeared to be safe and well tolerated, with no major safety concerns or clinically significant laboratory findings.
4.1 Pill Swallowing as a Barrier to Medication Adherence
By visual inspection of the concentration–time curves (Fig. 2), sprinkling viloxazine ER on applesauce and consuming it without chewing appeared to slightly decrease viloxazine exposure, but this change was well within the range of normal variability (as evidenced by the SDs and CVs reported in Table 1) and was not significant after a statistical comparison (Table 2). These results should reassure clinicians with patients seeking treatment for ADHD who also experience difficulty or aversion to swallowing pills (e.g., dysphagia). Difficulty swallowing pills is especially common among children, though it is also seen among the elderly, individuals with certain medical conditions (e.g., gastroesophageal reflux), and individuals with developmental and autism spectrum disorders with associated food limitations such as tactile sensitivities [35,36,37].
As it is a developmental disorder, treatment for ADHD usually occurs over the long term (e.g., over months or years) [1], presenting numerous opportunities for treatment nonadherence when medications have to be taken daily (or several times a day in the case of medium- or short-acting stimulants) [21, 22]. Alternative formulations such as liquids, chewables, or transdermal patches are relatively uncommon among ADHD treatments [13], limiting the clinician’s armamentarium considerably.
Unless explicitly stated otherwise, chewing the contents of a sprinkled capsule or crushing a pill may alter the protective/enteric coating or other features of the medication, bypassing protective design elements intended to control how and when the drug is metabolized [38, 39]. Such alterations have the potential to significantly alter the absorption pharmacokinetics and subsequently impact efficacy or safety outcomes [14, 15, 38,39,40]. As a result, they are generally inadvisable for most ADHD medications, particularly sustained, controlled, extended, or long-acting release drug formulations that may rely on coatings, beads, or other technologies to control drug absorption, metabolism, and exposure [38, 41].
The pharmacokinetics of several stimulant ADHD medications have been shown to be unaffected when administered as a sprinkle compared to an intact capsule [13, 38]. Notably, this is not the case for the nonstimulant ADHD drugs atomoxetine and guanfacine XR, as their prescribing information currently advises that they be consumed intact [11, 12]. Although many patients tolerate and respond well to stimulant therapy, an estimated 30% of patients are either nonresponsive or inadequately responsive to stimulants, experience limiting side effects, or are otherwise not candidates for such therapy [42]. For these patients, nonstimulant treatment options (either alone or in combination with stimulants) might be appropriate. The present data demonstrate that viloxazine ER may be the first nonstimulant ADHD medication that can be safely sprinkled on applesauce without any appreciable change in bioavailability, suggesting that viloxazine ER capsules might be a suitable option for individuals who are seeking nonstimulant ADHD treatment but are unable or unwilling to swallow whole pills.
4.2 Meals as a Source of Pharmacokinetic Variability
Consuming viloxazine ER capsules with a high-fat meal delayed median Tmax by 2 h (Table 1), consistent with studies demonstrating delayed gastric emptying and prolonged absorption with oral drugs when administered with food [17]. As with sprinkling, the resulting small decrease in overall viloxazine exposure in Fig. 2 was well within the range of normal variability (i.e., SD and CV, Table 1), and was not significant after a statistical comparison (Table 2). Although it is recommended that viloxazine ER capsules should be taken at approximately the same time each day, these data suggest that they can be taken without regard to mealtimes, which may support improved treatment adherence.
Consistent with FDA recommendations, this study used a high-calorie (800–1000 calories), high-fat (~50% of the calories) meal to test the impact of food on viloxazine bioavailability [29]. While the physiological mechanisms governing rate of digestion and metabolism are complex [17], a high-fat meal is thought to provide the greatest opportunity to elucidate any impact of food on the systemic availability of the drug [29]. Although the present study only examined the impact of a high-fat meal and did not probe other meal compositions (e.g., low fat), such variations are unlikely to result in either statistically or clinically significant changes to viloxazine bioavailability. Notably, in four recent phase 3 trials in children and adolescents in which viloxazine ER was shown to be safe and effective, participants were permitted to consume viloxazine ER capsules either with or without food [23,24,25,26].
4.3 Conclusions and Further Considerations
Under all three treatment conditions, viloxazine ER was well tolerated, with few reported or observed AEs. Although many medications may be associated with greater gastrointestinal AEs when taken on an empty stomach, this did not appear to be the case in the present study (Table 4). Importantly, the dose used in the present study (200 mg) was lower than the higher 400–600 mg/day range used in adolescent and adult studies [25,26,27], and lower than the highest approved dose (400 mg/day) in pediatric patients [43]. Despite this, the Tmax reported here (5 h) is consistent with previous single-dose studies using viloxazine ER 700 mg in adults [30, 33, 34], and chronic once-daily doses of 100, 200, 400, and 600 mg in children and adolescents [44]. Because the pharmacokinetics of viloxazine appear to be linear across the therapeutic range (data on file), the pharmacokinetic effects demonstrated here are likely to be true at the higher end of the dosing range.
In conclusion, this study found no difference in the relative bioavailability of viloxazine when the viloxazine ER capsule contents were sprinkled on applesauce under fasted conditions or administered as an intact capsule with a high-fat meal to subjects who were healthy adults.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC: American Psychiatric Publishing; 2013.
Danielson ML, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199–212.
Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9.
Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163(4):716–23.
Reynolds K, et al. Prevalence of psychiatric disorders in US older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74–81.
Stein DJ, et al. Lifetime prevalence of psychiatric disorders in South Africa. Br J Psychiatry. 2008;192(2):112–7.
Alegría M, et al. Prevalence of psychiatric disorders across Latino subgroups in the United States. Am J Public Health. 2007;97(1):68–75.
Kelly J, D’Cruz G, Wright D. Patients with dysphagia: experiences of taking medication. J Adv Nurs. 2010;66(1):82–91.
Stegemann S, Gosch M, Breitkreutz J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm. 2012;430(1–2):197–206.
Gardiner P, Dvorkin L. Promoting medication adherence in children. Am Fam Physician. 2006;74(5):793–8.
Eli Lilly and Company, LLC. Strattera (atomoxetine): prescribing information. Indianapolis: Eli Lilly and Company, LLC; 2017.
Shire US Inc. Intuniv (guanfacine extended-release): prescribing information. Lexington: Shire US Inc.; 2016.
Cutler AJ, Mattingly GW. Beyond the pill: new medication delivery options for ADHD. CNS Spectr. 2017;22(6):463–74.
Osterberg L, Urquhart J, Blaschke T. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–9.
Morrison A, Stauffer ME, Kaufman AS. Relationship between adherence rate threshold and drug ‘forgiveness.’ Clin Pharmacokinet. 2017;56(12):1435–40.
Cornish P. “Avoid the crush”: hazards of medication administration in patients with dysphagia or a feeding tube. Can Med Assoc J. 2005;172(7):871–2.
Davit BM, Conner DP. Food effects on drug bioavailability: implications for new and generic drug development. In: Krishna R, Yu L, editors. Biopharmaceutics applications in drug development. Boston, MA: Springer; 2008. p. 317–35.
Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213–24.
Sauer J-M, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet. 2005;44(6):571–90.
Martinez-Raga J, Knecht C, de Alvaro R. Profile of guanfacine extended release and its potential in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatr Dis Treat. 2015;11:1359.
Spencer L. Difficulty with pill swallowing is blamed for non-compliance. Paediatr Nurs. 2009;21(6):13–4.
Santer M, et al. Treatment non-adherence in pediatric long-term medical conditions: systematic review and synthesis of qualitative studies of caregivers’ views. BMC Pediatr. 2014;14(1):1–10.
Nasser A, et al. A phase 3, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended release) in the treatment of ADHD in school-age children. Clin Ther. 2020;42(8):1452–66.
Nasser A, et al. Once-daily SPN-812 200 and 400 mg in the treatment of ADHD in school-aged children: a phase III randomized controlled trial. Clin Ther. 2021;43(4):684–700.
Nasser A, et al. A phase 3, placebo-controlled trial of once-daily viloxazine extended-release capsules in adolescents with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2021;41(4):370–80.
Nasser A, et al. A phase 3 placebo-controlled trial of once-daily 400-mg and 600-mg SPN-812 (viloxazine extended-release) in adolescents with ADHD. Psychopharm Bull. 2021;51(2):43–64.
Supernus Pharmaceuticals Inc. Supernus announces positive results from phase III study for SPN-812 in adults with ADHD. 2020. https://ir.supernus.com/news-releases/news-release-details/supernus-announces-positive-results-phase-iii-study-spn-812-0.
Yu C. Metabolism and in vitro drug-drug interaction assessment of viloxazine. Xenobiotica. 2020;50(11):1285–300.
Center for Drug Evaluation and Research. Food-effect bioavailability and fed bioequivalence studies: guidance for industry. Silver Spring: Center for Drug Evaluation and Research; 2002.
Wang Z, et al. Impact of paroxetine, a strong CYP2D6 inhibitor, on SPN-812 (viloxazine extended-release) pharmacokinetics in healthy adults. Clin Pharmacol Drug Dev. 2021. https://doi.org/10.1002/cpdd.948.
Tulloch SJ, et al. SLI381 (Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparison of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22(11):1405–15.
Pentikis HS, et al. Methylphenidate bioavailability in adults when an extended-release multiparticulate formulation is administered sprinkled on food or as an intact capsule. J Am Acad Child Adolesc Psychiatry. 2002;41(4):443–9.
Faison SL, et al. Pharmacokinetics of co-administered viloxazine extended-release (SPN-812) and lisdexamfetamine in healthy adults. J Clin Psychopharm. 2021;41(2):155–62.
Faison SL, et al. Pharmacokinetics of co-administered viloxazine extended-release (SPN-812) and methylphenidate in healthy adults. Clin Drug Investig. 2020;41:149–59.
Marquis J, et al. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int J Clin Pharm. 2013;35(6):1130–6.
Schiele JT, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4):937–48.
Ghuman JK, et al. Behavioral training for pill-swallowing difficulties in young children with autistic disorder. J Child Adolesc Psychopharmacol. 2004;14(4):601–11.
Lee HS, et al. Sprinkle formulations—a review of commercially available products. Asian J Pharm Sci. 2020;15(3):292–310.
Siegel RA, Rathbone MJ. Overview of controlled release mechanisms. In: Seipmann J, Siegel RA, Rathbone MJ, editors. Fundamentals and applications of controlled release drug delivery. Boston, MA: Springer; 2012. p. 19–43.
Cattaneo D, et al. Comparison of the in vivo pharmacokinetics and in vitro dissolution of raltegravir in HIV patients receiving the drug by swallowing or by chewing. Antimicrob Agents Chemother. 2012;56(12):6132–6.
Parker C. Pharmacological treatments for ADHD. Prog Neurol Psychiatry. 2013;17(4):11–20.
Spencer T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35(4):409–32.
Supernus Pharmaceuticals, Inc. Qelbree (viloxazine extended-release capsules): prescribing information. Rockville, MD: Supernus Pharmaceuticals, Inc.; 2021.
Nasser A, et al. Population pharmacokinetics of viloxazine extended-release capsules in pediatric subjects with attention deficit/hyperactivity disorder. J Clin Pharmacol. 2021. https://doi.org/10.1002/jcph.1940 (online ahead of print).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This trial was fully sponsored by Supernus Pharmaceuticals, Inc. The protocol was designed by Supernus employees and the study was conducted by Worldwide Clinical Trials.
Availability of data and materials
The data are not available in a repository, but reasonable requests can be directed to Azmi Nasser at anasser@supernus.com.
Code availability
Not applicable.
Conflict of interest
A. Nasser, Z. Wang, A Kosheleff, L. Adeojo, O. Odebo, and T. Liranso are employees of Supernus Pharmaceuticals, Inc. S. Schwabe was an employee of Supernus Pharmaceuticals, Inc. at the time of this work.
Ethics approval
This trial was conducted in accordance with the Helsinki Declaration and the International Council for Harmonisation Note for Guidance on Good Clinical Practice. The trial conduct was reviewed and approved by IntegReview Institutional Review Board (Austin, TX, USA).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Subjects provided and signed informed consent regarding the publication of their data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Z., Kosheleff, A.R., Adeojo, L.W. et al. Impact of a High-Fat Meal and Sprinkled Administration on the Bioavailability and Pharmacokinetics of Viloxazine Extended-Release Capsules (QelbreeTM) in Healthy Adult Subjects. Eur J Drug Metab Pharmacokinet 47, 69–79 (2022). https://doi.org/10.1007/s13318-021-00729-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00729-6